www.elsevier.comrlocateranireprosci
Basic aspects of frozen storage of semen
W.V. Holt
)Institute of Zoology, Zoological Society of London, Regent’s Park, London NW1 4RY, UK
Abstract
Basic concepts of cryopreservation and the causes of cryoinjury are reviewed. The possible roles of cryoprotectants and additives are considered in the context of their putative interactions with the sperm plasma membrane. Modern approaches to the laboratory assessment of spermato-zoa after freeze-thawing are also briefly discussed. q2000 Elsevier Science B.V. All rights
reserved.
Keywords: Cryobiology; Spermatozoa; Cryoprotectant; Functional assessment
1. Introduction
The discovery of glycerol as a cryoprotectant marked a quantum advance in semen cryopreservation, but subsequent research has only made relatively small improvements to the basic techniques established in the early 1950s. Nevertheless, the influence of this technology upon the animal breeding industry, especially dairying, has been profound. The remarkable success with bull semen has not been matched in other mammals such as pigs, sheep and exotic species. Such species differences result from at least two
Ž .
sources of variability: i the physiology and biochemistry of the spermatozoa
them-Ž .
selves, and ii variations in the anatomy and physiology of sperm transport in the
Ž .
female reproductive tract. Surprisingly few viable bull spermatozoa tens of thousands are sufficient to achieve fertilization in the cow, while conversely the pig requires many millions. This quantitative difference between species is an important determinant of the fertility of cryopreserved semen and means that development of successful freezing
)Tel.:q44-171-449-6630; fax:q44-171-586-2870.
Ž .
E-mail address: [email protected] W.V. Holt .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
procedures necessarily involves more than the identification or application of novel cryoprotectants and additives. If large numbers of spermatozoa are required for concep-tion there will be less tolerance of poor sperm survival during cryopreservaconcep-tion. It could also be argued that species bearing large litters must generate a minimum number of fertilized eggs otherwise they fail to produce sufficient concentrations of pregnancy-re-cognition factors.
Having mentioned that the quirks of individual species can have major influences upon the success of artificial insemination procedures with cryopreserved semen, it has been left to the authors of accompanying reviews to deal with species-specific require-ments. This review will mainly treat the spermatozoon as a generic cell type which undergoes various stresses during cryopreservation leading to survival, cell death or functional impairment. The reader should be aware that because of space limitations the literature has not been exhaustively cited. The principles and practice of semen
Ž
cryopreservation have been reviewed several times Bwanga, 1991; Hammerstedt and Graham, 1992; Hammerstedt et al., 1990; Hofmo and Almlid, 1991; Salamon and
.
Maxwell, 1995a,b; Watson, 1979, 1990, 1995; Wolf and Patton, 1989 , and these authors refer to most of the original literature up to the early 1990s. The reviews by Salamon and Maxwell are notable for their inclusion of research performed in the former Soviet Union.
2. Basic principles of semen cryopreservation and cryoinjury
When cells are frozen they are subjected to stresses resulting from the water-solute interactions that arise through ice crystallization. Exposure of cells to the hyperosmotic, yet unfrozen, solution causes withdrawal of intracellular water, consequent cell
shrink-Ž .
age and possible influx of ions Mazur, 1984 . Thawing involves a reversal of these effects, and the consequent inward water flux may cause cell membrane disruption. The detrimental effects of slow freezing, and therefore prolonged exposure of cells to
Ž .
hyperosmotic conditions ‘‘solution effects’’; Mazur et al., 1970 , have been viewed as balancing the deleterious consequences of rapid freezing which encourage intracellular ice crystallization. Cytoplasmic disruption through intracellular ice formation may be
Ž .
further compounded by the growth of ice crystals during thawing recrystallization . It has been suggested that a compromise freezing rate exists where the damaging effects of
Ž .
these two different sources of cryoinjury can be minimized Watson, 1990 .
Ž .
In reviewing cell freezing hypotheses, Mazur 1984 argued that the microscopic architecture of the freezing environment was an important determinant of cell survival. Early cryomicroscopic observations showed that slowly frozen erythrocytes became
Ž .
sequestered in narrow channels between plates of ice Nei, 1970 . Subsequent observa-tions with fluorescence cryomicroscopy have shown that as spermatozoa can also lie across the channels, single cells are exposed to high and low salt concentrations
Ž .
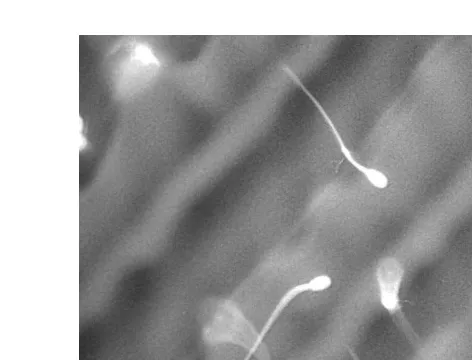
mem-Fig. 1. Ram spermatozoa surface-labelled with fluorescein isothiocyanate and cooled to y408C on the cryomicroscope stage at 308Crmin in the presence of 300mgrml rhodamine in phosphate-buffered saline.
Ž .
The fluorescence in the background highlights regions where rhodamine and other salts have become concentrated; conversely, dark areas indicate regions of low salt concentration. Single spermatozoa can span areas of both high and low salt concentration.
brane damage and loss of motility. If cell survival were dependent upon spatial orientation in relation to regions of heterogeneously distributed solute concentration, then different types of microstructure would alter the random likelihood of achieving optimal orientations.
Hypotheses of sperm cryoinjury must account for the known thermodynamic and structural properties of the sperm plasma membrane. It is well known that the sperm
Ž
plasma membrane contains an unusual array of lipids Lin et al., 1993; Parks et al.,
. Ž
1987 and that the plasma membrane is organized into different domains Friend, 1984;
.
Holt, 1984 . The phospholipids typically adopt unusual configurations, with a high proportion of plasmalogens that contain ether-linked fatty acids instead of the more usual ester linkages. Phospholipids account for 65–70% of the total, and a large proportion of these contain a docosahexaenoic acid side chain, which may confer membrane fluidity and instability. Possibly to counteract these destabilizing effects, sperm plasma membranes contain variable amounts of sterols. The sperm plasma membrane lipids respond to temperature changes by alterations in their physical phase state. Although regions of fluid and gel phase lipids coexist at physiological tempera-tures, reductions of temperature favour fluid to gel transitions; the presence of sterols is thought to inhibit these phase changes.
As spermatozoa are not adapted to undergo the temperature changes involved in cryopreservation, they cannot modify their lipid content to suit the environmental conditions. This useful strategy is widely used in nature to compensate for the changes
Ž
in ionic permeability and enzyme activity which result from phase transitions for
. Ž
.
al., 1989; Drobnis et al., 1993; Holt and North, 1984; 1986; Parks and Lynch, 1992 typically within the temperature range 17–368C. Their occurrence shows species depen-dence, which could go some way towards explaining the variations in cryopreservation sensitivity seen in spermatozoa from different species. It is also likely that during a typical freeze–thaw cycle, the sperm membranes must undergo phase transitions during both cooling and rewarming.
Ž .
Evidence that cold shock i.e. damage due to rapid cooling above 08C is caused by
Ž . Ž .
lipid phase transition effects was presented by Drobnis et al. 1993 . Holt et al. 1992 obtained some evidence that phase transitions might be involved in the manifestation of cryoinjury during the rewarming of cells after thawing. Ram spermatozoa were stained
Ž .
with fluorescein diacetate FDA , a fluorescent probe of cell membrane integrity, cooled
Ž .
to a series of minimum temperatures 58C, y108C andy208C and then rewarmed to 308C. Plasma membrane integrity was retained throughout cooling, but fluorescein leakage, indicating membrane disruption, occurred during the rewarming process. Conversely, performing similar experiments in the presence of external adenosine
Ž .
triphosphate ATP and a sperm reactivation medium, showed that spermatozoa rendered immotile by cooling could be restored to motility by an influx of ATP when the plasma membrane was breached. The threshold temperatures causing loss of membrane integrity were correlated with the minimum temperature reached during cooling. One interpreta-tion of this data is that as the post-thaw temperature increases, the plasma membrane is subjected to structural rearrangements involving lipids and proteins, the extent and nature of which are governed by interactions of temperature and solute effects during the freezing process.
Besides causing physical disruption of the plasma membrane by the induction of lipid packing faults, lipid phase transition effects cause non-linear kinetic responses in some enzymes, including some of the membrane ATPases whose activity depends upon the
Ž .
physical state of annular lipids Kimelberg, 1977 . It is likely that such effects are partly responsible for the poor control of intracellular calcium concentration which is evident
Ž .
at temperatures below about 178C Bailey et al., 1994; Robertson and Watson, 1986 .
Ž .
This is probably the rationale for including ethylenediaminetetra-acetic acid EDTA and citrate in some semen diluents; these would chelate calcium and diminish the concentra-tion gradient across the sperm plasma membrane. Intracellular calcium concentraconcentra-tions
Ž;0.1 mM are four orders of magnitude lower than those in the external milieux..
EDTA chelates other metallic ions, however, and might also act by inhibiting lipid peroxidation.
3. Cryoprotectants and additives
Ž
Many compounds have been tested for their efficacy as sperm cryoprotectants see
.
for example, Jeyendran and Graham, 1980; Molinia et al., 1994 , but most semen preservation protocols still favour glycerol in the cryoprotective media, following the
Ž .
example set by Polge et al., 1949 . In certain instances other cryoprotectants are
Ž .
Ž .
spermatozoa Jones, 1973 . The choice of cryoprotectant seems to have been a matter of trial and error in nearly all investigations; this is partly because a complete and satisfactory explanation for the action of cryoprotectants does not exist.
Cryoprotectant compounds can be roughly classified into groups, with differing modes of action. Glycerol, together with substances such as methanol, ethylene glycol, 1,2-propanediol, butanediol, acetamide and DMSO, belong to a group which permeate
Ž .
into the cellular cytoplasm. Lovelock and Polge 1954 proposed that the protective effects of glycerol were mediated by its colligative properties; depression of freezing point and the consequent lowering of electrolyte concentrations in the unfrozen fraction at any given temperature would help to counter the harmful ‘‘solution effects’’ imposed during the freezing process. Although this hypothesis has found support through a
Ž .
number of investigations see Mazur, 1984 , it is becoming increasingly apparent that this is not the only way in which glycerol might protect cells during freezing. It is also evident that glycerol is somewhat cytotoxic to spermatozoa. Species differences in ability to withstand glycerol exposure therefore interact in a complex fashion with the freezing rates used, and the degree of cryoprotection conferred.
Ž .
Hammerstedt and Graham 1992 , addressing the issue of poultry sperm cryopreser-vation, reviewed the actions of glycerol on spermatozoa, which in this instance include the abolition of fertilizing ability. Glycerol removal does, however, restore fertility. They pointed out that since glycerol reaches the interior of the cell it probably affects cytoplasmic viscosity, thereby changing rates of all diffusion limited processes. Previous
Ž .
evidence that cytoplasmic viscosity differs between species Hammerstedt et al., 1978 suggests that glycerol could have species-specific effects on spermatozoa. This argument can be applied more generally to other permeating cryoprotectants such as DMSO, ethylene glycol and methanol. Experimentally, it is also known that glycerol is able to
Ž .
insert into the membrane bilayer; Hammerstedt and Graham 1992 suggested that exposure of cells to 0.5M glycerol in cryoprotective media would yield an intramembra-nous concentration of about 1 mM. This might contribute to the alteration of cell membrane properties by inducing changes in lipid packing structure and hence the stability and water permeability of the cell membrane would be altered. Membrane fusogenicity and the responses of signal transduction pathways could also be affected by such changes, thus contributing to the possibility that post-thaw sperm longevity is
Ž .
reduced through accelerated capacitation Watson, 1995 .
Glycerol itself is known to be metabolized by ram, bull, boar and goat spermatozoa,
Žsee for example, Aalbers et al., 1961; Jones et al., 1992 ; the metabolic pathway differs.
from that operating in tissues such as the liver where glycerol is phosphorylated by a
Ž
kinase, being recruited instead by an NADP-dependent dehydrogenase Jones et al.,
. Ž .
In this context, it is worth noting that interspecific variation in glycerol tolerance can be very marked. Recent studies of marsupial spermatozoa have indicated that they have
Ž
an unusually high tolerance of glycerol Johnston et al., 1993; Molinia and Rodger,
.
1996; Rodger et al., 1991; Taggart et al., 1996 . Indeed, it seems that unless concentra-tions exceed about 10%, and approach 20%, there is little recovery of motility although the fertility of these spermatozoa has yet to be investigated. In contrast to these requirements for high glycerol concentrations, boar spermatozoa suffer loss of fertility if
Ž .
the concentration exceeds 3% Johnson, 1985 , an effect caused by increased acrosomal damage. Mouse spermatozoa, whose preservation has also been investigated recently,
Ž
seem unable to withstand more than about 1.75% glycerol Penfold and Moore, 1993;
.
Tada et al., 1990, 1993 . Some investigators have avoided glycerol completely for mouse spermatozoa, preferring instead a diluent based upon the non-permeating
cryopro-Ž .
tectant raffinose Nakagata and Takeshima, 1993 . Despite the possible perturbing actions of glycerol discussed above, there is at present no plausible explanation for these interspecific differences in cryoprotectant sensitivity.
While comparative studies of semen cryopreservation are likely to be valuable in providing explanations for the observed variations in cryosensitivity, there is some indication that between-strain studies of inbred mice might also be useful. Nakagata and
Ž .
Takeshima 1993 collected and cryopreserved spermatozoa from caudae epididymides of eight different strains of mice and then used them for in vitro fertilization assays. Post-thaw motilities ranged from 23% to 62%, and fertility in vitro ranged from 25.5% to 88.9%. The strain mostly used for transgenic work, C57BLr6N, consistently showed the worst post-thaw motility and fertility rates. Although it is premature to attribute these differences to biochemical properties of the cell, rather than to simpler explanations such as sperm head shape or flagellar efficiency, the intriguing possibility should be consid-ered that genetically determined differences in membrane properties are involved. The gross differences between species are undoubtedly under genetic control, but the subtle use of a within-species experimental model, where membrane organization and function is likely to be consistent, may permit more detailed investigations of, for example,
Ž .
membrane permeability and ion transport, to be performed. Willoughby et al. 1996
Ž .
included comparisons between two mouse strains outbred ICR and inbred B6C3F1 in their determinations of mouse sperm membrane properties, and failed to observe statistically significant inter-strain differences in the recovery of sperm motility after exposure to a range of anisosmotic conditions. Despite this formal result, their data seems to show that spermatozoa from the inbred strain had lower tolerance to extremes
Ž .
of osmolarity Willoughby et al., 1996 . A significant difference between strains was also observed in the maintenance and recovery of mitochondrial integrity after exposure to the same range of anisosmotic conditions. It would be interesting to confirm these results, identify the source of such variability if it really exists, and then correlate such differences across, rather than within, species.
Besides glycerol and the other penetrating cryoprotectants, sugars such as raffinose
Ž .
and lactose, polymers such as polyvinyl pyrollidone PVP and the amphipathic com-pounds glycine betaine, glutamine and proline have been identified as potentially cryoprotective. Raffinose has been used, with and without glycerol, for the preservation
Ž .
found useful in combination with pellet freezing methods, where it has been used for
Ž .
carnivore e.g. ferret, Howard et al., 1991; Giant panda, Moore et al., 1984 as well as
Ž .
for ram and boar spermatozoa Salamon and Lightfoot, 1969; Wilmut and Polge, 1977 . Sugars are thought to act by increasing the percentage of unfrozen water at any given temperature or reducing the concentration of salts in the unfrozen aqueous solution. Glycine betaine, proline and trehalose are thought to interact directly with membrane
Ž
lipids and proteins, altering their phase transition behaviour and hydration state Rudolph
.
et al., 1986 . Experimentally, however, these amphiphatic substances have only proved effective in the presence of glycerol and egg yolk when tested with ram and stallion
Ž .
spermatozoa Koskinen et al., 1989; Sanchez-Partida et al., 1992 .
In addition to these various cryoprotective compounds, egg yolk is routinely included in cryopreservation protocols for semen from domestic animals and many exotic species. Egg yolk is regarded as protecting against cold-shock, a lipid-phase transition effect
ŽDrobnis et al., 1993 . Given current needs for disease control and therefore the.
avoidance of biologically derived substances in cryoprotective media, there is a pressing requirement to find an egg yolk substitute. However, until the action of egg yolk in conferring membrane cryoprotection is better understood little progress in the search for
Ž .
alternatives can be made. Watson 1976 showed that the active component of egg yolk is a low-density lipoprotein, but direct evidence for its mode of action has remained elusive. Evidence from cryomicroscopic studies of ram spermatozoa showed that egg yolk protected against membrane damage and loss of motility induced below the
Ž . Ž .
extracellular freezing point y208C Holt et al., 1992 . In that study, the onset of membrane damage during thawing was detected by the loss of intracellular fluorescein where, in contrast to unprotected cells where fluorescein was lost soon after thawing, egg yolk inhibited fluorescein loss until the cells were rewarmed aboveq208C. In an earlier cryomicroscopic study of cold-shock effects in ram spermatozoa, it was evident that egg yolk prevented sperm flagellae from bending into a rigid ‘‘bow-like’’
configu-Ž .
ration during cooling Holt et al., 1988 . Furthermore, egg yolk abolished the tendency for these flagellae to undergo sudden, irreversible, midpiece bending through 1808when the temperature declined to about 12–148C. As the sudden bending phenomenon could not be induced by detergent-mediated membrane permeabilization, it was suggested that a localized membrane lesion permitted influx of ions which activated the axonemal mechanism in a highly specific region.
The direct modulation of sperm plasma membrane lipid phase transition behaviour by interaction with egg yolk is an attractive idea, but one which has little evidence in its favour. Freeze-fracture electron microscopy failed to demonstrate that egg yolk influ-enced the extent of intramembranous particle aggregation induced when ram, bull or
Ž
boar spermatozoa were cooled and stored at 0–58C De Leeuw et al., 1990; Holt and
.
North, 1984 . Taking particle aggregation as evidence of phase transitions having occurred, no prevention of the effect by the presence of egg yolk was evident. If modulation of phase transition behaviour does not occur, the alternative is likely to be egg yolk binding to the cell surface, modification of membrane permeability and
Ž .
through increased phosphorylation could affect the cells osmotic behaviour and response to permeating cryoprotectants such as glycerol.
Several investigators have concluded that the addition of surfactant to egg yolk diluents improves the post-thaw sperm motility, acrosomal integrity, survival and
Ž .
fertility for further details, see Bwanga, 1991 . Most diluents for boar semen contain Orvus ES paste, a detergent. The benefits of its use have also been documented for
Ž
several large ruminant species e.g. zebra, elephant, scimitar-horned oryx, Eld’s deer,
.
wildebeest and greater kudu . The consensus explanation for this beneficial effect is that the detergent modifies egg yolk particles, thus facilitating a more efficient interaction
Ž .
with the sperm plasma membrane Pontbriand et al., 1989 . If this were true, it is presumably reasonable to expect equally improved performance with all species. How-ever, very few studies of semen preservation in primates, carnivores and other taxa have
Ž .
examined the value of including detergent. Penfold and Moore 1993 developed a cryopreservation diluent for mouse spermatozoa, and included 0.1% sodium lauryl sulphate. These authors specifically commented that the detergent solubilized protective lipids in the egg yolk and did not act directly upon the sperm plasma membrane. In
Ž .
contrast, Kaplan and Mead 1992 evaluated semen diluents for the Western spotted skunk and noted that BF5 containing sodium dodecyl sulphate performed badly in the comparison. In this case, glycerol also proved less effective than DMSO as a cryoprotec-tant.
A number of studies have implicated membrane lipid peroxidation as a cause of
Ž .
defective sperm function, both in natural male infertility for review, see Aitken, 1995
Ž
and after semen cryopreservation for a detailed discussion of this topic, see Salamon
.
and Maxwell, 1995a . Attempts to overcome peroxidation during semen cryopreserva-tion have included processing under anaerobic condicryopreserva-tions, addicryopreserva-tion of antioxidants and the inclusion of chelating agents. Confirming the effectiveness of these strategies has
Ž . Ž
been somewhat problematic see Salamon and Maxwell, 1995a . Glutathione Slaweta
. Ž .
and Laskowska, 1987 and dithiothreitol Rao and David, 1984 have both been reported as providing protection against peroxidation during cryopreservation, with improvements
Ž .
in post-thaw motility and acrosomal integrity. Butylated hydroxytoluene BHT , a free radical scavenger known to interact with biological membranes altering their fluidity and phase transition behaviour, has been shown to decrease the permeability of bovine sperm
Ž .
membranes Hammerstedt et al., 1976 . Little benefit on cold-shock resistance has subsequently been observed with ram spermatozoa; this is perhaps not surprising as BHT reportedly enhances freeze–thaw damage and membrane fragility in mammalian
Ž .
cells Law et al., 1986; Shertzer et al., 1991 .
In addition to the choice of cryoprotectant and various potential additives, semen diluents must be prepared in an aqueous medium. Some commonly used formulations, especially those with high sugar content, do not contain a pH buffer even though components such as egg yolk can affect the solution pH. Many media include sodium
Ž Ž . .
citrate, tris tris hydroxymethyl aminomethane or zwitterionic buffers such as TES
ŽN-tris hydroxymethyl methyl-2-aminoethane sulphonic acid . Tris titrated with TESŽ . . ŽTEST media has proved a particularly successful choice for wild species owing to its.
Ž . Ž
freezing Pursel and Johnson, 1975 , contains the TEST combination Graham et al.,
.
1972 . The comparative merits of buffer systems were discussed in some detail by
Ž . Ž .
Watson 1990 and Salamon and Maxwell 1995a .
4. Practical aspects of semen cryopreservation
The practical details of semen processing for cryopreservation have been described
Ž .
previously Pursel and Park, 1985; Salamon and Maxwell, 1995a; Watson, 1990 .
Ž .
Semen is either packaged in straws 0.25 and 0.5 ml for freezing and storage, or frozen as pellets on shallow depressions in dry ice. Straws are either frozen in the vapour phase above liquid nitrogen or in a controlled-rate freezing machine. The practical require-ments of artificial insemination techniques have considerable influence over the choice of technique. Bull spermatozoa are routinely packaged in 0.25-ml straws; these contain a
Ž .
known number of live spermatozoa typically 10–15 million which can be inseminated directly from the straw after thawing. Boar semen, on the other hand, has to be frozen in much larger quantities and pellets of approximately 200 ml volume are frequently preferred. These can be stored frozen in 10–15 ml tubes, each tube providing sufficient
Ž .
spermatozoa for one insemination. Larger straws Weitze et al., 1991 and flattened
Ž .
plastic bags Bwanga et al., 1991 have been introduced for coping with the higher volume requirements.
Laparoscopic insemination pipettes for sheep, deer and exotic ruminants have been developed with the 0.25 ml plastic straw in mind, especially as lower sperm numbers are needed for this mode of insemination than for the transcervical route. Typically, the straw is fitted into the insemination pipette immediately after thawing, and insemination can be performed within seconds. The same principle applies to the pipettes used for transcervical insemination in cattle, where either of the two standard straw sizes can be used. A disadvantage of this approach is that there is no easy way to thaw the samples while simultaneously reducing the cryoprotectant concentration. This is where the pellet technique offers a clear advantage because, where desired, thawing can be rapidly performed by pouring the pellets into a solution specifically formulated for the purpose; ‘‘wet thawing’’. Thawing of straws is usually performed by immersion in a warm waterbath; this has its own advantages in that standardized temperature and time combinations can be used. Some experimentation with thawing at relatively high
Ž .
temperatures 60–708C has been undertaken. The comparative merits of different
Ž .
thawing techniques have been discussed in detail by Salamon and Maxwell 1995a and
Ž .
Pursel and Park 1985 . It is generally considered that fast rewarming rates are required for best sperm recovery. This has been attributed to the possibility that small intra-cellular ice crystals formed in some cells during freezing might grow during a slow rewarming process.
Systematic studies of sperm plasma membrane properties in relation to cryopreserva-tion have provided some new insights into the factors which determine cell survival during the freeze–thaw process. Particularly interesting from a practical viewpoint is the realization that if cryoprotectant addition and removal are carried out in a number of steps, the excessive cell volume excursions which cause membrane rupture can be
Ž .
Commercially produced semen freezing machines have been available for a number of years. In principle, these should be of considerable help for both handling large numbers of samples by a standardized method, and should also permit accurate cooling of the samples at the desired rates. While these systems may meet the first objective, they have so far proved inadequate to meet the second. This is because during the
Ž .
freezing process, the samples release sufficient heat latent heat of fusion to cause a sharp increase in temperature; no successful method of dissipating this heat rapidly has so far been developed, and sample temperature consequently does not decrease in tandem with the fall in chamber temperature. In fact, the sample temperature can remain static for 2–3 min before cooling is resumed. Several investigators have shown that this period between freezing and resumption of cooling, the freezing point plateau, is
Ž .
detrimental to sperm survival. Parkinson and Whitfield 1987 showed that reduction of this plateau improved the fertility of bull spermatozoa, and similar results have been
Ž .
obtained with boar semen Pursel and Park, 1985; Bwanga et al., 1991 . The introduc-tion of larger volume freezing methods such as the maxi-straw or plastic bag is likely to exacerbate this problem and there is clearly a need for the development of more effective temperature control systems.
5. Disease control issues
Concern over the potential for transmission of viral infection between samples stored in liquid nitrogen has recently been expressed in the United Kingdom, particularly in relation to samples of human semen. Fears arose initially when transmission of hepatitis to a patient was attributed to stored bone marrow cells that had been contaminated when an infected sample was damaged within the same sample storage container. An independent review panel was convened following this case to assess disease transmis-sion risks between stored samples. The panel’s recommendation that all straws of human semen should henceforth be stored in the vapour, rather than the liquid phase of the nitrogen container caused consternation in laboratories with semen storage facilities. This position has now been abandoned, partly through difficulties of implementation.
This episode highlighted a problem with regard to semen storage which has hardly ever been addressed. In fact, there is virtually no data upon which to base an assessment, except for some experiments performed recently at The Royal Veterinary College,
Ž .
London Russell et al., 1997 to investigate the potential for viral leakage from sealed
Ž .
6. Assessment of frozen–thawed semen
While it is obvious that freezing and thawing causes sperm damage, it is less apparent that some relatively minor damaging effects may entirely abolish the fertility of individual spermatozoa. In order to fertilize an egg, a spermatozoon must retain the capacity to reach and penetrate the oocyte, and thus needs not only a functional flagellum for propulsion but also the appropriate membrane surface chemistry to avoid either engulfement by phagocytes in the female reproductive tract or irreversible binding by epithelial cells. Furthermore, the spermatozoon must be capable of binding to the zona pellucida and responding with an acrosome reaction when the appropriate sig-nalling pathway is stimulated. This implies in turn that the sigsig-nalling pathway itself, and the associated structures such as the plasma and outer acrosomal membranes, must remain intact and undamaged throughout cryopreservation. Zona penetration is followed by sperm–oolemma fusion. The mechanism for egg activation, which either involves
Ž .
transfer of a cytoplasmic factor oscillin; Parrington et al., 1996 or stimulation of another signalling pathway, must also remain undamaged. Finally, the sperm chromatin should be available for decondensation, not having suffered inappropriate additional stabilization or destabilization during cryopreservation. Damage to any one of the physiological units that govern these attributes would render a spermatozoon unable to fertilize the oocyte; the spermatozoon would be infertile. This level of subtlety in determining the proportion of spermatozoa which remain completely unaffected by cryopreservation would explain the surprising discrepancy in sperm numbers needed to achieve comparable fertility rates with fresh or frozen bull sperm; 10 times more frozen
Ž .
than fresh spermatozoa were required Shannon, 1978 . At the time that data were published, standard sperm doses per insemination were 25 million frozen and 2.5 million fresh spermatozoa. Although greater economy of sperm dose has been developed in the intervening years, the same 10:1 ratio still holds; sperm doses in New Zealand are now 10–15 million cryopreserved spermatozoa per insemination straw vs. 1–1.5 million
Ž .
fresh spermatozoa Vishwanath et al., 1996 .
6.1. Sperm function tests
Many technical approaches to sperm function testing have been developed over the
Ž
past 10–15 years for reviews, see Amann, 1989; Critser and Noiles, 1993; Oehninger et
.
al., 1992 , and it is increasingly possible to combine the techniques in order to assess different aspects of function simultaneously. The functional aspects most readily exam-ined include plasma membrane, acrosomal and mitochondrial integrity, ability to undergo the acrosome reaction in response to appropriate stimuli and sperm motility. Flow cytometry is frequently used as an alternative to microscopy, but the latter is still essential if details of cell structure have to be examined.
Although the eosinrnigrosin technique is still used in many routine laboratories as a means of plasma membrane assessment, it is being superseded by fluorescent tests
ŽHarrison and Vickers, 1990; Johnson et al., 1996 . Typically, these involve the. Ž .
latter can either be the non-fluorescent ester of a fluorochrome, e.g. carboxyfluorescein diacetate, which generates the fluorochrome upon intracellular cleavage by an esterase, or a membrane-permeant fluorescent compound which has affinity for DNA, such as
Ž .
SYBR-14 Garner and Johnson, 1995 . Damaged and intact cells are therefore revealed in different colours, and the proportion of intactrdamaged cells, sometimes called the liverdead ratio, is estimated by either microscopy or flow cytometry. Mitochondrial assessment can be estimated using rhodamine 123, a fluorescent dye that binds to the inner mitochondrial membrane.
The mechanisms involved in some of the fluorescent methods, and consequently their validity, have sometimes been questioned. For instance, when stained by the SYBR-14rPI technique, the intact cell nuclei appear green, since SYBR-14 binds to chromatin. Cells change colour and nuclei become red once membrane damage has occurred.
Ž .
Johnson et al., 1996 discussed these changes in terms of ‘‘DNA viability’’ and membrane potential, but in this instance the mode of action remains unclear. This particular method has nevertheless proved useful in conjunction with freeze–thaw techniques as cell damage can be detected directly even in the presence of egg yolk
ŽMedrano and Holt, 1996 . Some techniques seem difficult to implement in a given.
laboratory. The fluorescent dye Hoechst 33258, which is unable to permeate the plasma membrane of intact cells, has been used as a marker of cell membrane integrity in
Ž .
glutaraldehyde-fixed cells De Leeuw et al., 1991 . This technique has proved somewhat idiosyncratic in the hands of different groups, even though it has the potential advantage of being combined with other fluorescent probes for the determination of acrosomal status. Rhodamine 123 is regarded as binding only to actively metabolizing
mitochon-Ž
dria; however, since sperm mitochondria continue to fluoresce belowy208C Holt et
.
al., 1988 when viewed directly by cryomicroscopy, it seems likely that it will continue to fluoresce once the dye is bound even if metabolic activity is extremely low.
Since cryopreservation induces physical damage in some cells, the proportion of undamaged acrosomes is widely assessed. As acrosomal integrity does not necessarily reflect plasma membrane integrity, combining the two tests provides a more discrimina-tory assessment than either test on its own. Although phase contrast microscopy of fixed spermatozoa has been used extensively to evaluate spermatozoa, generating data about
Ž .
the proportions of normal acrosomal ridges %NAR seen in many publications, this approach has been largely overtaken by the use of fluorescent probes. Many lectins, proteins that interact with the glycoconjugates of the acrosomal membranes or matrix,
Ž .
have been tested for use in acrosomal evaluation for review, see Cross, 1995 . These include peanut agglutinin, Concanavalin A, Pisum sativum agglutinin and Ricinus communis agglutinin. Monoclonal antibodies are also used for acrosomal evaluation, but they are more time consuming. Using these techniques with cells cryopreserved in egg yolk diluents requires a post-thaw washing step, otherwise, the yolk interferes with lectin or antibody binding. This practical issue probably means that the frequency of damaged acrosomes is always artificially increased by the washing procedure.
Acrosomal and plasma membrane integrity can be regarded functionally as well as in terms of structure. The combined ability of the acrosome-plasma membrane complex to respond to acrosome reaction inducers, or to bind and penetrate the zona pellucida is an
Ž .
induce the acrosome reaction in bull spermatozoa. They observed a high correlation between 90-day non-return rates and the extent of acrosome reaction induction in a fertility trial using frozen semen. This study was remarkable in that the correlation was found even though the acrosome reactions were detected with unfrozen spermatozoa, suggesting that the freezing itself did not change the responsiveness of the structurally intact cells, and implying that acrosome reacting ability may be correlated with the capacity to reach the site of fertilization. A similar approach was taken by Graham and
Ž .
Foote 1987 , who used liposomes instead of heparin to induce acrosome reactions. In human clinical investigations, the principle of examining acrosomal responses to various inducing substances, e.g. ionophore A23187 and more recently progesterone, has been developed although its validity has yet to be confirmed. This type of approach has been combined with the zona-free hamster egg penetration test to assess acrosomal respon-siveness and ability to fuse with the oolemma.
Ž .
An indirect test of capacitation status, using the antibiotic chlortetracycline CTC ,
Ž
has recently provided a new insight into the functional status of spermatozoa for
.
review, see Fraser, 1995 . With CTC as a fluorescent probe, spermatozoa show various fluorescent patterns which have been correlated with predominantly incapacitated, capacitated or acrosome reacted sperm populations. Cooled and frozen–thawed
sperma-Ž
tozoa apparently become more ‘‘capacitated’’ as determined by this test; Watson 1995,
.
1996 has argued that this effect shows that the cryopreservation process is in some respects analogous to capacitation, which may account for the shortened lifespan of spermatozoa, and the brief period of capacitation required to promote fertilization in vitro, after freeze-thawing.
A slightly different approach to testing the functional integrity of the plasma membrane-acrosome complex has been evaluation of the sperm interaction with isolated zonae pellucidae. As acrosome reactions are physiologically induced by zona pellucida proteins, incubation of spermatozoa with fresh, frozen or salt-stored zonae has been developed as a test. The end-points can be either the number of sperm bound to the zona surface, or the number which undergo the acrosome reaction and commence penetration
Žsee, for example, Fazeli et al., 1993 . This type of assay was developed further with the.
Ž .
introduction of the hemi-zona assay Fazeli et al., 1995 , where one-half of a zona pellucida is reacted with a reference semen sample while the other is reacted with the sample under test.
As acrosomes contain proacrosin, the precursor of acrosin which is implicated in zona binding and penetration, it has been suggested that spontaneous conversion of proacrosin to acrosin during cryopreservation may depress fertility. A recent study
ŽPalencia et al., 1996 , using a labelled serine protease-inhibitor to detect active acrosin,.
Ž .
semen samples in a fertility clinic Holt et al., 1985, 1989; Irvine and Aitken, 1986 . Amplitude of lateral head displacement, an indicator of the power exerted by the flagellum, has also been identified as being predictive of fertility, and the ability of
Ž .
individual spermatozoa to penetrate cervical mucus Jeulin et al., 1986 . It seems likely that this type of analysis is still at an early stage of development, and that considerably more information will become available when methods for the analysis of sperm population data are finalized. Most studies have worked with population mean values, which may well obscure the true subpopulation structures to be found within samples. Recently, efforts to develop multivariate clustering techniques for the analysis of sperm
Ž . Ž .
populations have made some progress see, Holt, 1996 . Davis et al. 1995 used pre-freeze kinematic measurements as predictors of the proportion of spermatozoa that would survive cryopreservation. Few similar studies have been applied to animal
Ž .
spermatozoa and the results are conflicting. Bailey et al. 1994 were unable to show that computer-assisted semen analysis could predict the fertility of frozen bull semen, but recent studies with liquid-stored boar semen demonstrated that measurements of sperm survival in vitro under capacitating conditions, were indicative of conception rate
Ž .
and litter size Holt, 1995 .
The above description of sperm function tests is not intended to be exhaustive. However, it would not be complete without mentioning that the relative values of some
Ž
of these functional tests have been examined in heterospermic insemination trials for
.
review, see Dziuk, 1996 . This approach involves inseminating doses of semen that originate from different males, then comparing relative fertility values with the outcomes of the laboratory tests. Using this technique to study cryopreserved boar semen,
Ž .
Hammitt et al. 1989 obtained high correlations between fertility and the zona-free hamster egg penetration test, sperm motility in the presence and absence of caffeine, and the proteolytic activity of individual acrosomes. Interestingly, acrosomal morphology
Ž .
showed no correlation with relative fertility. When Budworth et al. 1988 studied the motility of frozen-thawed bull semen, no significant relationships between objectively measured sperm motion parameters and the results of homospermic inseminations were
Ž .
detected all r values -0.1 . However, the motion parameters:curvilinear velocity
ŽVCL and straightline velocity VSL accounted for approximately 70% of variance in. Ž .
much accuracy; however, it is probably reasonable to remark that the use of such tests in combination can help to identify subfertile individuals, ejaculates or cryopreserved samples, and can therefore be regarded as useful for quality assurance.
7. Concluding remarks
In this review, an attempt has been made to combine the provision of information about current practices in sperm cryobiology with the identification of unsolved prob-lems and possible ways forward. Some would argue that the time is ripe to develop
Ž
revolutionary new approaches to semen preservation for a speculative review, see Holt,
.
1997 ; however, it would be unrealistic to expect the imminent emergence or re-emer-gence of techniques such as freeze-drying or the in vitro culturing of testicular explants. It is reasonable to expect that the current trend of evaluating aspects of sperm membrane permeability to water and cryoprotectants will eventually lead to accurate predictions of optimum freezing rates for different species; but there are some relevant caveats which must be recognized and incorporated into future studies. The first is that the major discrepancies that currently exist between theoretical and actual optimum freezing rates
ŽGao et al., 1992, 1993 suggest the likelihood of an error, either in the theoretical basis.
of the arguments upon which this approach is based, or in the assumptions involved in the practical elucidation of the theory. The structural complexity of the sperm plasma membrane may be one cause of the problem, as it is unlikely that any single descriptor of membrane permeability or activation energy can apply to the entire cell. The second major drawback to this approach is that inter-animal, within species variations in the ability of spermatozoa to survive cryopreservation are evident in many publications. For example, this is a recurrent observation in the studies of mouse spermatozoa by
Ž .
Willoughby et al. 1996 and is a well-known observation for pig, stallion and human spermatozoa. For such cases, the derivation of a single optimum freezing rate is unlikely ever to prove satisfactory. The application of molecular techniques for the elucidation of these inter-animal differences is likely to provide a fruitful and novel direction for increasing our understanding of sperm cryoinjury, even if it cannot necessarily demon-strate how to overcome the problems.
References
Aalbers, J.G., Mann, T., Polge, C., 1961. Metabolism of boar semen in relation to sperm motility. J. Reprod. Fertil. 2, 42–53.
Aitken, R.J., 1995. Mechanisms and prevention of lipid peroxidation in human spermatozoa. In: Fenichel, P.,
Ž .
Parinaud, J. Eds. , Human Sperm Acrosome Reaction vol. 236 John Libby Eurotext, Montrouge, France, pp. 339–353.
Amann, R.P., 1989. Can the fertility potential of a seminal sample be predicted accurately? J. Androl. 10, 89–98.
Budworth, P.R., Amann, R.P., Chapman, P.L., 1988. Relationships between computerized measurements of motion of frozen-thawed spermatozoa and fertility. J. Androl. 9, 41–54.
Bwanga, C.O., 1991. Cryopreservation of boar semen: 1. A literature-review. Acta Vet. Scand. 32, 431–453. Bwanga, C.O., Einarsson, S., Rodriguez-Martinez, H., 1991. Cryopreservation of boar semen. II: Effect of cooling rate and duration of freezing point plateau on boar semen frozen in mini- and maxi-straws and plastic bags. Acta Vet. Scand. 32, 455–461.
Critser, J.K., Noiles, E.E., 1993. Bioassays of sperm function. Semin. Reprod. Endocrinol. 11, 1–16. Cross, N., 1995. Methods for evaluating the acrosomal status of human sperm. In: Fenichel, P., Parinaud, J.
ŽEds. , Human Sperm Acrosome Reaction vol. 236 John Libby Eurotext, Montrouge, France, pp. 277–285..
Crowe, J.H., Hoekstra, F.A., Crowe, L.M., Anchordoguy, T.J., Drobnis, E., 1989. Lipid phase transitions measured in intact cells with Fourier transform infrared spectroscopy. Cryobiology 26, 76–84.
Davis, R.O., Drobnis, E.Z., Overstreet, J.W., 1995. Application of multivariate cluster, discriminate function, and stepwise regression analyses to variable selection and predictive modeling of sperm cryosurvival. Fertil. Steril. 63, 1051–1057.
De Leeuw, A.M., Den Daas, D.H.G., Woelders, H., 1991. The fix vital stain method: simultaneous determination of viability and acrosomal status of bovine spermatozoa. J. Androl. 12, 112–118. De Leeuw, F.E., Chen, H.C., Colenbrander, B., Verkleij, A., 1990. Cold-induced ultrastructural changes in
bull and boar sperm plasma membranes. Cryobiology 27, 171–183.
Drobnis, E.Z., Crowe, L.M., Berger, T., Anchordoguy, T.J., Overstreet, J.W., Crowe, J.H., 1993. Cold shock damage is due to lipid phase-transitions in cell-membranes — a demonstration using sperm as a model. J. Exp. Zool. 265, 432–437.
Dziuk, P.J., 1996. Factors that influence the proportion of offspring sired by a male following heterospermic insemination. Anim. Reprod. Sci. 43, 65–88.
Fazeli, A.R., Holt, C., Steenweg, W., Bevers, M.M., Holt, W.V., Colenbrander, B., 1995. Development of a sperm hemizona binding assay for boar semen. Theriogenology 44, 17–27.
Fazeli, A.R., Steenweg, W., Bevers, M.M., De Loos, F.A.M., Van den Brock, J., Colenbrander, B., 1993. Development of a sperm zona pellucida binding assay for bull sperm. Vet. Rec. 132, 14–16.
Fraser, L.R., 1995. Mechanisms regulating capacitation and the acrosome reaction. In: Fenichel, P., Parinaud,
Ž .
J. Eds. , Human Sperm Acrosome Reaction vol. 236 John Libby Eurotext, Montrouge, France, pp. 17–33. Friend, D.S., 1984. Membrane organization and differentiation in the guinea pig spermatozoa. In: Van
Ž .
Blerkom, J., Motta, P.M Eds. , Ultrastructure of Reproduction. Martinus Nijhoff, Boston, pp. 75–85. Gao, D.Y., Mazur, P., Kleinhans, F.W., Watson, P.F., Noiles, E.E., Critser, J.K., 1992. Glycerol permeability
of human spermatozoa and its activation-energy. Cryobiology 29, 657–667.
Gao, D.Y., Ashworth, E., Watson, P.F., Kleinhans, F.W., Mazur, P., Critser, J.K., 1993. Hyperosmotic tolerance of human spermatozoa — separate effects of glycerol, sodium-chloride, and sucrose on spermolysis. Biol. Reprod. 49, 112–123.
Gao, D.Y., Liu, J., Liu, C., McGann, L.E., Watson, P.F., Kleinhans, F.W., Mazur, P., Critser, E.S., Critser, J.K., 1995. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum. Reprod. 10, 1109–1122.
Garner, D.L., Johnson, L.A., 1995. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 53, 276–284.
Graham, E.F., Crabo, B., Brown, K.I., 1972. Effects of some switterion buffers on the freezing and storage of spermatozoa: 1. Bull. J. Dairy Sci. 55, 372–378.
Graham, J.K., Foote, R.H., 1987. Dilaurylphosphatidylcholine liposome effects on the acrosome reaction and in vitro penetration of zona-free hamster eggs by bull spermatozoa: a fertility assay for frozen–thawed semen. Gamete Res. 16, 147–158.
Hammerstedt, R.H., Amann, R.P., Rucinsky, T., Morse, P.I., Lepock, J., 1976. Use of spin labels and electron spin resonance spectroscopy to characterize membranes of bovine sperm: effect of butylated hydroxy-toluene and cold-shock. Biol. Reprod. 14, 381–397.
Hammerstedt, R.H., Graham, J.K., 1992. Cryopreservation of poultry sperm: the enigma of glycerol. Cryobiology 29, 26–38.
Hammerstedt, R.H., Keith, A.D., Snipes, W., Amann, R.P., Arruda, D., Griel, L.J., 1978. Use of spin labels to evaluate effects of cold shock and osmolarity and osmolality on sperm. Biol. Reprod. 18, 686–696. Hammitt, D.G., Martin, P.A., Callanan, T., 1989. Correlations between heterospermic fertility and assays of
porcine semen quality before and after cryopreservation. Theriogenology 32, 385–399.
Harrison, R.A.P., Vickers, S.E., 1990. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil. 88, 343–352.
Hazel, J.R., 1989. Cold adaptation in endotherms: regulation of membrane function and cellular metabolism.
Ž .
In: Wang, L.C.H. Ed. , Advances in Cmparative and Evironmental Physiology vol. 4 Springer-Verlag, Berlin, pp. 1–50.
Hofmo, P.O., Almlid, T., 1991. Recent developments in freezing of boar semen with special emphasis on
Ž .
cryoprotectants. In: Johnson, L.A., Rath, D. Eds. , Boar Semen Preservation II. Paul Parey, Berlin, pp. 111–122.
Holt, C., 1995. An investigation of boar sperm motility using a novel computerized analysis system. PhD thesis, London.
Holt, W.V., 1984. Membrane heterogeneity in the mammalian spermatozoon. Int. Rev. Cytol. 87, 159–194. Holt, W.V., 1996. Can we predict fertility rates? Making sense of sperm motility. Reprod. Domest. Anim. 31,
17–24.
Holt, W.V., 1997. Alternative strategies for the long-term preservation of spermatozoa. Reprod. Fertil. Dev. 9, 309–319.
Holt, W.V., Head, M.F., North, R.D., 1992. Freeze-induced membrane damage in ram spermatozoa is manifested after thawing — observations with experimental cryomicroscopy. Biol. Reprod. 46, 1086–1094. Holt, W.V., Moore, H.D.M., Hillier, S.G., 1985. Computer-assisted measurement of sperm swimming speed in
human semen: correlation with in vitro fertilization assays. Fertil. Steril. 44, 112–119.
Holt, W.V., Morris, G.J., Coulson, G., North, R.D., 1988. Direct observation of cold-shock effects in ram spermatozoa with the use of a programmable cryomicroscope. J. Exp. Zool. 246, 305–314.
Holt, W.V., North, R.D., 1984. Partially irreversible cold-induced lipid phase transitions in mammalian sperm plasma membrane domains: freeze-fracture study. J. Exp. Zool. 230, 473–483.
Holt, W.V., North, R.D., 1986. Thermotropic phase transitions in the plasma membrane of ram spermatozoa. J. Reprod. Fertil. 78, 445–457.
Holt, W.V., Shenfield, F., Leonard, T., Hartman, T.D., North, R.D., Moore, H.D.M., 1989. The value of sperm swimming speed measurements in assessing the fertility of human frozen semen. Hum. Reprod. 4, 294–297.
Howard, J.G., Bush, M., Morton, C., Morton, F., Wentzel, K., Wildt, D.E., 1991. Comparative semen
Ž .
cryopreservation in ferrets Mustela putorius furo and pregnancies after laparoscopic intrauterine insemi-nation with frozen thawed spermatozoa. J. Reprod. Fertil. 92, 109–118.
Irvine, D.S., Aitken, R.J., 1986. Predictive value of in vitro sperm function tests in the context of and AID service. Hum. Reprod. 1, 539–545.
Jeulin, C., Feneux, D., Serres, C., Jouannet, P., Guillet, R.F., 1986. Sperm factors related to failure of human in-vitro fertilization. J. Reprod. Fertil. 76, 735–744.
Jeyendran, R.S., Graham, E.F., 1980. An evaluation of cryoprotective compounds on bovine spermatozoa. Cryobiology 17, 458–464.
Johnson, L.A., 1985. Fertility results using frozen boar spermatozoa: 1970 to 1985. In: Johnson, L.A., Larsson,
Ž .
K. Eds. , Deep Freezing of Boar Semen. Proc. 1st Int. Conf. Deep Freezing of Boar Semen Swedish Univ. Agric. Sciences, Uppsala, pp. 199–222.
Johnson, L.A., Maxwell, W.M.C., Dobrinsky, J.R., Welch, G.R., 1996. Staining sperm for viability assess-ment. Reprod. Domest. Anim. 31, 37–47.
Johnston, S.D., McGowan, M.R., Carrick, F.N., Tribe, A., 1993. Preliminary investigations into the feasibility
Ž .
of freezing koala Phascolarctos cinereus semen. Aust. Vet. J. 70, 424–425.
Jones, A.R., Chantrill, L.A., Cokinakis, A., 1992. Metabolism of glycerol by mature boar spermatozoa. J. Reprod. Fertil. 94, 129–134.
Jones, R.C., 1973. Collection, motility and storage of spermatozoa from the African elephant, Loxodonta
Ž .
africana. Nature London 243, 38–39.
Kaplan, J.B., Mead, R.A., 1992. Evaluation of extenders and cryopreservatives for cooling and
cryopreserva-Ž .
Kimelberg, H.K., 1977. The influence of membrane fluidity on the activity of membrane-bound enzymes. In:
Ž .
Poste, G., Nicolson, G.L. Eds. , Dynamic Aspects of Cell Surface Organization vol. 3 ElsevierrNorth-Holland, Amsterdam, pp. 205–293.
Koskinen, E., Junnila, M., Katila, T., Soini, H., 1989. A preliminary study on the use of betaine as a cryoprotective agent in deep-freezing of stallion semen. Zentralbl. Veterinaermed., Reihe A 36, 110–114. Law, P., Campbell, S., Lepock, J., Kruuv, J., 1986. Effects of butylated hydroxytoluene on membrane lipid
fluidity and freeze–thaw survival in mammalian cells. Cryobiology 23, 317–322.
Lin, D.S., Connor, W.E., Wolf, D.P., Neuringer, M., Hachey, D.L., 1993. Unique lipids of primate spermatozoa — desmosterol and docosahexaenoic acid. J. Lipid Res. 34, 491–499.
Lovelock, J.E., Polge, C., 1954. The immobilization of spermatozoa by freezing and thawing and the protective action of glycerol. Biochem. J. 58, 618–622.
Mazur, P., Leibo, S.P., Farrant, J., Chu, E.H.Y., Hanna, M.G. Jr., Smith, L.H., 1970. Interactions of cooling rate, warming rate and protective additive on the survival of frozen mammalian cells. In: Wolstenholme,
Ž .
G.E.W., O’Connor, M. Eds. , The Frozen Cell, Churchill, London, pp. 69–88.
Mazur, P., 1984. Freezing of living cells: mechanisms and implications. Am. J. Physiol. 247, C125–C142. Medrano, A., Holt, W.V., 1996. Protective effects of glycerol during cold shock in boar spermatozoa. A
cryomicroscope study using propidium iodide and SYBR-14. Reprod. Domest. Anim. 31, 281–282. Molinia, F.C., Evans, G., Maxwell, W.M.C., 1994. Incorporation of penetrating cryoprotectants in diluents for
pellet-freezing ram spermatozoa. Theriogenology 42, 849–858.
Molinia, F.C., Rodger, J.C., 1996. Pellet-freezing spermatozoa of 2 marsupials — the tammar wallaby,
Macropus-eugenii, and the brushtail possum, TrichosurusÕulpecula. Reprod. Fertil. Dev. 8, 681–684.
Moore, H.D.M., Bush, M., Celma, M., Garcia, A.-L., Hartman, T.D., Hearn, J.P., Hodges, J.K., Jones, D.M.,
Ž
Knight, J.A., Monsalve, L., Wildt, D.E., 1984. Artificial insemination in the Giant Panda Ailuropoda
. Ž .
melanoleuca . J. Zool. London 203, 269–278.
Nakagata, N., Takeshima, T., 1993. Cryopreservation of mouse spermatozoa from inbred and F1-hybrid strains. Exp. Anim. 42, 317–320.
Nei, T., 1970. Mechanism of haemolysis of erythrocytes by freezing, with special reference to freezing at
Ž .
near-zero temperatures. In: Wolstenholme, G.E.W., O’Connor, M. Eds. , The Frozen Cell. Churchill, London, pp. 131–147.
Oehninger, S., Toner, J., Muasher, S.J., Coddington, C., Acosta, A.A., Hodgen, G.D., 1992. Prediction of fertilization in vitro with human gametes: is there a litmus test? Am. J. Obstet. Gynecol. 167, 1760–1767. Okamura, N., Onoe, S., Sugita, Y., Paquinon, M., Dacheux, F., Dacheux, J.L., 1991. Water insoluble fraction of egg yolk maintains porcine sperm motility by activating adenylate cyclase. Mol. Reprod. Dev. 28, 136–142.
Palencia, D.D., Garner, D.L., Hudig, D., Holcombe, D.W., Burner, C.A., Redelman, D., Fernandez, G.C.J., Abuelyaman, A.S., Kam, C.M., Powers, J.C., 1996. Determination of activable proacrosinracrosin in bovine sperm using an irreversible isocoumarin serine protease inhibitor. Biol. Reprod. 55, 536–542. Parkinson, T.J., Whitfield, C.H., 1987. Optimisation of freezing conditions for bovine spermatozoa.
Theri-ogenology 27, 781–797.
Parks, J.E., Arion, J.W., Foote, R.H., 1987. Lipids of plasma-membrane and outer acrosomal membrane from bovine spermatozoa. Biol. Reprod., 1249–1258.
Parks, J.E., Lynch, D.V., 1992. Lipid-composition and thermotropic phase-behavior of boar, bull, stallion and rooster sperm membranes. Cryobiology 29, 255–266.
Parrington, J., Swann, K., Shevchenko, V.I., Sesay, A.K., Lai, F.A., 1996. Calcium oscillations in mammalian
Ž .
eggs triggered by a soluble sperm protein. Nature London 379, 364–368.
Penfold, L.M., Moore, H.D.M., 1993. A new method for cryopreservation of mouse spermatozoa. J. Reprod. Fertil. 99, 131–134.
Polge, C., Smith, A., Parkes, A., 1949. Revival of spermatozoa after vitrification and dehydration at low
Ž .
temperatures. Nature London 164, 166.
Pontbriand, D., Howard, J.G., Schiewe, M.C., Stuart, L.D., Wildt, D.E., 1989. Effect of cryoprotective diluent and method of freeze-thawing on survival and acrosomal integrity of ram sperm. Cryobiology 26, 341–354.
Pursel, V.G., Park, C.S., 1985. Freezing and thawing procedures for boar spermatozoa. In: Johnson, L.A.,
Ž .
Larsson, K. Eds. , Deep Freezing of Boar Semen. Swedish Univ. Agric. Sciences, Uppsala, pp. 147–166. Rao, B., David, G., 1984. Improved recovery of post-thaw motility and vitality of human spermatozoa
cryopreserved in the presence of dithiothreitol. Cryobiology 21, 536–541.
Robertson, L., Watson, P.F., 1986. Calcium transport in diluted or cooled ram semen. J. Reprod. Fertil. 77, 177–185.
Rodger, J.C., Cousins, S.J., Mate, K.E., 1991. A simple glycerol-based freezing protocol for the semen of a marsupial TrichosurusÕulpecula, the common brushtail possum. Reprod. Fertil. Dev. 3, 119–125.
Rudolph, A.S., Crowe, J.H., Crowe, L.M., 1986. Effects of three stabilizing agents — proline, betaine, and trehalose — on membrane phospholipids. Arch. Biochem. Biophys. 15, 134–143.
Russell, P.H., Lyaruu, V.H., Millar, J.D., Curry, M.R., Watson, P.F., 1997. The potential transmission of
Ž .
infectious agents by semen packaging during storage for artificial insemination. Anim. Reprod. Sci. 47 4 , 337–342.
Salamon, S., Lightfoot, R.J., 1969. Freezing of ram spermatozoa by the pellet method. 1. The effect of diluent composition on the survival of spermatozoa. Aust. J. Biol. Sci. 22, 1527–1546.
Salamon, S., Maxwell, W.M.C., 1995a. Frozen storage of ram semen: I. Processing, freezing, thawing and fertility after cervical insemination. Anim. Reprod. Sci. 37, 185–249.
Salamon, S., Maxwell, W.M.C., 1995b. Frozen storage of ram semen: II. Causes of low fertility after cervical insemination and methods of improvement. Anim. Reprod. Sci. 38, 1–36.
Sanchez-Partida, L.G., Maxwell, W.M.C., Paleg, L.G., Setchell, B.P., 1992. Proline and glycine betaine in cryoprotective diluents for ram spermatozoa. Reprod. Fertil. Dev. 4, 113–118.
Shannon, P., 1978. Factors affecting semen preservation and conception rates in cattle. J. Reprod. Fertil. 54, 519–527.
Shertzer, H.G., Bannenberg, G.L., Rundgren, M., Moldeus, P., 1991. Relationship of membrane fluidity, chemoprotection and the intrinsic toxicity of butylated hydroxytoluene. Biochem. Pharmacol. 42, 1587– 1593.
Slaweta, R., Laskowska, T., 1987. The effect of glutathione on the motility and fertility of frozen bull sperm. Anim. Reprod. Sci. 13, 249–253.
Tada, N., Sato, M., Yamanoi, J., Mizorogi, T., Kasai, K., Ogawa, S., 1990. Cryopreservation of mouse spermatozoa in the presence of raffinose and glycerol. J. Reprod. Fertil. 89, 511–516.
Tada, N., Sato, S., Amann, E., Ogawa, S., 1993. Effect of prefreezing equilibration and postthawing centrifugation on the fertilizing-capacity of frozen mouse epididymal spermatozoa. Cryo-Lett. 14, 195–206. Taggart, D.A., Leigh, C.M., Steele, V.R., Breed, W.G., Temple-Smith, P.D., Phelan, J., 1996. Effect of cooling and cryopreservation on sperm motility and morphology of several species of marsupial. Reprod. Fertil. Dev. 8, 673–679.
Vishwanath, R., Pitt, C.J., Shannon, P., 1996. Sperm numbers, semen age and fertility in fresh and frozen bovine semen. Proc. N. Z. Soc. Anim. Prod. 56, 31–34.
Watson, P.F., 1976. The protection of ram and bull spermatozoa by the low density lipoprotein fraction of egg yolk during storage at 58C and deep freezing. J. Therm. Biol. 1, 137–141.
Ž .
Watson, P.F., 1979. The preservation of semen in mammals. In: Finn, C.A. Ed. , Oxford Reviews of Reproductive Biology vol. 1 Clarendon Press, Oxford, pp. 283–350.
Ž .
Watson, P.F., 1990. Artificial insemination and the preservation of semen. In: Lamming, G. Ed. , Marshall’s Physiology of Reproduction vol. 2 Churchill Livingstone, Edinburgh, London, pp. 747–869.
Watson, P.F., 1995. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod. Fertil. Dev. 7, 871–891.
Watson, P.F., 1996. Cooling of spermatozoa and fertilizing capacity. Reprod. Domest. Anim. 31, 135–140. Weitze, K.F., Stampa, E., Richter, L., Willmen, T., Waberski, D., 1991. Fertility of frozen boar semen:
Ž .
influence of packaging, number of inseminations and seminal plasma. Reprod. Domest. Anim. Suppl. 1 , 139–142.
Whitfield, C.H., Parkinson, T.J., 1992. Relationship between fertility of bovine semen and in vitro induction of acrosome reactions by heparin. Theriogenology 38, 11–20.
Wilmut, I., Polge, C., 1977. The low temperature preservation of boar spermatozoa: II. The motility and morphology of boar spermatozoa frozen and thawed in diluent which contained only sugar and egg yolk. Cryobiology 14, 479–482.