www.elsevier.comrlocateranireprosci
Reproductive aspects and storage of semen in
Camelidae
P.W. Bravo
a,), J.A. Skidmore
b, X.X. Zhao
c aCentro Experimental La Raya, UniÕersidad Nacional San Antonio Abad, Cusco, Peru
b
The Camel Reproduction Centre, Dubai, United Arab Emirates
c
Department of Veterinary Medicine, Gansu Agricultural UniÕersity, Lanzhou, Gansu, People’s Republic of China
Abstract
The characteristics of male and female reproductive tracts and reproductive physiology in camelids are described. An account is given on methods of collection, characteristics and storage
Ž .
of semen, and fertility after artificial insemination AI with fresh, liquid-stored and frozen–thawed
lamoid and camel semen.q2000 Published by Elsevier Science B.V.
Keywords: Camel; Dromedary; Bactrian; Llama; Alpaca; Vicuna
1. Introduction
There are two genera within the Camelidae family, Camelus and Lama. The genus
Ž .
Camelus consists of Camelus dromedarius, dromedary camel one hump and C.
Ž .
bactrianus, Bactrian camel two humps . Both species are also known as Old World
camelids. The genus Lama constitutes of four species: two domesticated, Lama glama,
llama; L. pacos, alpaca; and two wild species, L. guanicoe, guanaco, and L.Õicugna,
vicuna. They are collectively known as South American or New World camelids, and for the purpose of this review, as lamoids. All camelids live on harsh conditions, camels in desert areas, and lamoids on high altitude terrains. All of them have 37 pairs of chromosomes. Both camels and lamoids can interbred within their genera and the offspring is fertile.
)Corresponding author. Present address: Dept. Animal Science, Brighan Young University, Provo, UT
84602, USA.
0378-4320r00r$ - see front matterq2000 Published by Elsevier Science B.V. Ž .
Ž .
Progress on artificial insemination AI , semen preservation and related techniques in camelids has been slow in comparison to other livestock species. As there is relatively limited information in the literature on the anatomy and physiology of the male and female camelid, first, a short account on these aspects is given.
2. The male reproductive tract
The testes of Camelidae are ovoid in shape and are found in the scrotum in a perineal position, as in the dog and pig. The testes of camels are descended at birth and very small, but an increase in size occurs at the onset of puberty, with the maximum weight attained at 10–14 years of age. In lamoids, the testes may not be in the scrotum at birth, but descended by 6 months of age. In mature alpaca and llama, the testes weigh 18 and 24 g, respectively, which represents approximately 0.02–0.03% of the total body weight. Testicular size and weight for llamas, alpacas and vicunas are given in Table 1.
The camel testes vary in length from 7 to 10 cm in dromedary and 12 to 14 cm in
Ž
Bactrian and weigh 80–100 g each, the left tending to be bigger than the right Tayeb,
.
1951–1952; Zhao, 1995 . It should be noted that there are seasonal changes in the size of the testes, which become enlarged and protrude when male camels are sexually active in the rutting season.
The diameter of the seminiferous tubules in the alpaca is between 174 and 237mm
ŽCasas et al., 1993–1996 . In the camel, the seminiferous tubules have smaller diameter.
when the male is not in rut. The season does not have an effect on the size of spermatozoa, although it can effect their number. The estimated production of camel spermatozoa is 8.1=106 sperm cellsrday at the end of spring, and it drops to 4.2=106
dayy1 at the end of summer. There are no differences in the size of Camelidae
Table 1
Ž . Ž .
Mean testicular size length=transverse axis and weight g of llamas, alpacas and vicunas of different ages
Žns158 alpacas, 54 llamas, 6 vicunas.
a b c
Ž .
Age months Llama Alpaca Vicuna
Ž .
Size cm Weight Size Weight Size
spermatozoa, with the total length being 50mm, of the head 5.3mm, and the tail 37mm
ŽPalomino, 1962; Merlian et al., 1979 . The cycle of the seminiferous epithelium of the.
Ž .
male llama series of changes with well-defined cellular associations has been defined as eight stages with the relative frequencies of cell populations being 9.8%, 12.5%, 17.7%, 14.1%, 5.8%, 8.1%, 13.0% and 18.9%, for stages 1 through 8, respectively
ŽDehlon and von Lawzewitch, 1987 ..
The epididymis is located on the anterior edge of the testis and extends from the interior extremity to just above the upper edge. Macroscopically, it can be divided into head, body and tail regions. Histologically, the epididymis has six segments differenti-ated by roman numerals. Segment I is short where ductuli efferentia join the epididymis. Segments II and III show maximal epithelial height and mitotic activity, respectively,
Ž .
and some lactate dehydrogenase LDH activity. Epithelial cells in segment IV contain periodic acid-Schiff activity, amylase and neuramidase-resistant secretory granules. Segment V shows a strong acid phosphatase and LDH activities. Segment VI has moderate acid phosphatase and high LDH activities, as well as maximum sperm storage
ŽDehlon and von Lawzewitsch, 1994 ..
The vas deferens, enclosed in the spermatic cord, measures 35–40 cm in lamoids and 45–50 cm in camels. It has two portions, the proper vas deferens and a fusiform
Ž .
dilation, the ampulla. A distinct ampulla is not observable in camels Zhao, 1995 . In lamoids, the ampulla is approximately 4 mm in diameter and is located on the dorsal
Ž .
aspect of the bladder Osorio and San Martin, 1966 . Histological studies of the ampulla reveal a dilation of the submucosa with the presence of glandular acini. Observations in adult alpacas do not reveal large numbers of spermatozoa in the ampulla; hence, it is not a place for storage of spermatozoa as occurs in bulls and rams. This may be the reason why semen collection by electroejaculation is not reliable.
The penis is fibroelastic with its sigmoid-shaped flexure in front of the scrotum, not behind it as in the bull. The length is 35–40 cm in lamoids and 37–60 cm in camels and
Ž . Ž .
the diameter decreases from its root 22 cm towards the glans penis 4 cm . The glans penis is 8.5 cm long in camels and ends in a cartilaginous process, which supposedly directs the penis through the cervix of the female during copulation. It is curved along its vertical plane, giving it a hook shape with a definite neck between the glans of the body of the penis. These observations are important when designing an artificial vagina
ŽAV . The urethral opening is located at the tip of a second and smaller urethral process..
The detachment of the glans penis from the prepuce in lamoids seems to be related to the secretion of testosterone. The initiation of penis–prepuce detachment in llamas appears at 13–15 months and continues from the tip caudally. By 21–22 months of age, the penis is completely detached and the testosterone concentrations are almost at adult values. In alpacas, 12.2% of males shows an initiation of detachment by 12 months of age and by 24 and 34 months of age, the penis is free from any attachment to the prepuce in 59.5% and 94.4% of males, respectively.
The accessory sex glands are the prostate and the bulbourethral glands, as camelids do not have vesicular glands. The prostate gland has two components, a compact and diffuse part, the two forming an H-shape which lies dorsal to the pelvic urethra. The capsule of the prostate is thick and supplied with smooth muscles fibres. The bul-bourethral glands are two compact, almond-shaped structures and are located on either side of the terminal portion of the pelvic urethra. They may be palpated digitally via the rectum. The capsule surrounding these glands contains striated muscle.
3. The female reproductive tract
The size and shape of camelid ovaries vary with age and with their content of
Ž .
follicles and corpora lutea CL . In nulliparous females, the ovaries are oval or circular, flattened laterally and with an irregular surface resulting from many small follicles of
Ž .
3–5 mm in diameter Elwishy, 1992 . Each ovary weighs between 3 and 4 g in
Ž
dromedaries, 5 and 15 g in Bactrians and 1.9 and 2.4 g in lamoids Sato and Montoya,
.
1990 . Mature follicles and current CL project from the main contour of the ovary and give it an exaggerated, lobular form. Each ovary is enclosed in a long conical, pocket-like fold of mesosalpinx called the bursa oÕarii, the apex of which forms a large
Ž .
circular orifice within which lie the fimbriae of the oviduct Nawito et al., 1967 . The length of oviducts is 17–28 cm in camels and 11–21 cm in lamoids. The isthmus is less coiled than the ampulla and ovarian parts of the tube, and the fimbria lies in the bursa at a short distance from the ovary. Each oviduct opens into the uterine horn via a narrow orifice at the summit of a protuberant papilla which can be as much as 3–4 mm in height. Unlike other mammals, the oviducts are enlarged at the uterine end and this
Ž
unique arrangement allows prolonged storage of large numbers of spermatozoa Bravo
.
et al., 1996a .
The uterus is bicornuate with a short body of only 2.0–3.5 cm in dromedaries and lamoids, and 8.5–9.5 cm in Bactrians. In camels, the two uterine horns diverge and taper anteriorly to form a T shape with the uterine body, but the left horn is distinctly bigger
Ž .
and longer than the right Smuts and Bezuidenhout, 1987; Chen et al., 1985 . In lamoids, the uterine horns also diverge to form a Y shape with the uterine body. The endometrium of the uterine body and horns is smooth and no cotyledons are present.
4. Ovarian physiology
4.1. Follicular phase of the oÕarian cycle
All camelids are induced ovulators, i.e. they ovulate after mating or the administra-tion of a hormone with luteinizing activity. Hence, the follicular phase is constant and interrupted by a luteal phase after ovulation. If mating does not occur, follicles grow and regress constantly in a wave-like fashion. Therefore, it is more appropriate to use the term follicular wave pattern, rather than oestrous cycle. In lamoids, follicles become dominant when they are 6 mm in diameter. Dominance is exerted on the cohort of follicles on the ipsilateral and contralateral ovaries. A follicular wave lasts, on average,
Ž .
10–12 days range 7–14 and the dominant follicle alternates between the ovaries in
Ž .
85% of the cases Bravo and Sumar, 1989; Bravo et al., 1990 . Follicle waves overlap
Ž
and this determines constant and long periods of sexual receptivity of the female San
.
Martin et al., 1968; Bravo et al., 1994 .
Based on observations of follicular waves in the dromedary camel and on
determina-Ž .
tions of plasma oestradiol concentrations Elias et al., 1984 , the mean duration of the
Ž .
oestrous cycle has been reported to be 17.2–23.4 days in India Joshi et al., 1978 , 24.2
Ž .
days in Egypt, 28 days in Sudan Musa and Aubusineina, 1978 , and 16–22 days in Bactrians. However, oestrous behaviour can be difficult to detect since the female may show receptivity to the male outside the confines of oestrus when she has mature follicles in her ovaries. The dromedary will ‘succumb’ to being mated at any stage of her ovarian cycle, even during early pregnancy and during the luteal phase that follows a
Ž .
sterile mating Arthur et al., 1985 . Studies using real time ultrasonography have shown
Ž .
that these waves of follicular activity are clearly divisible into three stages: i the
Ž . Ž .
growth phase lasting a mean "s.d. of 10.9"3 days; ii a mature phase of 7.6"4.2
Ž .
days; and iii a regression phase of 11.9"4.2 days with the mature follicles reaching a
mean maximum diameter of 2"0.3 cm, or may in some cases continue to grow to as
Ž .
large as 6 cm in diameter Skidmore et al., 1995 .
4.2. OÕulation
There are various stimuli that induce ovulation in camelids, but the most common
Ž
triggering mechanism is mating by an intact andror vasectomized male Fernandez-Baca
.
et al., 1970; Marie and Anouassi, 1987 . In Bactrians, dromedaries and alpacas, mechanical stimulation of the cervix and vagina does not trigger ovulation, nor does mounting alone. However, the presence of whole semen deposited deep in to the vagina
Ž . Ž .
induced ovulation in Bactrians Chen et al., 1985 and alpacas Rios, 1989 and even the
Ž
intramuscular injection of semen, or seminal fluid provokes ovulation in Bactrians Zhao
. Ž . Ž .
et al., 1990, 1992a . Similarly, luteinizing hormone 300 IU , hCG 100–200 IU and
Ž . Ž
GnRH analogue 250–500mg have been shown to trigger ovulation in Bactrians Zhao
. Ž .
et al., 1990 and, in dromedaries, both hCG 3000 IU and 20 mg GnRH analogue
Ž . Ž
the presence of 1–1.9 cm diameter growing follicles Musa et al., 1990; McKinnon
.
and Tinson, 1992; Skidmore et al., 1996 . In lamoids, it was found that 750 IU hCG was
sufficient to induce ovulation in 100% of alpacas and 80–800 mg GnRH provoked
ovulation in 80% of llamas and alpacas. In lamoids, the minimum ovulatory size follicle has been determined as 7 mm and the follicles should be in growing phase. Ovulation cannot be induced in the presence of smaller follicles due to the lack of release of LH by the pituitary gland and luteinization of the follicle occurs if female lamoids are bred in
Ž .
the presence of regressing follicles Bravo et al., 1991 .
4.3. Synchronisation of female camels
When preparing camels for AI, it is necessary to be able to control follicular growth and synchronise ovulation in groups of animals. This poses particular problems in the camels due to the absence of a cyclical CL that would exist in spontaneously ovulating
species such as the horse and cow, and would be lysable with PGF2a or one of its
analogues. There are few published reports on the use of progestagens to control follicular growth in the camel. Treating them with PRIDS has been found to be unreliable, because removal of the PRID may be followed very rapidly by spontaneous
Ž .
ovulation in some animals Marie and Anouassi, 1987; Cooper et al., 1992 . Daily intramuscular injections of 100 mg progesterone-in-oil for 10–15 days has been shown to limit follicular growth and is particularly effective at hastening the regression of large
Ž .
unovulated follicles McKinnon and Tinson, 1992; McKinnon et al., 1994 . However, as growth rates of individual follicles can be highly variable between different animals, attempts to synchronise them with progesterone — so that they only have a few small follicles present at the end of treatment — is not always beneficial as it does not necessarily mean that they will all have a mature ovulable follicle between 1 and 1.9 cm in their ovaries at the same time.
Synchronisation of female lamoids for AI has not been explored. Whenever insemina-tion was intended, sexually receptive females to vasectomised males andror intact males have been used. Then, ovulation has been provoked by mating to a vasectomised male
or administration of hCG, andror GnRH.
5. Methods of semen collection
Collection of semen from male camelids is complicated by the position during
Ž .
mating, long duration of copulation 5–50 min , and intrauterine semen deposition. Copulation takes place with the female in recumbent position, the male squatting behind and the male dictates the duration of copulation.
5.1. Collection of semen in lamoids
A human condom, containing a small steel ball at its end, is inserted into the vagina of a sexually receptive female by the aid of a glass rod, then a small amount of air is blown through a tube to adhere the condom to the vaginal wall and an adhesive tissue can be used to attach the outside of the condom to the vulva of the female. The disadvantages of this method are that males do not copulate for the normal length of
Ž
time and the females become uncooperative after a few attempts Mogrovejo, 1952;
.
Johnson, 1989; Sucapuca, 1991 .
When a sterile sponge is used for semen collection, it is inserted into the cranial
Ž .
vagina San Martin et al., 1968 ; however, the samples collected from 39 males
contained no spermatozoa in 20% of cases.
Collection of semen through a fistula in the penile urethra has the disadvantage of
Ž .
post-operative care and impairment of the male von Kubiceck, 1974 .
For semen collection by electroejaculation, sedation or general anaesthesia of the
Ž .
animal may be required Johnson, 1989; McEvoy et al., 1992 . The glans penis should be exteriorized with the aid of gauze and the semen collected into a pre-warmed tube. After insertion of the probe into the rectum, electrical stimulation is initiated at the lowest setting and gradually increased to 4–6 s of stimulation, followed by 4 s resting period. Several collection tubes should be at hand because of the possibility of urine
Ž .
contamination Fernandez-Baca and Calderon, 1963–1966 . Due to the short duration of ejaculation, the semen obtained is often of poor quality.
The post-copulatory vaginal aspiration is easily applicable and is a non-invasive method, but the semen samples obtained usually are incomplete and often contaminated with blood.
Collection of semen by an AV mounted inside a dummy is more natural and reliable
Ž .
than the other methods Sumar and Leyva, 1981; Moscoso, 1996; Bravo et al., 1997a . The difficulty is that male llamas and alpacas have to be trained to the dummy, but after acceptance, they keep the normal ejaculation time and the ejaculates are similar to those deposited into the female reproductive tract. The characteristics of lamoid semen collected by the various methods are shown in Table 2.
5.2. Collection of semen in camels
The accepted methods of semen collection in camels are by AV and by
electroejacu-Ž
lation. For collection by AV, a modified bull vagina 30 cm long, 5 cm internal
.
diameter has given the best results. However, care must be taken to avoid the ejaculate making contact with the rubber liner of the AV, since this has been shown to adversely affect sperm motility. Hence, a shortened AV may be used, allowing the semen to pass directly into the collection flask. Alternatively, an additional disposable plastic inner liner may be inserted to avoid contact with the rubber material. The AV is filled with
water at 55–608C to give an internal temperature of 38–408C and a clear glass
Ž .
water-jacketed 35–378C semen vessel is attached to the apex of the cone-shaped
Table 2
Mean characteristics of lamoid semen collected by different methods
Method of Volume pH Motility Concentration Normal Reference
Ž . Ž . Ž .
collection ml % ml spermatozoa
Ž .%
Condom 1.9 8.3 low 33,320 41 Mogroveja, 1952
Condom 0.7 7.3 slow 83,750 – Sucapuca, 1991
Electroejaculation 1.4 7.0 low 31,688 – Fernandez-Baca and
Calderon, 1963–1966
Electroejaculation 1.0 7.1 48.2 38,705 Cardenas et al., 1987
Urethral fistula 6.6 7.5 – 600,000 – von Kubiceck, 1974
Vaginal sampling 1.2 7.4 slow – 10–78 Neely, 1993
AV 2.8 – fair 192,000 – Sumar and Leyva, 1981
AV 0.5 – low 145,000 – Quispe, 1987
AV 2.3 7.1 30.6 339,040 – Cardenas et al., 1987
AV 1.7 – slow 150,000 – Garnica et al., 1993
a
AV 3.0 8.1 23.7 100,000 39.7 Lichtenwalner et al., 1996b
AV 1.9 7.8 85 147,500 75.9 Bravo et al., 1997a
a
Values for llama.
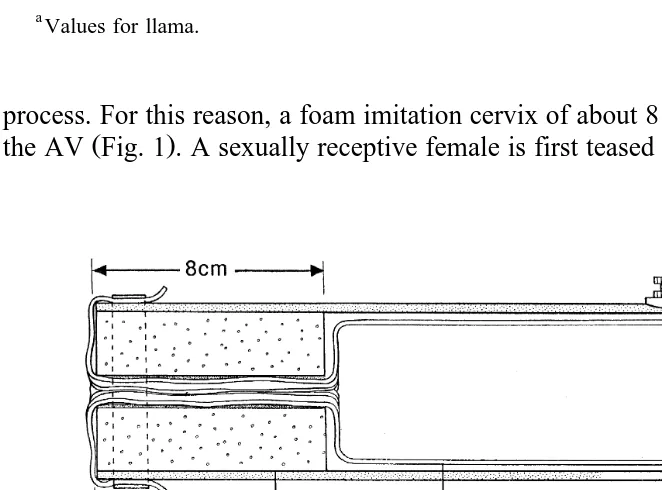
process. For this reason, a foam imitation cervix of about 8 cm in length is placed inside
Ž .
the AV Fig. 1 . A sexually receptive female is first teased by the male in order to make
olfactory contact. The male is then led up from behind to the sitting female with the operator sitting on the left side of the female. As soon as the male has sat down on the female and makes a few thrusts, the operator grasps the male’s sheath and directs his penis into the AV, and holds it there by manual pressure at the base of the scrotum. The male will make several thrusts, interspersed by periods of rest, until ejaculation is completed. The ejaculate usually occurs in fractions and this whole process can take between 5 and 10 min, although it may occasionally last for 20 min or even longer.
Electroejaculation may be employed if collection by AV cannot be achieved, using a
Ž .
standard bovine ejaculator Standard Precision Electronics, Denver . The male is secured
Ž .
in sternal recumbency and then turned on his side as described by Tingari et al. 1986 . Depending on the temperament of the individual male, the collection can be done with
Ž
or without the use of sedative and analgesic Domosedan Detomidine hydrochloride,
Ž . .
30–35mgrkg body weight bwt , i.v., or 70–80mgrkg bwt, i.m. , which is superior to other sedatives such as xylazine and acepromazine for obtaining semen by
electroejacu-Ž .
lation Jochle et al., 1990 . Ejaculation can be achieved by using the rectal probe lubricated with a copious amount of jelly to ensure good contact with the mucosa and giving two sets of stimulation, each of 10–15 pulses of 3–4 s duration at 12 V and 180 mA with a rest of 2–3 min between the two series of impulses. The semen is collected into a flask held at the prepucial orifice with occasional milking of the prepuce to expel all the semen. The volume of semen recovered by electroejaculation is usually less than that obtained by AV, but the other semen parameters are similar.
6. Characteristics of semen
6.1. Ejaculation and semen characteristics of lamoid semen
Ž
Ejaculation in lamoids is a continuous process throughout copulation dribble
ejacula-.
tion and it occurs with urethral pulses that may vary during copulation. It was observed
Ž y1.
that in llamas during the first 4 min, 11 urethral pulses 3.5 min occurred randomly,
Ž .
but were not associated with body strains. Then, a cluster of contractions 18.5
occurred, which were accompanied of body contractions. Thus, llamas ejaculate 18–19
Ž .
times during 22 min time of copulation Lichtenwalner et al., 1996a .
Lamoid semen is highly viscous, which makes handling and estimation of concentra-tion difficult. The problem may be overcome by using hydrolytic enzymes for liquefac-tion of semen. Trypsin at 1:250 concentraliquefac-tion was effective in liquefying alpaca semen. Trypsin at 1:500 concentration had partially liquefied the semen, and no liquefaction occurred with trypsin at 1:1000 and 1:2000 concentration. The percentage of live
Ž .
spermatozoa was not different for trypsin placed in the collecting tube 61% or added to
Ž .
the semen after ejaculation 62.9%; Bravo et al., 1997b . In a further attempt to
Ž .
eliminate the viscosity of semen in 2–3 min, collagenase 0.5 mgrml seemed to be a
better agent than trypsin. The reaction was irreversible, i.e. the liquefied semen did not
Ž .
Table 3
a Ž .
Biochemical components of lamoid and Bactrian camel semen mean"s.e.m.
b
Components Llama adult Alpaca Lamoid range Bactrian camel
3 years 6 years
Ž .
Chloride mEqrl 402"10 348"32 404"34 263–491 173"59
Ž .
Calcium mgrdl – 18"1 18"3 13–31 13"5
Ž .
Inorganic phosphate mgrdl 10" 12"2 8"0.4 7–17 13"1
Ž .
Total nitrogen mgrdl 623"23 548"50 647"32 398–697 –
Ž .
Adapted from Garnica et al. 1993 and Fuentes 1990 .
b Ž .
Zhao et al. 1992b .
In alpacas, the volume of ejaculate varies from 0.8 to 3.1 ml and sperm concentration
from 82,000 to 250,000rml, with the seminal plasma constituting about 85% of the
ejaculate. Mass motility is not present as it is seen in ram semen. In lamoids, individual motility and percentage of motile spermatozoa in a microscope field range from 69% to 91%. However, due to the viscosity of the semen, live spermatozoa appear to oscillate rather than progress in a forward direction. Morphologically, between 70.6% and 84.1% of spermatozoa is normal with 6.7% abnormal heads, 12.3% abnormal tails and 3.8%
Ž .
with cytoplasmic droplets Bravo et al., 1997a . The study of Lichtenwalner et al.
Ž1996b on seminal characteristics of male llamas revealed 23.7% motile, 39.7% normal.
Ž .
and a high proportion 60.3% of abnormal spermatozoa, with abnormal heads
account-Table 4
Mean characteristics of camel semen collected by two methods Method of collection
Concentration=10 ml 325 465 331 462
Ž .
Motility % 51 68 50 69
a
Ž . Ž .
Stained dead sperm % 18 14 19 15
b Ž .
Sperm showing morphological abnormalities % 28 24 27 24
Ž .
Sperm showing acrosome abnormalities % 8.5 7.5 8.1 7.7
a
Ž .
Eosin color test Bartmann, 1959 .
b
Ž .
Ž .
ing for 20.1%, followed by abnormal acrosome structure 12.9% and the presence of
Ž .
cytoplasmic droplets 11.1% . Most of the biochemical components of semen are
comparable to those of other farm animals with the exception of small amounts of
Ž . Ž .
fructose 4–6 mgrdl and citric acid 4.3 mgrdl which may be due to the absence of
Ž .
vesicular glands Garnica et al., 1995 . There is no significant difference regarding the
Ž .
biochemical components between the 3- and 6-year-old alpaca males Table 3 .
6.2. Characteristics of camel semen
Semen of camels is of grey to milky white colour, and as in lamoids, has a viscous consistency immediately after collection. If it is allowed to stand for 10–15 min, it partially liquefies and the spermatozoa attain motility. Semen used for AI or storage should have at least the characteristics shown in Table 4, as recommended also by
Ž . Ž . Ž .
Tingari et al. 1986 , Taha Ismail 1986 and Merkt et al. 1990 .
7. AI and storage of semen
7.1. Use of fresh undiluted and diluted semen in lamoids
AI in lamoids has been impeded by difficulties in semen collection and semen handling. Nonetheless, there is some progress and a brief discussion is given below.
Ž .
The first attempt on AI Fernandez-Baca and Novoa, 1968 involved the use of 42 female alpacas that were inseminated with fresh undiluted semen from two vicunas and
Ž .
four paco vicunas cross between alpaca and vicuna . Semen samples were obtained by electroejaculation and the females were induced to ovulate by copulation with vasec-tomized males. Insemination was done immediately after induction of ovulation and the semen was deposited at the point of bifurcation between the left and right uterine horns. A cria was born after 340 days of gestation.
The second study to determine the best time for insemination after induction of
Ž
ovulation used 96 alpacas and fresh semen collected by electroejaculation Calderon et
.
al., 1968 . The females were slaughtered 3 days after insemination and 75% had fertilised eggs when insemination was carried out between 35 and 45 h after induction of ovulation, in contrast to 12.5% when females were inseminated immediately or 18 h after induction of ovulation. The fertilization rate decreased to 58.3% when insemination occurred 52 h after induction of ovulation.
Ž .
Ž .
In a report by Bravo et al. 1997a , using semen collected by AV, 20 alpacas were inseminated via the cervix with undiluted semen and 20 alpacas by laparoscopy into the uterine horns with diluted semen. A mean fertility rate of 60% was obtained with no
Ž .
differences in fertility for methods of semen deposition. Pacheco 1996 used semen
Ž . Ž .
collected by AV and diluted with egg yolk 10% –citrate 3% for insemination of 80 alpacas. The semen was deposited into the uterine horns by cervix fixation through the rectum and ovulation was induced by treatment with GnRH 24 h before insemination. The fertility rate was 60%.
Ž .
In a study by Quispe 1996 , 8 million motile spermatozoa appeared to be sufficient
Ž .
for AI in alpacas. In the report of De la Vega 1996 , semen collected by AV was
Ž . Ž .
diluted with phosphate-buffered saline 8% and mixed with cria alpaca serum 10% . The semen was deposited through the cervix at the bifurcation of the uterine horns. The
Ž .
pregnancy rate was 54r133 40% . The best fertility rate was obtained when
insemina-tion took place 30 h after inducinsemina-tion of ovulainsemina-tion with a vasectomized male. In an
Ž .
incubation test 358C for viability of spermatozoa, egg yolk–citrate proved to be more
Ž
efficient than saline–phosphate or skim milk as diluting media for alpaca semen Bravo
.
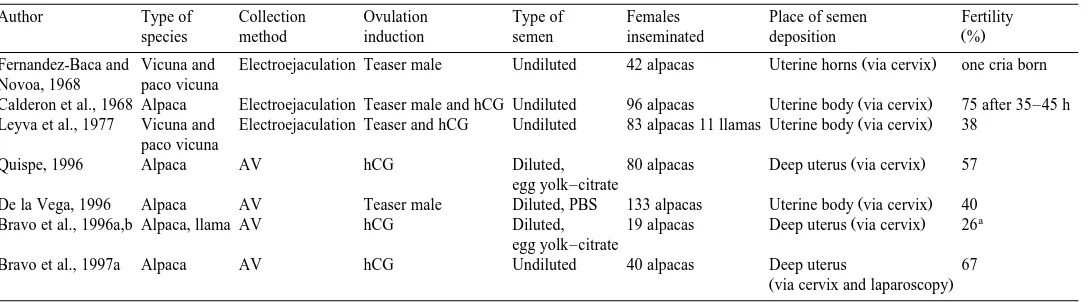
et al., 1997b . The methodologies and results of AI in lamoids are summarized in Table 5.
7.2. Frozen storage of semen in lamoids
There are few reports on freezing lamoid semen. In the report of McEvoy et al.
Ž1992 , llama semen collected by electroejaculation was frozen in straws after one-step. Ž .
dilution 1:2 with Tris–egg yolk–glycerol diluent. Only 10% of spermatozoa showed
Ž .
motility after thawing. On the other hand, Graham et al. 1978 observed 50%, 45% and 30% motile spermatozoa before freezing, after thawing, and after 8 days of frozen storage, respectively.
Ž .
A further report on freezing lamoid semen is by Bravo et al. 1996b . Semen from
Ž
three alpacas and two llamas was collected by AV and liquefied with collagenase 1
.
mgrml to facilitate semen handling. For two-step dilation of semen sodium, citrate
Ž2.9% –egg yolk 10% diluent was used. The second diluent fraction containing 7%. Ž .
glycerol was added at 15-min intervals in three equal parts when the semen diluted in
the first step was undergoing cooling to 48C in a refrigerator. The cooled semen was
loaded into 0.25 ml straws and equilibrated for 2 h. After freezing in liquid nitrogen vapor, the straws were plunged into liquid nitrogen. At the time of semen collection, 80% of spermatozoa showed oscillatory motility, and after final dilution and cooling
Žbefore freezing , 60% of spermatozoa was progressively motile. The semen was kept.
for 9 months before use. After thawing in water bath at 358C for 8 s, 30–40% of sperm
cells had oscillatory movement. The frozen–thawed semen was used for the insemina-tion of 19 female alpacas of which five were diagnosed pregnant 5 months later by ultrasonography, and gave birth to three male and two female crias.
7.3. Use of fresh and liquid-stored camel semen
For fresh use and liquid storage of dromedary semen, a number of extenders have
Ž .
()
Bra
Õ
o
et
al.
r
Animal
Reproduction
Science
62
2000
173
–
193
185
Table 5
Different methodologies and results of artificial insemination in lamoids
Author Type of Collection Ovulation Type of Females Place of semen Fertility
Ž .
species method induction semen inseminated deposition %
Ž .
Fernandez-Baca and Vicuna and Electroejaculation Teaser male Undiluted 42 alpacas Uterine horns via cervix one cria born Novoa, 1968 paco vicuna
Ž .
Calderon et al., 1968 Alpaca Electroejaculation Teaser male and hCG Undiluted 96 alpacas Uterine body via cervix 75 after 35–45 h
Ž .
Leyva et al., 1977 Vicuna and Electroejaculation Teaser and hCG Undiluted 83 alpacas 11 llamas Uterine body via cervix 38 paco vicuna
Ž .
Quispe, 1996 Alpaca AV hCG Diluted, 80 alpacas Deep uterus via cervix 57
egg yolk–citrate
Ž .
De la Vega, 1996 Alpaca AV Teaser male Diluted, PBS 133 alpacas Uterine body via cervix 40
a
Ž .
Bravo et al., 1996a,b Alpaca, llama AV hCG Diluted, 19 alpacas Deep uterus via cervix 26
egg yolk–citrate
Bravo et al., 1997a Alpaca AV hCG Undiluted 40 alpacas Deep uterus 67
Žvia cervix and laparoscopy. a
Ž . Ž .
commercial extenders: Green buffer IMV, L’Aigle, France , with 20% v:v egg yolk
Ž . Ž .
added, Laiciphos IMV and Androhep Waberski et al., 1989 , or a diluent containing
Ž . Ž . Ž .
11% w:v lactose and 20% v:v egg yolk Anouassi et al., 1992 , as compared with
Ž . Ž
other extenders such as skimmed milk Kenny et al., 1975 or glucose-EDTA Martin
.
and Klug, 1979 . As the semen collection procedure can be rather prolonged, it may be advantageous to add about 1–2 ml of extender into the collection vessel before collection. In general, the ratio of diluent to semen should be 3:1 with a diluent
temperature of 30–358C when it is added slowly to the semen. Further work is required
to determine the optimum number of spermatozoa per insemination in the dromedary
camel, but initial studies would indicate that at least a minimum of 100=106 motile
Ž .
spermatozoa are needed Anouassi et al., 1992 and fertility can be improved if
6 Ž .
300=10 motile spermatozoa are inseminated J.A. Skidmore, unpublished data .
The diluted semen can be stored in a refrigerator at 48C for 24–36 h or in an
Ž .
equitainer Hamilton Thorn, Canvers, MA, USA , where the cans used to cool the equitainer are placed in a freezer for at least 24 h before use. The semen is sealed in a plastic universal which is wrapped in two thermal ballast bags at room temperature and placed within a plastic cup inside the equitainer before closing the lid. The semen is used for insemination 24 h later, provided it contains at least 35–40% motile spermato-zoa. While pregnancy rates of 50–60% have been reported in camels inseminated with
Ž .
fresh diluted semen within 30 min of collection Anouassi et al., 1992 , the conception rate decreased to 25–30% in camels inseminated with semen cooled–stored for 24 h. All
the pregnancies were achieved with cooled semen diluted in Green bufferq20% egg
Ž .
yolk J.A. Skidmore, unpublished data .
7.4. Frozen storage of camel semen
Ž .
Split samples of semen were used by Seime et al. 1990 to compare different freezing methods by assessing post-thaw morphology, motility and viability of sperma-tozoa in 1% sodium chloride solution at 388C. They found that the best freezing method for dromedary semen was a modification of the technique developed for boar semen by
Ž .
Westendorf et al. 1975 . Details of the method used are shown below.
Ž . Ž . Ž .
Ø Dilution of semen at 25–308C 1:1, vrv with lactose 11% –egg yolk 20% cooling
diluent.
Ø Cooling to 158C in 2.5 h.
6 Ž
Ø Second dilution to sperm concentration of 150=10 rml approximately 2r3 of total
.
volume with cooling diluent.
Ø Cooling to 58C in 1.5 h.
6 Ž
Ø Further dilution to sperm concentration of 100=10 rml approximately 1r2 of total
.
volume with the above lactose–yolk diluent containing 6% glycerol and 1.5% OEP
ŽEquex ; final glycerol concentration approximately 2%..
Ž .
Ø Filling of 4 ml straws Fa. Makrotub, Landshut, Germany with semen diluted to
Freezing of straws:
Ø from 58C toy1208C by suspending in liquid nitrogen vapour for 20 min;
Ø fromy1208C toy1968C by plunging into liquid nitrogen.
Attempts to freeze dromedary semen using a combination of both Green and Clear
Ž . Ž .
buffers IMV have been carried out by Skidmore et al. unpublished adopting the following protocol:
Ž .
Ø Dilution of semen at 25–308C 1:1, vrv with Green buffer containing 20% egg
yolk.
Ø Cooling to 48C in 2 h.
Ø Second dilution by addition of equal volume of Clear buffer containing 20% egg yolk
and 11% glycerol; final glycerol concentration approximately 3.0%.
Ø Equilibration at 48C for 30 min.
Ø Filling of 0.25 ml straws with semen diluted to final rate.
Ø Freezing of straws:
( from 48C toy1408C by suspending in liquid nitrogen vapour for 20 min;
( fromy1408C toy1968C by plunging into liquid nitrogen.
For freezing Bactrian semen, different glucose-, sucrose- and lactose-based diluents
Ž .
were examined by Chinese workers Zhao, 1995; Zhao et al., 1990, 1996b .
In a comparison of different diluents using split semen samples frozen in ampoules,
Ž .
the SYG-2 extender 73 ml of 12% sucrose–20 ml egg yolk–7 ml glycerol gave the best results regarding post-thaw motility, survival and acrosome integrity of Bactrian
Ž .
spermatozoa Zhao et al., 1996b .
The SYG-2 medium is used for two-step dilution by the following procedure:
Ž .
Ø First dilution 1:3 at 35–378C with non-glycerolated diluent.
Ø Holding the diluted semen at 208C for 2 h.
Ø Cooling and holding at 48C for 4 h.
Ž . Ž .
Ø Addition 1:1 of glycerolated 7% diluent.
Ø Holding at 48C for 10 min.
Ø Transfer of diluted semen in 2 ml ampoules.
Ø Suspension of ampoules in liquid nitrogen vapour for 10 min.
Ø Plunging the ampoules into liquid nitrogen.
7.5. Thawing of camel semen
Ž .
Thawing is best carried out in a water bath: small 0.25 ml straws at 408C for 10 s,
Ž .
Ž .
of thawed dromedary semen was improved by freezing in small straws 0.25 ml , but the use of larger straws was recommended to ensure deposition of an adequate amount of semen in the uterus, which is necessary for inducing ovulation. However, this necessity
has been overcome these days by treatment of camels with 3000 IU hCG or 20 mg
Buserelin 24 h before the insemination.
7.6. Fertility results for frozen–thawed camel semen
Although remarkable fertility has been reported for Bactrians inseminated with frozen–thawed semen, such results have not yet been obtained for dromedaries. Post-thaw motility has looked promising, but the pregnancy results to date do not reflect this.
The pregnancy rates obtained with frozen–thawed Bactrian semen after different ‘‘systems’’ of insemination are shown in Table 6. It should be noted that the mean pregnancy rate of 95% after insemination with frozen–thawed semen was higher than the pregnancy rates of 60–65% obtained over 8 years after natural mating. It should also be mentioned that by supplementation of semen with GnRH, it was possible to use the
Ž .
semen of one male for 450 inseminations Zhao, 1994; Zhao et al., 1996a .
7.7. Methods and timing of insemination
Ž
Female lamoids that are receptive to the male assume a prone position ventral
.
recumbency after a short period of chasing by the male, or adopt a copulatory position next to mating alpacas. For the purpose of AI, all sexually receptive females are mated
Table 6
Ž
Pregnancy rates in Bactrian camel after AI with frozen–thawed semen semen frozen with SYG-2 diluent;
.
Zhao et al., 1996a
a
Insemination system Number of Number of Number of females
females females pregnant at days
Ž .
inseminated ovulated 60–90 %
b
Ž . Ž .
Double AI 4q4 ml at 24-h interval 71 68 68 95.8
Ž .
Single AI 24 h after 1000 IU hCG injection 10 10 10 100
Ž .
Single AI 24 h after 200 IU LH injection 10 10 10 100
Ž . Ž .
Single AI with double dose 8 ml 10 10 10 100
Ž . Ž .
Single AI with single dose 4 ml 5 4 4 80
Ž .
Single AI with semen diluted 10-fold 45 45 45 100
c
q200mg GnRHrdose
Ž .
Single AI with semen diluted 15-fold 149 149 138 93
d
q200mg GnRHrdose
Ž .
Totals and mean 300 287 285 95
a
Ž .
Dose of inseminate: 4 ml two 2.0 ml ampoules .
b
Total number of motile sperm inseminated: 1.48=109. c
Total number of motile sperm inseminated: 290=106. d
to a vasectomized male or the females are checked for follicular size by ultrasonogra-phy. A second alternative to induce ovulation is the administration of 750 IU hCG or
80–800 mg GnRH. So far, the best time for insemination is 36 h after induction of
ovulation. In fact, 75% of females had fertilised eggs when slaughtered 3 days after insemination in contrast to 40% when insemination was done at the time of induction of
Ž . Ž .
ovulation Calderon et al., 1968 . This report has been confirmed by Quispe 1996 who obtained 60% pregnancy rate by inseminating 36 h after induction of ovulation. Insemination is carried out with the female in standing position. Preferably, two people
restrain the animal and the operator andror inseminator empties the rectum from fecal
material. The perineum is cleaned and the cervix held through the rectum while an insemination pipette loaded with semen is inserted into the uterine horns for deposition of semen.
Oestrous female camels can be detected by teaser males. However, more accurate records can be obtained by rectal palpation combined with teasing or by ultrasonography of the uterus and ovaries. When using ultrasonography or rectal palpation, the ovarian follicles should be noted and not be smaller than 12 mm in diameter. Insemination can
Ž .
be carried out with the female in a standing position dromedary or restrained in a
Ž .
sitting position dromedary and Bactrian . The perineal region should be cleaned and the semen deposited into the cranial part of the cervix or the body of the uterus. This can be achieved by using an insemination gun passed through the vagina and cervix with one hand in the rectum holding the cervix, similar to the technique used in cattle. An alternative method is to use a porcine insemination catheter together with a tubular speculum. The screw-like end of the catheter facilitates its passage through the annular rings of the cervix in the female camel.
In the dromedary camel, ovulation occurs between 36 and 48 h after GnRH of hCG injection and the optimum time for insemination with either fresh, liquid-stored or frozen–thawed semen is 24 h after treatment. Good results have been obtained with fresh semen when insemination occurred 24 h after mating with a vasectomised male
ŽAnouassi et al., 1992 ..
In the Bactrian camel, ovulation occurs 30–48 h after mating or treatment with hormones and the appropriate time for insemination with fresh semen is the first day of sexual receptivity. When frozen–thawed semen is used, it is advisable to inseminate
Ž .
twice at 24-h interval. Chen et al. 1985 reported that the incidence of ovulation was 87% after deep vaginal and 100% after uterine insemination. There is no information regarding the optimum number of spermatozoa per insemination. All Bactrian females
6 y1 Ž .
ovulated after insemination with sperm numbers of 5–8=10 ml Xu et al., 1985
and pregnancy rates of 50% have been obtained in dromedary camels with 100=106
Ž .
motile spermatozoa Anouassi et al., 1992 .
8. Conclusions
use of traditional systems of reproductive management in most breeding herds. These age-old methods make it difficult to ensure that an optimum number of females is pregnant at the end of the season and they may also lead to widespread venereal infections with consequent decreased fertility. The technique of AI can be employed to overcome some of these problems, especially to impregnate as many females as possible at the start of the breeding season, thereby giving them the best chance to conceive again as soon as possible after parturition. In addition, successful freezing of semen and its transport to several different countries would lead to the genetic improvement of Camelidae stock worldwide.
Acknowledgements
The authors are grateful to Mr. M. Billah for his help with the collection and processing of dromedary semen and to Dr. S. Salamon for his considerable and critical contribution to the revision of the manuscript.
References
Anouassi, A., Adnani, M., Raed, E.I., 1992. Artificial insemination the camel requires induction of ovulation
Ž .
to achieve pregnancy. In: Allen, W.R., Higgins, A.J., Mayhew, I.G., Snow, D.H., Wade, J.F. Eds. , Proc.
Ž .
1st Int. Camel Conf.. R&W Publications Newmarket , UK, pp. 175–178.
Arthur, G.H., Rahim, A.T.A., Hindi, A.S.A., 1985. Reproduction and genital diseases of the camel. Br. Vet. J. 141, 650–659.
Bartmann, G., 1959. Untersuchungen uber die Supravitalfarbung an Bullenspermien im Vergleich zu deren¨ Bewegunsverhalten im Nativpraparat under Berucksichtigung verschiedener Samenverdunnungen und¨ ¨ aufbewahrungen. Thesis, School of Veterinary Medicine, Hannover.
Bravo, P.W., Sumar, J., 1989. Laparoscopy examination of the ovarian activity in alpacas. Anim. Reprod. Sci. 21, 271–279.
Bravo, P.W., Fowler, M.E., Stabenfeldt, G.H., Lasley, B.L., 1990. Ovarian follicular dynamics in the llama. Biol. Reprod. 43, 579–585.
Bravo, P.W., Stabenfeldt, G.H., Fowlder, M.E., Lasley, B.L., 1991. The effect of follicle size on pituitary and ovarian responses to copulation in domesticated South American camelids. Biol. Reprod. 47, 884–888. Bravo, P.W., Stabenfeldt, G.H., Fowler, M.E., Lasley, B.L., Frey, R.E., 1992. Testes development and
Ž .
testosterone concentration in the llama Lama glama . 12th ICAR, The Hague, The Netherlands. Bravo, P.W., Moscoso, J., Ordonez, C., Alarcon, V., 1996a. Transport of spermatozoa and ova in female˜
alpaca. Anim. Reprod. Sci. 43, 173–179.
Bravo, P.W., Fowler, M.E., Lasley, B.L., 1994. The postpartum llama: fertility after parturition. Biol. Reprod. 51, 1084–1088.
Bravo, P.W., Ordonez, C., Alarcon, V., 1996b. Processing and freezing of semen of alpacas and llamas. 13th˜
Ž .
Int. Congr. Anim. Reprod., Sydney 2 pp. 2–3, Abstract .
Bravo, P.W., Flores, U., Garnica, J., Ordonez, C., 1997a. Collection of semen and artificial insemination of˜ alpacas. Theriogenology 47, 619–626.
Bravo, P.W., Enriquez, E., Ordonez, C., 1997b. The effect of trypsin and three extenders on alpaca semen.˜ Allpak’s 6, 19–21.
Ž .
Calderon, W., Sumar, J., Franco, E., 1968. Avances de la inseminacion artificial de las alpacas Lama pacos . Rev. Fac. Med. Vet., Lima 22, 19–35.
Ž
Casas, H., San Martin, M., Copaira, M., 1993–1996. Aspectos histologicos testiculo de la alpaca Lama
.
pacos . Rev. Fac. Med. Vet., Lima 18–20, 233–238.
Ž
Chen, B.X., Yuen, Z.X., Rao, G.W., 1985. Semen-induced ovulation in the Bactrian camel Camelus
.
bactrianus . J. Reprod. Fertil. 73, 335–339.
Cooper, M.J., Skidmore, J.A., Allen, W.R., Wensworth, S., Billah, M., Ali-Chaudry, M., Billah, A.M., 1992. Attempts to stimulate and synchronize ovulation and superovulation in dromedary camels for embryo
Ž .
transfer. In: Allen, W.R., Higgins, A.J., Mayhew, I.G., Snow, D.H., Wade, J.F. Eds. , Proc. 1st. Int.
Ž .
Camel Conf.. R&W Publications Newmarket , UK, pp. 175–178.
De la Vega, D., 1996. Efecto de la concentracion espermatica y la hora de inseminacion artificial con semen fresco sobre el porcentaje de gestacion en alpacas. MVZ Thesis, Fac. Med. Vet. Univ. Nac. Altiplano, Peru, 54 pp.
Ž .
Dehlon, G., von Lawzewitch, I., 1987. Reproduction in the male llama Lama glama , a South American camelid: I. Spermatogenesis and organization of the intertubular space on the mature testis. Acta Anat. 129, 59–66.
Dehlon, G., von Lawzewitsch, I., 1994. Ductus epididymis compartment and morphology of epididymal spermatozoa in llamas. Anat. Histol. Embryol. 23, 217–225.
Elias, E., Rodrack, E., Yagil, R., 1984. Estradiol concentrations in the serum of one the humped camel
ŽCamelus dromedarius during the various reproductive stages. Gen. Comp. Endocrinol. 56, 258–264..
Elwishy, A.B., 1992. Functional morphology of the ovaries of the dromedary camel. In: Allen, W.R., Higgins,
Ž .
A.J., Mayhew, I.G., Snow, D.H., Wade, J.F. Eds. , Proc. 1st. Int. Camel Conf.. R&W Publications
ŽNewmarket , UK, pp. 149–154..
Fernandez-Baca, S., Calderon, W., 1963–1966. Metodos de coleccion de semen de la alpaca. Rev. Fac. Med. Vet., Lima 18–20, 13.
Ž .
Fernandez-Baca, S., Novoa, C., 1968. Primer ensayo de inseminacion artificial Lama pacos con semen de
Ž .
vicuna VicugnaÕicugna . Rev. Fac. Med. Vet., Lima 22, 9.
Fernandez-Baca, S., Madden, D.H., Novoa, C., 1970. Effect of different mating stimuli on induction of ovulation in the alpaca. J. Reprod. Fertil. 22, 261.
Fuentes, C., 1990. Concentracion de los principales componentes quimicos del plasma seminal de la llama. MVZ Thesis, Fac. Med. Zootec., Univ. Nac. Altiplano, Puno, Peru, 49 pp.
Garnica, J., Achata, R., Bravo, P.W., 1993. Physical and biochemical characteristics of alpaca semen. Anim. Reprod. Sci. 32, 85–90.
Garnica, J., Flores, E., Bravo, P.W., 1995. Citric acid and fructose concentrations in seminal plasma of the alpaca. Small Rumin. Res. 18, 95–98.
Graham, E.F., Schmell, M.K.L., Eversen, B.K., Nelson, D.S., 1978. Semen preservation in nondomestic mammals. Symp. Zool. Soc. London 43, 153–182.
Jochle, W., Merkt, H., Sieme, H., Musa, B., Baldreldin, H., 1990. Sedation and analgesia with detomidine
Ž .
hydrochloride Domosedan in camelids for rectal examinations and electroejaculation. Proc. Unite de Coordination pour l’Elevage Camelin. Workshop: Is It Possible to Improve the Reproductive Performance of the Camel? Paris. pp. 263–271.
Ž .
Johnson, L.W., 1989. Llama reproduction. In: Johnson, L.W. Ed. , Llama Medicine, The Veterinary Clinics of North America. Saunders, Philadelphia, PA, pp. 159–182.
Joshi, C.K., Vyas, K.K., Poreck, P.K., 1978. Studies on the oestrous cycle in Bikaneri she-camels. Indian J. Anim. Sci. 48, 141–145.
Karras, W., 1952. Waermewasterbad and Registrarur ihe Entwicklung and Amaendung. Disch Tierderstl.
Ž .
Wochenedir 59 Suppl. 2 60–62, 68–69.
Kenny, R.M., Bergmann, R.V., Cooper, W.L., Morse, G.W., 1975. Minimal contamination techniques for breeding mares: technique and preliminary findings. Proc. Annu. Conf. Am. Assoc. Equine Pract. 21, 327–336.
von Kubiceck, J., 1974. Samenentnahme biem Alpaka durch eine Harnohrenfistel. Z. Tierzucht 90, 335. Leyva, V., Franco, E., Sumar, J., 1977. Inseminacion artificial en camelidos sudamericanos. Mem. Primera
reunion cientifica anual de la Asoc Peruana. Prod. Anim., 32–39, Lima, Peru.
Lichtenwalner, A.B., Woods, G.L., Weber, J.A., 1996b. Seminal collection, seminal characteristics and pattern of ejaculation in llamas. Theriogenology 46, 293–305.
Marie, M., Anouassi, A., 1987. Induction of luteal activity and progesterone secretion in the non-pregnant
Ž .
one-humped camel Camelus dromedarius . J. Reprod. Fertil. 80, 183–192.
Martin, J.C., Klug, E., 1979. Zur Samenubertragung biem Pferd; Samenkonservierung in Kunststoffrohrchen.¨ ¨ Prakt. Tierarzt 3, 196–199.¨
McEvoy, T.G., Kyle, C.E., Young, P., Adam, C.L., Bourke, D.A., 1992. Aspects of artificial breeding and establishment of pregnancy in South American camelids. Proc. 12th Int. Congr. Anim. Reprod., The Hague vol. 4 pp. 1963–1965.
McKinnon, A.O., Tinson, A.H., 1992. Embryo transfer in dromedary camels. In: Allen, W.R., Higgins, A.J.,
Ž . Ž .
Mayhew, I.G., Snow, D.H., Wade, J.F. Eds. , Proc. 1st Int. Camel Conf. R&W Publications Newmarket , UK, pp. 203–208.
McKinnon, A.O., Tinson, A.H., Nation, G., 1994. Embryo transfer in dromedary camels. Theriogenology 41, 145–150.
Merlian, C.P., Sikes, J.D., Read, B.W., Bower, W.J., Knox, D., 1979. Comparative characteristics of spermatozoa and semen from a Bactrian camel, dromedary and llama. J. Zool. Anim. Med. 10, 22–25. Merkt, H., Rath, D., Musa, B.E., El-Naggar, M.A., 1990. Reproduction in camels. FAO Anim. Prod. Health,
Paper 82.
Mogrovejo, D., 1952. Estudios del semen de la alpaca. BS Thesis, Fac. Med. Vet., Lima, 21 pp.
Moscoso, R., 1996. Caracteristicas seminales y numero de contracciones pene-uretrales en el eyaculado de
Ž . Ž .
alpacas Lama pacos y lamas Lama glama . BS Thesis, Fac. Agron. Zootec., Univ. Nac. San Antonio Abad, Cusco, Peru, 89 pp.
Ž .
Musa, B.E., Aubusineina, M.E., 1978. The oestrous cycle of the camel Camelus dromedarius . Vet. Rec. 102, 555–557.
Ž
Musa, B.E., Merkt, H., Seime, H., Hago, B.A., Hoppen, H.O., 1990. The female camel Camelus dromedarius and artificial insemination. Proc. Unite de Coordination pur’l Elevage Camelin. Workshop: Is It Possible to Improve The Reproductive Performance Of The Camel? pp. 257–261, Paris.
Nawito, M.F., Shalash, M.R., Hoppe, R., Bakha, A.M., 1967. Reproduction in the female camel. Bull. Anim.
Ž .
Sci. Res. Inst. Cairo 2, 82.
Neely, D.P., 1993. Reproductive aspects of the male llama. Proc. 4th. Annu. Hudson-Walker, Theriol. Conf., 791.
Osorio, E., San Martin, M., 1966. Aspecto histologico del epididimo, conducto deferente y glandulas sexuals
Ž .
accesorias del aparato reproductor musculino de la alpaca Glama lama pacos . Arch. Inst. Biol. Andina 1, 128–141.
Pacheco, C., 1996. Efecto de la tripsina y colagenasa sobre el acrosoma espermatozoide y su relacion con la fertilidad del semen de alpaca. MVZ Thesis, Prog. Med Vet. Zootec. Univ. Catol. Santa Maria, Arequipa, Peru, 52 pp.
Palomino, H., 1962. Espermograma y dimensiones de los espermatozoides de la alpaca. MV Thesis, Fac. Med. Vet. UNMSM, Peru, 16 pp.
Quispe, F., 1987. Evaluacion de las caracteristicas fisicas del semen de la alpaca durante la epoca de empadre. MVZ Thesis, Fac. Med. Vet. Zootec., Univ. Nac. Altiplano, Puno, Peru, 75 pp.
Ž .
Quispe, G., 1996. Inseminacion artificial en alpacas Lama pacos con semen diluido a diferentes concentra-ciones: I. Zootec. Thesis, Fac. Agron. Zootec., Univ. Nac. San Antonio Abad, Cusco, Peru, 103 pp. Rios, M., 1989. Presencia de un factor de induccion de la ovulacion en el semen de la alpaca y toro. MV
Thesis, Fac. Med. Vet., UNMSM. Peru, 23 pp.
San Martin, M., Copaira, M., Zuniga, J., Rodrigues, R., Bustinza, G., Acosta, L., 1968. Aspects of reproduction in the alpaca. J. Reprod. Fertil. 16, 395–399.
Ž .
Sato, A., Montoya, L., 1990. Aparato reproductor de la alpaca Lama pacos , Anatomia macroscopica. Rev. Camelidos Sudamericanos 7, 23.
Skidmore, J.A., Billah, M., Allen, W.R., 1995. The ovarian follicular wave pattern in the mated and non-mated
Ž .
dromedary camel Camelus dromedarius . J. Reprod. Fertil. 49, 545–548, Suppl.
Skidmore, J.A., Billah, M., Allen, W.R., 1996. The ovarian follicular wave pattern and indication of ovulation
Ž .
in the mated and non-mated dromedary camel Camelus dromedarius . J. Reprod. Fertil. 306, 185–192. Smuts, M.M.S., Bezuidenhout, A.J., 1987. Anatomy of the Dromedary. Oxford Science Publications,
Clarenden Press, UK.
Sucapuca, V., 1991. Caracteristicas fisicas del semen de la alpaca obtenido por el metodo del perservativo. MVZ Thesis, Univ. Nac. Altiplano, Puno, Peru, 49 pp.
Ž .
Sumar, J., Leyva, V., 1981. Coleccion de semen mediante vagina artificial en la alpaca Lama pacos . Resumenes IV Conv. Int. Sobre Camelidos Sudamericanos, Punta Arenas, 3.
Ž .
Taha Ismail, S.T., 1986. Reproduction in the male dromedary Camelus dromedarius . Theriogenology 29, 1407–1419.
Tayeb, M.A.F., 1951–1952. L’appareil genital male du chameua. Rev. Elev. Med. Vet. Pays. Trop. 5,ˆ 203–212.
Tingari, M.D., El-Manna, M.M.M., Rahim, A.T.A., Ahmed, A.K., Hamad, M.H., 1986. Studies on camel semen: I. Electroejaculation and some aspects of semen characteristics. Anim. Reprod. Sci. 12, 213–222. Urquieta, B., Caceres, J., Cepeda, R., De los Reyes, M., Raggi, L.A., Rojas, J.R., 1991. Estudio morfoen-docrinologico testicular en vicuna en epoca estival e invernal. Resumenes VII Conv. Int. de especialistas en˜ Camelidos Sudamericanos. San Salvador de Jujuy, Argentina 17, p. 31.
Waberski, D., Weitze, K.F., Rath, D., Sallmann, H.P., 1989. Wirkung von bovinem Serumalbumin und Zwitterionenpuffer auf flussigkonservirten Ebersamen. Zuchthygiene 24, 126–133.
Westendorf, P., Richter, L., Treu, H., 1975. Zur Teifgefrierung von Ebersperma: Labor-und Besmungsergeb-nisse mit dem Hulsenberger Pailletten Verfahren. Dtsch. Tieraerztl. Wschr. 82, 261–267.¨
Xu, Y.S., Wang, H.Y., Zeng, G.Q., Jiang, G.T., Gao, Y.H., 1985. Hormone concentrations before and after
Ž .
semen-induced ovulation in the bactrian camel Camelus bactrianus . J. Reprod. Fertil. 74, 341–346.
Ž .
Zhao, X.X., 1994. Artificial insemination and pregnancy diagnosis in the bactrian camel Camelus bactrianus . J. Arid Environ. 26, 61–65.
Zhao, X.X., 1995. Ecophysiology and Reproduction of the Camelidae. Gansu Science and Technology Press, Lanzhou, Gansu, China, pp. 222–242.
Zhao, X.X., Pan, G.W., Huang, Y.M., Gao, Y.H., Chen, B.X., 1990. Studies on the ovulation inducing factor in the seminal plasma of the bactrian camel. Proc. Unite de Coordination pour l’Elevage Camelin. Workshop: Is It Possible to Improve the Reproductive Performance of the Camel? pp. 192–210, Paris. Zhao, X.X., Huang, Y.M., Chen, B.X., 1992a. Biological activity of gonadotrophin-releasing hormone-like
factors in the seminal plasma of the bactrian camel. In: Allen, W.R., Higgins, A.J., Mayhew, I.G., Snow,
Ž . Ž .
D.H., Wade, J.F. Eds. , Proc. 1st Int. Camel Conf.. R&W Publications Newmarket , UK, pp. 163–168. Zhao, X.X., Huang, Y.M., Chen, B.X., 1992b. Studies on the semen characteristics of bactrian camel semen.
Chin. J. Anim. Sci. 28, 13–15.
Zhao, X.X., Chen, B.X., Huang, Y.M., 1996a. Artificial insemination with deep-frozen semen in the Bactrian
Ž .
camel Camelus bactrianus . Research Progress in Animal Industry and Animal Products Processing. Compiled by the Chinese Association of Agricultural Sciences. Presented at the First China International Annual Meeting on Agricultural Science and Technology, March 19–22, 1996. Chinese Agriculture Press, Beijing, pp. 237–242.