www.elsevier.comrlocateranireprosci
Storage of ram semen
S. Salamon, W.M.C. Maxwell
)Department of Animal Science, UniÕersity of Sydney, Sydney, NSW 2006, Australia
Abstract
Storage of ram semen in liquid and frozen state, the diluents used for both methods, processing, cooling, freezing and thawing of semen are reviewed. Factors influencing the fertility of stored semen and methods used for improvement are discussed, and fertility results of long-term frozen stored ram semen are also given. q2000 Elsevier Science B.V. All rights
reserved.
Keywords: Spermatozoa; Liquid and frozen storage; Diluents; Protective agent; Freezing; Thawing; Cervical and intrauterine insemination
1. Introduction
Ž .
Investigations on artificial insemination AI of sheep were initiated at the beginning
Ž .
of the twentieth century by Ivanov 1907, 1912 , whose studies on diluting media and reproduction led to the development and the practical application of AI with farm animals. After the First World War, further intensive studies, under the leadership of Milovanov, were undertaken in the former Soviet Union, and by the early 1930s AI with fresh and diluted semen was used on a large scale in sheep breeding programmes.
The need to fertilise large numbers of ewes with semen of outstanding rams required transport of semen from the points or centres of collection to the sites of insemination at more distant farms. The necessity to use the rams over extended periods, or at different times of the year, stimulated research on storage of spermatozoa under artificial conditions.
This could be achieved by methods that reduced or arrested the metabolism of spermatozoa and thereby prolonged their fertile life. Accordingly, investigators
exam-Ž . Ž .
ined storage of semen in i a liquid unfrozen state, using reduced temperatures or
)Corresponding author. Tel.:q61-29-351-4864; fax:q61-29-351-3957.
Ž .
E-mail address: [email protected] W.M.C. Maxwell .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
Ž .
other means to depress sperm metabolism; and ii in a frozen state which involved preservation at sub-zero temperatures. Comprehensive reviews have been published on
Ž . Ž
liquid Maxwell and Salamon, 1993 and frozen storage of ram semen Salamon and
.
Maxwell, 1995a,b .
2. Liquid storage of semen
Ž
The main methods of storage of semen in a liquid state are storage at reduced 0–5 or
.
10–158C and at ambient temperatures by reversible inactivation of spermatozoa.
2.1. Storage at reduced temperatures
Ž .
Milovanov 1940, 1951 described the early work and discussed the problems of
Ž
storage of ram spermatozoa in a chilled state. Whereas British investigators Chang and
.
Walton, 1940 and some Soviet workers claimed that 10–158C was the ‘‘optimum’’
Ž .
temperature for liquid storage, most Soviet investigators reported that survival of ram and bull spermatozoa was better after storage at 0–58C.
When semen was stored at a low temperature, care had to be taken not to subject the spermatozoa to cold shock. This caused irreversible changes in sperm cells cooled to temperatures close to 08C. The detrimental effects of cold shock, discovered in 1931
ŽMilovanov, 1951 , were overcome either by gradual cooling of semen from room.
temperature, or by addition of lipid to the diluent. The value of lipids from various
Ž .
sources egg yolk, testicles, corpus luteum, brain and soy beans in the protection of spermatozoa against cold shock was initially reported by Milovanov and Selivanova in
Ž . Ž .
1932 cited by Milovanov, 1962 . Lardy and Phillips 1939 and Phillips and Lardy
Ž1940 applied these observations in the development of the egg yolk–phosphate diluent.
for the preservation of bull semen. Another generally accepted storage medium, the egg
Ž .
yolk–citrate diluent, was elaborated later by Salisbury et al. 1941 and Willett and
Ž .
Salisbury 1942 .
Before 1940, the main diluent used in the former Soviet Union for liquid storage of
Ž . Ž .
ram semen was glucose–phosphate–phospholipid soy extract medium GFO-5 . This was subsequently replaced by glucose–citrate–egg yolk as the generally accepted
Ž . Ž
diluent for ram semen Milovanov, 1962 . The egg yolk component and more likely its
.
high molecular weight low density lipoprotein fraction , apart from providing protection against cold shock, has been shown to reduce the loss of acrosomal enzymes and prevent degenerative changes in the acrosome during liquid storage. The composition of the citrate diluent as used nowadays is:
Ž .
2.37 g sodium citrate 2H O2 0.50 g glucose
15 ml egg yolk 100,000 i.u. penicillin 100 mg streptomycin
Although fructose is the only simple carbohydrate present in ram semen, spermatozoa can also metabolise glucose and mannose when these sugars are included in the storage diluent. No other sugars act as energy sources, but a range of sugars have been examined for their ability to preserve the motility of spermatozoa. Most investigators
Ž
used glucose and fructose as sugar components of the diluent. Some other sugars e.g.
.
sucrose and lactose may only act extracellularly to maintain the osmotic pressure of the diluent and membrane integrity of the sperm cells during storage.
Attempts to replace the traditional citrate buffer with glycine or addition of glycine to the citrate buffer gave contradictory results regarding the survival of spermatozoa, and were abandoned. Other diluents investigated, such as citrate–glycol–egg yolk–mucinase
ŽSokolovskaja et al., 1956 and citrate–boric acid–egg yolk Yoshioka et al., 1951 ,. Ž .
were variants of the above citrate diluent and remained only in experimental use. During the early and mid-1970s, several studies concentrated on the use of organic buffers, on the basis that these have better buffering capacity than phosphate or citrate, are relatively non-toxic to living cells, and may penetrate the sperm cell thereby acting as intracellular buffers against pH shifts, or may increase the tolerance of the cell to an intracellular increase in monovalent cations. Organic buffers often increase the overall tonicity of the diluent, which is important when the semen is stored.
Ž . Ž .
Diluents containing tris hydroxymethyl aminomethane Tris as the main component
Ž
have been examined for storage of semen from the bull, boar and ram reviewed by
.
Visser, 1975 . Tris concentrations ranging from 10 to 50 mM were reported to have little or no effect on motility and metabolism of ram spermatozoa. Subsequently, higher concentrations of tris were reported to be advantageous in diluents for chilled storage. More research is required, particularly on those buffers with efficient hydrogen ion buffering capacity in the ‘‘sperm tolerant’’ range of pH 6.5–7.5, for example Tes
wN-Tris hydroxymethyl -methylaminoethane-sulfonic acid , HepesŽ . x w
N-2-hydroxyethyl-X
x w Ž . x
piperazine-N -2-ethanesulfonic acid , Mops 3- N-morpholino propane sulfonic acid ,
w Ž . x Ž . Ž
Mes 2 N-morpholino ethane sulfonic acid and Pipes piperazine-N, N-bis 2-ethane
.
sulphonic acid .
Ž
Nowadays, the tris-based medium is a recommended extender for ram semen Evans
.
and Maxwell, 1987 , and its composition is as follows:
3.63 g tris 0.50 g fructose 1.99 g citric acid 14 ml egg yolk 100,000 i.u. penicillin 100 mg streptomycin
glass-distilled water to 100 ml
Before dilution of semen, the whole, skim or reconstituted milk should be heated at 92–958C for 8–10 min to inactivate the lactenin in the protein fraction which is toxic to spermatozoa. The skim milk powder must be carefully selected because some samples may not be satisfactory for preparation of diluent. The reconstituted milk diluent is prepared by dissolving 9 g of skim milk powder in 100 ml of glass-distilled water. To
Ž
control microbial growth, antibiotics e.g. 1000 i.u. sodium penicillin and 1 mg
.
streptomycin sulphate should be added to each milliliter of whole, skim and reconsti-tuted milk diluents.
Some investigators found that skim milk was better than whole milk for storage of semen at 2–58C. When combined with antibiotics, it was as effective as egg yolk–glu-cose–citrate diluent for chilled storage of ram semen. Addition of 5% egg yolk and 1% glucose to the skim milk was claimed to improve the viability of spermatozoa during
Ž .
chilled storage Feredean et al., 1967 . Regarding reconstituted skim milk, French workers found that viability and fertility of ram spermatozoa was better after storage for
Ž .
8–16 h at 158C than at 58C Colas et al., 1968, 1974 .
Ž .
In past years, commercially available ultra-heat-treated UHT ‘‘long-life’’ milk has proved a satisfactory diluent for fresh semen and also for maintaining the viability of spermatozoa during liquid storage. The UHT milk is sterile, does not require heating and may be used directly as a diluent without further treatment; a freshly opened carton of UHT milk is required for use each day.
Ž .
Recent studies on the suitability of a ‘‘chemically defined’’ diluent RSD-1 , with and without combination with antioxidants, for liquid storage of ram semen have yet to
Ž .
improve fertility over other diluents Upreti et al., 1997 .
2.2. Storage at ambient temperatures by reÕersible inactiÕation of spermatozoa
The first experiments on the inhibitory effect of carbon dioxide on the motility of spermatozoa of different species were performed in 1924 by Krshyshkovsky and Pavlov
Žcited by Milovanov, 1962 . More than three decades later, this method was re-examined. Ž .
and a new extender for bull semen, called the Illini Variable Temperature IVT diluent,
Ž .
was elaborated Salisbury and VanDemark, 1961 . The diluent required gassing with carbon dioxide before use.
Another diluent, also developed for storage of bull semen at ambient temperature, the
Ž .
Cornell University Extender CUE , is a self-gassing medium in which the carbon
Ž
dioxide is derived from the reaction of citric acid and sodium bicarbonate Foote et al.,
.
1958 . Both diluents have been modified for use with ram semen; they were saturated with carbon dioxide by gassing to pH 6.3 for about 10 min before use, or were ‘‘self-carbonating’’ as a result of their carbonate or bicarbonate and acid content.
Ž .
As an alternative to gassing, diluents containing volatile organic oxalic and acetic
Ž .
acids were also examined Habibulin, 1963 .
2.3. Fertility of semen after liquid storage and factors influencing it
difficult, as they do not provide sufficient information on essential experimental procedures. Details such as type or composition of diluent, temperature of storage, dilution rate, dose of inseminate, type of teaser rams used for the detection of oestrous
Ž .
ewes vasectomised or aproned entire rams , number of inseminations within one oestrus
Ž .
and the method of determining the fertility lambing data were often not stated. Several papers were in the form of reports of selected data from large-scale field insemination programmes, rather than from proper experiments.
Ž .
In critical studies reviewed by Maxwell and Salamon, 1993 , fertility declined rapidly when semen stored for more than 24 h was used for cervical insemination. The decrease in fertility was at a rate of 10–35% per day of storage. Thus, while 68–75% of ewes lambed from insemination with fresh semen in a single cycle, the lambing rates for semen stored for 24, 48 and 72 h were 45–50%, 25–30% and 15–20%, respectively.
Irrespective of the diluent, dilution rate, temperature or conditions of storage, the spermatozoa deteriorated as the duration of storage increased. The main changes that occurred during storage included reduction in motility and morphological integrity of spermatozoa. These changes may be contributed to by the accumulation of the toxic
Ž .
products of metabolism, mainly of reactive oxygen species ROS formed through lipid peroxidation of the membranes of spermatozoa. The above events are accompanied by a decline in transport and survival of spermatozoa in the female reproductive tract and reduction in fertility.
The cervix constitutes the initial barrier to the ascent of spermatozoa. By comparison to fresh spermatozoa, a relatively small proportion of the stored cells deposited at the external os cervix during insemination penetrate the cervical canal and migrate through the tract of the ewe to the site of fertilisation. Establishment of an adequate cervical sperm population is important with the insemination of sheep, as spermatozoa may continuously ascend to the oviducts from this reservoir. However, spermatozoa function-ally affected during liquid storage may not migrate, or may migrate slowly, and their survival in the female tract is also reduced to about half that of fresh spermatozoa
ŽLopyrin, 1971 . Attempts to improve the transport of spermatozoa from the posterior.
Ž .
cervix to the oviducts of oestrous ewes by prostaglandins PGF2a or PGE2 added to
Ž
the stored semen have given conflicting results reviewed by Maxwell and Salamon,
.
1993 .
Ž .
It is also possible that the process of liquid storage like that of frozen storage advances the maturation of sperm membranes, thus increasing the proportion of capaci-tated and acrosome reacted cells. Capacicapaci-tated spermatozoa have reduced viability and limited fertile life; or they may be rendered incapable of fertilisation if they are further
Ž
aged in the female reproductive tract after cervical insemination Maxwell and Watson,
.
1996 .
Early embryonic mortality is considered to be a further, or even one of the main causes of low fertility. The condition of gametes at the time of fertilisation is known to affect embryonic survival. An increase in abnormalities of embryonic development associated with ageing of spermatozoa has been observed for several species, and considerable attention has been focused on possible changes in the haploid genome of
Ž .
female tract resulting in asynchrony between the age of the spermatozoa and ova
ŽSalamon et al., 1979 . Thus, there was a steeper decline with age of semen in.
embryonic survival after single than after double insemination, pointing to the impor-tance of time of semen deposition into the cervix to avoid additional ageing of spermatozoa.
The problem of low fertility after cervical insemination with liquid stored ram semen still remains unresolved. Increased depth of cervical insemination may improve the fertility, but it has so far been problematic in achieving sufficient penetration and obtaining acceptably high conception rates. A number of attempts have also been made
Ž
to penetrate the cervical canal and to deposit the semen in the uterus see Sections 3 and
.
3.6 . Improvement of the techniques proposed for transcervical insemination and further research in this area seem warranted.
The fertility of liquid stored semen can be improved by carefully timed intrauterine insemination by laparoscopy, and when semen containing antioxidants is used. Surgical uterine insemination after laparotomy resulted in acceptable fertility with chilled semen stored for 8 days, and some spermatozoa retained their fertilising capacity for up to 10
Ž . Ž .
days Salamon et al., 1979 . Several antioxidants, such as superoxide dismutase SOD ,
Ž .
catalase CAT , cytochrome c and glutathione peroxidase had beneficial effects on maintenance of motility and acrosome integrity of spermatozoa during chilled storage when added to the storage diluent. In fertility tests, using laparoscopic insemination,
Ž . Ž .
SOD 800 Urml and CAT 200 Urml added to tris–glucose–egg yolk diluent improved the fertility and extended the ‘‘useful’’ storage time to 14 days, when egg
Ž
fertilisation and pregnancy rates of 50% and 33% have been obtained Maxwell and
.
Stojanov, 1996 .
Storage of semen packaged in encapsulated form, as proposed for bovine semen by
Ž . Ž .
Nebel et al. 1993 , has been examined in the ram by Maxwell et al. 1996b . After 8 days storage at 58C, the viability and acrosome integrity of ram spermatozoa was lower
Ž . Ž
for encapsulated in D-lysine and poly D-lysine microcapsules than for control
unen-.
capsulated semen. In a fertility test, using semen stored at 58C for 20 h for intrauterine insemination, there was no difference between encapsulated and control semen regarding
Ž .
fertilisation rates 67% of 33 vs. 79% of 44 . Improvement of the viability of encapsulated spermatozoa and attempts to use micro-encapsulated ram semen for cervical insemination remain future tasks.
3. Frozen storage of semen
Ž .
The first records on freezing ram semen are by Bernstein and Petropavlovsky 1937
Ž . Ž
who successfully used glycerol solution 1 M, 9.2% for storage of mammalian rabbit,
. Ž .
guinea pig, ram, bull, boar, stallion and avian fowl and duck spermatozoa aty218C;
Ž .
glycerol at 18% concentration had a toxic effect on spermatozoa. Smirnov 1949, 1950
Ž . Ž
during 1947–1950 investigated vitrification without glycerol of small volumes 0.05–
.
0.10 ml of ram and bull semen; in a fertility trial in 1949, of 19 ewes receiving three to
Ž .
Ž .
cryoprotective property of glycerol was brought into prominence by Polge et al. 1949 whose finding gave a big impetus to research on cryopreservation of mammalian semen.
Considerable experimentation was conducted on frozen storage of ram semen during the 1950s. Initially the method and diluent for freezing bull semen was applied to ram
Ž
semen with poor fertility results 5% lambing: Blackshaw and Emmens, 1953; Dauzier,
.
1956 . It became obvious that the ‘‘bull method’’ had limitations when adopted for ram semen. Over the years of research, a wealth of information has been accumulated on aspects of frozen storage of ram semen such as various diluents, cryoprotective agents, processing, freezing and thawing methods, cryogenic damage and fertility of ram spermatozoa. These aspects will be discussed in the sections that follow.
3.1. Diluents
The diluents used for preservation of ram semen, as for other species, generally should have adequate pH and buffering capacity, suitable osmolality, and should protect spermatozoa from cryogenic injury. The diluents discussed here have been grouped on the basis of their chronological use or development, with observations given on their efficiency with regard to fertility.
3.1.1. Citrate–sugar-based diluents
Opinions of investigators differed on the ‘‘suitable’’ type of sugar to be included in citrate medium. Using the post-thawing survival of spermatozoa as the criterion, some investigators have chosen arabinose, others fructose or glucose, as components of the citrate diluent. Due to the decrease in osmotic pressure caused by the glycerol in the extender, hypertonic citrate–glucose–yolk or citrate–fructose–yolk diluents with an osmotic pressure of 8–12 atm were generally used. Ram semen, with a mean osmotic pressure of 7.7 atm, tolerated well the above range in diluent tonicity. Also, ram spermatozoa can tolerate a glucose or fructose concentration twice that of isotonicity due to the capacity of monosaccharides to permeate the sperm cells, and thus equalise the osmotic gradient.
Ž . Ž
The lambing results for semen frozen in ampoules with citrate–glucose or
.
fructose –yolk diluents in experiments conducted under controlled conditions varied
Ž
between 17% and 40% after one to three inseminations reviewed by Salamon and
.
Maxwell, 1995a . The relatively high lambing rates claimed by some workers in the former Soviet Union could not be confirmed and were severely questioned on the basis
Ž .
of experimental artefacts Lopyrin, 1971 .
The citrate–sugar-based diluents were seldom used for freezing ram semen after the late 1960s.
3.1.2. Milk diluents
re-ported better post-thaw sperm survival after freezing with reconstituted skim milk than citrate–yolk, fructose- or lactose-based diluents. Addition of egg yolk to heated ho-mogenised milk did not increase post-thaw sperm survival. Freezing in hoho-mogenised milk resulted in better recovery of spermatozoa than when frozen in Norman–Johnson
ŽN-J-1 and N-J-2 media Patt and Nath, 1969 , but not when frozen in test-yolk, which. Ž .
also prevented the leakage of glutamic oxaloacetic transaminase from spermatozoa
ŽHinshelwood et al., 1980 . In some laboratory studies, milk preparations were included.
in synthetic diluents containing sugars and electrolytes.
Ž . Ž .
The INRA-patented milk–citrate medium was used by several mainly French workers as a glycerolated portion of the two-step dilution method, and was added to the
Ž .
cooled and partially diluted with lactose–yolk semen. Skim milk has been used as both portions of the two-step dilution, and freezing of semen for practical use in Sweden
ŽSoderquist et al., 1996 .
¨
.The fertility results after cervical insemination with milk-frozen semen have been
Ž . Ž .
variable reviewed by Salamon and Maxwell, 1995a . Some were poor 0–23% , others
Ž . Ž .
moderate 30–45% . Reports on lambing rates of 50–75% or higher should be regarded with caution.
3.1.3. Lactose-based diluents
Successful use of lactose as the main component of diluents for freezing bull semen stimulated its application for ram semen. The initial in vitro studies with lactose-based diluents gave contradictory results, and the first fertility tests were also not convincing. In subsequent tests, the lambing results for lactose–yolk frozen semen were poor
Ž12–29% or moderate 40–50% . Lactose–yolk was used for both the non-glycerolated. Ž .
and glycerolated diluent portions, or only for the non-glycerolated portion followed by glycerolated INRA medium.
Ž .
Disaccharides lactose and saccharose were found more effective in lowering the crystallisation temperature during freezing than monosaccharides, and this was increased
Ž .
when they were combined with EDTA–Na and diethylamine Platov, 1988 . Inclusion2
of gum arabic, a complex polysaccharide, in a lactose-based diluent has also been
Ž . Ž .
examined Platov, 1977 . Gum arabic is a high molecular weight )37,000 , water-soluble substance secreted by acacia plants, and at 1.5–15.0% concentration in the diluent, is well tolerated by spermatozoa and has extracellular cryoprotective properties.
Ž .
In the studies of Platov 1977 , the semen was diluted in two steps after collection, first with non-glycerolated lactose–yolk and then, after mixing, with lactose–gum arabic– yolk–glycerol or with lactose–yolk–EDTA–Na -diethylamine–gum arabic–glycerol.2
In further studies, for practical application, Platov et al. elaborated and recommended
Ž .
the so-called VNII-plem system Russian Animal Research Institute, Lesnie Poljany for
Ž .
processing and freezing of ram semen Platov, 1988 . According to this system, the semen is diluted in two steps: first, 1.5- to 2-fold with non-glycerolated extender at 288C; second, 2-fold at 248C with glycerolated medium. For both steps of dilution, either
Ž . Ž . Ž . Ž . Ž .
lactose 9% –dextran 5% –EDTA–Na2 0.135% –tris 0.105% –yolk 20% q
Ž . Ž .
antibiotics q14% glycerol for the second step is used Variant 1 , or lactose
Ž12% –yolk. Ž20%.qantibiotics for the first step and glycerolated Ž17%. lactose
Ž . Ž .
second step Variant 2 . The semen is cooled to 28C in 2 h, then pelleted 0.2 ml on dry ice or a polymer plate aty808C toy908C.
In fertility tests comparing the diluent variants of Platov, the claimed lambing results varied from 26% to 60%.
Ž . Ž
Arabonic acid extracted from tree gums substituted for gum arabic Sadykov et al.,
. Ž
1985 and inclusion of 5–6% polyglukine or 10% reopolyglukine glucose polymers
. Ž .
with high molecular weight: 30–60,000 in lactose–yolk Zheltobjuk et al., 1981 did not give better fertility than Platov’s diluents. However, insemination with semen frozen in lactose–dextran-based diluents resulted in more acceptable lambing rates.
3.1.4. Saccharose-based diluents
Saccharose was used as the main component of synthetic diluents because it was found to protect the acrosome integrity of spermatozoa better than glucose, fructose or
Ž .
lactose Milovanov and Sokolovskaja, 1980b . Synthetic antioxidants have generally been added to saccharose diluents to inhibit the peroxidation of sperm phospholipids, particularly of unsaturated fatty acids. Peroxidation may be prevented by processing in
Ž .
anaerobic conditions, and inclusion of antioxidants and chelating agents e.g. EDTA in
Ž .
the diluent. According to Nauk 1991 , lipid peroxidation, as measured by the
malon-Ž .
dialdehyde MDA; a secondary peroxide product content of thawed semen, is not
Ž .
prevented by the freeze–thawing procedure. However, Erochin and Derjazencev 1991 reported that MDA content was similar in fresh-diluted and thawed ram semen, and it increased only during post-thawing incubation at 378C.
A substantial amount of work on the inhibition of peroxidation in saccharose-based diluents has been done by workers in the former Soviet Union. Inhibition of
peroxida-Ž .
tion by providing anaerobic conditions hydrogen or nitrogen atmosphere during processing of semen for freezing proved effective, but technically difficult. Therefore, attention was focused on the use of antioxidants, first of natural or synthetic tocopherol
Žvitamin E . Tocopherol was most effective when included in saccharide-based diluents.
containing glucose, EDTA–Na2 and tris, and when the semen was processed in anaerobic conditions. Subsequently, the diluent was replaced by a saccharose–EDTA–
Ž
CaNa2 complex medium EDTA–Na2 saturated with Ca ions; Milovanov and
.
Sokolovskaja, 1980a , and several synthetic antioxidants were examined such as
buty-Ž . Ž . Ž .
lated hydroxytoluene BHT , 6-ditrat-butyl-1,4-kresol ‘‘ionol’’ or ‘‘topanol’’ DTBK ,
Ž . Ž
monoethanolamine ‘‘kolamine’’ , phosphoethanolamine, and echinochrome A an
ex-.
tract from sea urchins with activity 10 times higher than DTBK .
Most workers claimed that processing and freezing of semen in diluents containing the above antioxidants improved lambing rates after cervical insemination. Other investigators, however, reported that BHT and vitamin E gave little protection to ram spermatozoa, although it was found that BHT could stabilise the membrane
phospho-Ž .
lipids and decrease the permeability of the sperm membrane Hammerstedt et al., 1978 ,
Ž .
and that DTBK has a cryoprotective effect Milovanov et al., 1985 .
Several variants of the saccharose-based diluents served as a basis of the so-called
Ž .
VIZ system All-Union Animal Research Institute, Moscow; Milovanov et al., 1985 . The features of this system, recommended by the authors for practical use were: 3- to
Ž .
Ž0.84% –DTBK antioxidant 0.5% –egg yolk 10% –glycerol 5%. Ž . Ž . Ž .qantibiotics,
cool-Ž . Ž .
ing to 2–48C in 3 h, and pellet freezing 0.2 ml on a polymer plate or dry ice . The claimed lambing rates for semen frozen in the saccharose-based diluents with anti-oxidants varied from 19% to 55%.
Ž
Saccharose-based diluents were used also in the KazNITIO system Kasakh Research
.
and Technological Institute for Sheep Breeding, Alma-Ata; Kasymov, 1984 and the
Ž
KirgNPOZ system Kirghiz Association for Animal Research and Production; Sadykov
.
et al., 1985 . The composition of diluents used in the above two systems are described
Ž .
by Salamon and Maxwell 1995a .
3.1.5. Raffinose-based diluents
Raffinose as a diluent component was brought into prominence by the observation of
Ž .
Nagase and Graham 1964 with bull semen, that sugars of high molecular weight provide better protection to spermatozoa during fast freezing than do those of low molecular weight. The better cryoprotective effect of trisaccharides in comparison to
Ž .
mono- and disaccharides has also been reported when freezing ram semen. Nauk 1991 found the order of efficiency in stabilising the protein–lipid complex of the cell membrane to be: raffinose)saccharose)lactose)fructose)glucose, and the protec-tive effect of sugars in the higher rank was better when they were combined in certain proportions with other sugars, than when used alone.
Comprehensive studies on combinations of sodium citrate with various concentrations
Ž .
of sugars arabinose, glucose, lactose, raffinose led to the elaboration of the raffinose
Ž9.9% –citrate 2H O; 2% –egg yolk 15% –glycerol 5% medium for pellet freezing. Ž 2 . Ž . Ž .
Ž .
of ram semen Salamon and Lightfoot, 1969; Lightfoot and Salamon, 1969a . These workers found that the optimum osmotic pressure varied with the type of diluent, and that hypertonic media were required for successful freezing of ram semen by the pellet method. The optimum osmotic pressure for the raffinose–citrate–yolk diluent was 375–485 mosM. The high osmotic pressures due to increased raffinose concentrations were less harmful to the survival of spermatozoa than high citrate concentrations. The lambing results were 40–50% after cervical inseminations with semen frozen in this
Ž
diluent using 150–180 million motile sperm for single and double insemination;
.
Lightfoot and Salamon, 1970a,b; Salamon and Lightfoot, 1970 .
Inclusion of glutamic acid and methionine in raffinose–citrate–yolk resulted in
Ž
modest lambing after both single and double insemination 31.3% and 39.8%; Smirnov
.
et al., 1978 , and lambing was not improved when tris buffer was added to a
Ž .
raffinose–glucose–saccharose diluent Ivakhnenko and Aibasov, 1982 . However, it was
Ž .
claimed Ostashko and Kantsedal, 1976 that, as with bull semen, addition of the
Ž .
lysomal fermentb-glucuronidase 150 Urml to raffinose–citrate–yolk improved
post-Ž .
thawing survival and fertility of pellet-frozen ram semen 54.3% vs. 40.8% control . The
Ž .
lambing was also improved in comparison to the control 37% when peroxidation was
Ž .
inhibited by processing the semen in a hydrogen atmosphere 44% and also in the
Ž .
presence of an antioxidant 48% in the combined
raffinose–lactose–fructose–mag-Ž .
nesium–yolk medium Nauk, 1991 .
Ž
Raffinose formed the basis of the so-called VNIIOK freezing system All-Union
.
Ž .
use by Zheltobrjuk et al. 1987 . The system consisted of 2.5- to 3-fold dilution of
Ž . Ž .
semen at 25–308C with a diluent containing raffinose 15% –saccharose 3% –glucose
Ž0.5% –PVP 3%, molecular weight 8000–20,000 –tris 0.07% –yolk 20% –glycerol. Ž . Ž . Ž . Ž4.2%.qantibiotics, cooling to 2–48C in 3–4 h before pellet freezing 0.15–0.20 ml onŽ .
Ž .
a polymer plate y858C toy958C .
3.1.6. Tris-based diluents
Tris in diluents for freezing ram semen was reported by several workers in the early 1970s. Systematic examination of tris as a basic diluent component for pellet freezing
Ž .
was undertaken by Salamon and Visser 1972 . In their studies, ram spermatozoa tolerated a range of tris concentrations from 250 to 400 mM, and glucose was a more suitable sugar component in the tris medium than fructose, lactose or raffinose. In
Ž . Ž . Ž .
fertility tests, semen diluted 5-fold with tris 300 mM –glucose 27.75 mM –citric
Ž . Ž . Ž .
acid 94.7 mM –egg yolk 15% –glycerol 5% qantibiotics and pellet-frozen on dry
Ž
ice, yielded lambing rates from 30–57% after cervical insemination Visser and
Sala-.
mon, 1973, 1974a .
Ž .
Fischer 1990 found that tris with an osmolality of 375 mosMrkg containing 2% egg yolk was the best in preserving acrosomal integrity and motility after thawing.
Ž .
Combination of 30–290 mM sugars mono- and disaccharides with 100–300 mM tris
Žmaintaining 325 mosmol osmolality. did not influence post-thawing motility and
Ž .
fertility of spermatozoa Molinia et al., 1994 . However, there are also reports that tris performed better in vitro when combined with lactose rather than glucose, saccharose or
Ž .
milk Zamfirescu et al., 1980 , although trisqskim milkqorvus-es-paste offered some
Ž .
protection to acrosome integrity Tekin and Gunzel, 1986 .
¨
w Ž .
In studies in vitro, Triladil a tris-based diluent proved to be of equal value or a better freezing medium than lactose–yolk and saccharose–lactose–yolk, and addition of 2% bovine serum albumin to Triladilw
improved its protective effect on acrosome integrity. However, after insemination the conception rate was better for semen frozen in
w Ž .
saccharose-based medium than in Triladil Marinov et al., 1983 . Subsequently,
w Ž
Triladil -frozen semen yielded promising fertility after transcervical insemination
Hal-.
bert et al., 1990 .
Tris–fructose was used as the first diluent part in the two-step method of Fiser et al.
Ž1987 , and tris has also been included as a component in some lactose- and saccharose-. Ž .
based diluents. The tris–glucose diluent elaborated by Salamon and Visser 1972 is
Ž
widely used and recommended for the frozen storage of ram semen Salamon, 1976;
.
Evans and Maxwell, 1987 .
3.1.7. Other diluents
Zwitterion buffers such as tes, hepes and pipes have been used with varying success as the basis of diluents for freezing ram semen. Fertility of semen frozen in test medium
Žtes titrated with tris varied from poor to satisfactory 13–67% lambing . Test combined. Ž .
Noteworthy among other substances examined are dextran and hydroxyethyl starch.
Ž
When these were added to a combined medium tes–citrate–glycine–lactose–
.
raffinose–fructose–citric acid and used as the non-glycerolated diluent portion, fol-lowed by glycerolated milk–citrate, more effective cryoprotection was provided to spermatozoa than by using lactose–yolk as the first and INRA medium as the second
Ž .
diluent portion Fiser, 1975 .
3.2. ProtectiÕe agents and methods of addition to semen
3.2.1. Glycerol
Glycerol is the most commonly used protective substance in diluents for freezing ram semen. For semen frozen by the slow ‘‘conventional’’ method, and using mainly hypertonic diluents, most investigators found that the optimal glycerol concentration was within the range of 6–8%; spermatozoa frozen rapidly by the pellet method survived
Ž .
best with 3–4% glycerol in the diluent. Graham et al. 1978 reported that glycerol levels above 6% were detrimental to post-thawing survival of spermatozoa. The action of glycerol on ram and bull spermatozoa seems to be different, as it more easily
Ž .
penetrates ram spermatozoa Nauk et al., 1970 . It was suggested that the optimal glycerol concentration in diluted semen is also related to its final concentration relative
Ž .
to the spermatozoa Colas, 1975 .
The level of glycerol included in diluents for frozen storage of ram semen is
Ž .
ultimately limited by its toxicity Fahy, 1986 , which in turn depends on cooling and freezing rate, diluent composition, and method of addition of glycerol. Examination of
Ž
the combined effects of glycerol concentrations and cooling rates 0–8% and pelleting on dry ice and into liquid nitrogen, Visser and Salamon, 1974c; 0–16% and cooling at
.
1–1008Crmin, Fiser and Fairfull, 1984 showed that the higher the cooling rate, the lower the optimum glycerol concentration, and the best post-thaw sperm survival rates observed by the latter investigators were for 4–6% glycerol and a freezing rate of 10–1008Crmin.
Apart from the cooling rate, the optimum glycerol concentration also depends on the
Ž
composition of the diluent and in particular on its osmotic pressure Salamon, 1968;
.
Lightfoot and Salamon, 1969a . Furthermore, the glycerol concentration may be influ-enced by the egg yolk level in the diluent, as increased concentrations of egg yolk may
Ž .
reduce the required concentration of glycerol Watson and Martin, 1974 .
There are reports indicating that inclusion of antioxidants with a cryoprotective effect in the diluent may also allow the use of a relatively low glycerol concentration of 3.5%,
Ž .
2.5% or 2.0% Varnavskij and Varnavskaja, 1976; Milovanov et al., 1985 .
Ž .
Glycerol can be added to the semen in a separate diluent fraction two-step dilution ,
Ž .
or by a single addition of the diluent containing glycerol one-step method . Initially, it was found better to add the glycerolated diluent portion at 298C than at 58C, but in subsequent tests addition at 58C was more suitable. Addition of glycerolated diluent at 4–58C was preferred by most workers, but some found no difference between addition at either 328C or 38C or at 228C or 58C.
The glycerol-containing diluent generally was of the same type as that used for initial
Ž .
workers found that post-thawing recovery and fertility of spermatozoa were better when the partially diluted semen was glycerolated with INRA medium than with the initial lactose–yolk extender, and lambing was also better when glycerolisation occurred at 48C
Ž .
rather than at 308C Colas, 1975; Colas and Brice, 1975 .
Ž .
Colas 1975 suggested that glycerol may be slightly toxic to spermatozoa even at a concentration of 4% and its harmful effect is less when added at a temperature close to 08C. Early workers using two-step dilution recommended a gradual addition of the glycerol-containing diluent at 2–58C. However, it has been found that single addition of
Ž .
the glycerolated portion before freezing at 58C was as effective, or better than, the gradual addition in divided portions.
Ž .
Contrary to the work recommending the use of two-step dilution, Salamon 1968 and
Ž .
Lightfoot and Salamon 1969a reported that a single dilution of semen with
glycerol-Ž .
containing diluent at 308C ‘‘one-step’’ method was as good as two-step dilution, but the efficiency of the former method depended on the sugar component of the diluent. One-step addition of glycerol at 308C is a practical and widely used method for dilution
Ž .
of ram semen for frozen storage Salamon, 1976; Evans and Maxwell, 1987 .
While glycerol offers cryoprotection to spermatozoa, it may also cause structural damage during pre-freeze processing. Consequently, it was suggested that glycerol should be added no more than 20–30 min before freezing. Effective cryoprotection after
Ž .
short 5–10 s contact with glycerol has been demonstrated for bull and boar, and also
Ž .
for ram semen 0–5 min , which supports the earlier view that penetration of glycerol into the cell is not essential for protection. Removal of glycerol from thawed semen by centrifugation or by dialysis had no effect on lambing.
Successes in obtaining fertility with ram semen frozen without glycerol have been
Ž . Ž .
reported by Lopatko 1976 and Abdelhakeam et al. 1991a who claimed 26% and 52% lambing rates, respectively. For freezing ram semen without glycerol, Abdelhakeam et
Ž .
al. 1991b diluted the semen at 58C, 3 h after collection, with hypertonic test buffer containing 25–30% egg yolk and 10% maltose monohydrate. The efficacy of the procedure remains to be confirmed.
Several agents other than glycerol have also been examined for their cryoprotective
Ž .
action on ram spermatozoa. Such agents were dimethylsulfoxide DMSO , ethylene glycol, albumin, low molecular weight polyols, polymeric compounds, surfactants, high
Ž
concentrations of sugars of various types, compatible solutes proline, glycine betaine,
. Ž
taurine and antifreeze proteins from polar fish reviewed by Salamon and Maxwell,
.
1995a . None of the agents examined proved better than glycerol.
3.2.2. Egg yolk
Egg yolk is a common constituent of semen diluents, protects the spermatozoa against cold shock and confers protection during freezing and thawing. It is believed to act at the level of the cell membrane, and to have a bigger effect for bull than ram spermatozoa.
Ž
concentration, although the effect depended on the composition of the diluent Salamon
.
and Lightfoot, 1969 . Some investigators examined egg yolk concentrations as low as
Ž .
1.5–3.75%, but others Graham et al., 1978 found that low levels were unsatisfactory.
Ž .
The saccharose–EDTA–CaNa complex diluent allowed the use of low 4–5% egg2
Ž .
yolk concentrations and even its elimination was suggested Milovanov et al., 1985 . On
Ž .
the other hand, in the attempt of Abdelhakeam et al. 1991b to freeze ram semen without glycerol, it was necessary to increase the egg yolk to 25–30% in the test diluent for successful freezing.
Ž .
Egg yolk, although sometimes partially replaced by substance s with similar activity, is still likely to remain an important component of diluents for freezing ram semen, particularly due to its protective effect on the plasma membrane.
3.3. Processing and freezing of semen
The success of deep freezing depends to a notable degree on the rate of dilution of semen. Originally, semen was diluted to protect spermatozoa during cooling, freezing and thawing, but the rate of dilution was often changed for technical reasons, such as to increase the number of females which could be inseminated with each ejaculate, or to standardise the number of spermatozoa in each dose of frozen–thawed semen.
The pre-freezing dilution rate used by early workers varied from 11- to 26-fold, but
Ž .
the majority of subsequent investigators used 2- to 6-fold vrv dilution rates. As the diluent composition was not adjusted for the different rates of dilution, it could not be determined whether any effect on spermatozoa was due to dilution per se, or to variation of the diluent components in the diluted semen. For freezing ram semen nowadays,
Ž .
generally 2- to 5-fold vrv pre-freezing dilution rates are used, with the diluent
Ž
composition adjusted for the rate of dilution Salamon, 1976; Evans and Maxwell,
.
1987 .
After dilution, the semen is cooled to a temperature close to 08C. Cooling is a period of adaptation of spermatozoa to reduced metabolism. Traditionally, equilibration has been regarded as the total time spermatozoa remain in contact with glycerol before freezing, during which it penetrates into the sperm cell to establish a balanced intra-cellular and extraintra-cellular concentration. It should not be overlooked that the term ‘‘equilibration’’ includes the concentration balance not only of glycerol, but also of the other osmotically active diluent components. Glycerol has been implicated in some undesirable phenomena occurring during equilibration such as changes in the structure and biochemical integrity of spermatozoa and acceleration of the acrosome reaction. Although these may be overlooked in the light of the other favourable cryogenic properties of glycerol, it should also be noted that this medium may, to a certain extent, adversely affect the fertility.
With the two-step method of dilution the contact with glycerol begins at 2–58C. However, when one-step dilution is adopted, glycerolisation begins at 308C. In both methods, a period of cooling to 2–58C is required, the duration of which generally varies from 1 to 3 h. Rapid cooling of extended semen from 308C to about 158C may have no effect on survival of spermatozoa, but fast cooling from 308C to 108C, 58C or 08C
Ž .
In the case of one-step dilution to the final rate at 308C with the glycerolated
Ž .
medium, a combined cooling–equilibration storage is involved. In the studies on pellet
Ž .
freezing of ram semen Lightfoot and Salamon, 1969a , the optimum period of storage
Žequilibration. after one-step dilution and cooling to 58C depended on the sugar composition of the diluent and the glycerol concentration. The presence of raffinose in the diluent allowed a decrease in equilibration from 2.5 to 1 h. Nowadays the one-step method of dilution with glycerolated medium is generally used and it is recommended to
Ž
freeze the semen after 1.5 to 2 h cooling to 58C Salamon, 1976, Evans and Maxwell,
.
1987 . There seems to be no convincing evidence supporting either a longer coolingrequilibration period, or the need for the more complicated two-step dilution procedure.
The cooling rate of diluted semen from temperatures just above 08C can significantly influence the survival of spermatozoa after freezing. Ram semen frozen in ampoules has generally been cooled at the slow ‘‘conventional’’ rate applied for bull semen. This method is seldom used today, and freezing is done in PVC straws and minitubes or in pellet form.
Freezing of semen in straws is performed by suspension in liquid nitrogen vapour, and the velocity of cooling is regulated by the distance of the straws from the level of the liquid nitrogen. Ram spermatozoa tolerate a range of freezing velocities in straws. Thus, suspension of straws in liquid nitrogen vapour betweeny758C and y1258C had
Ž .
no effect, but vapour at y558C reduced the survival of spermatozoa Colas, 1975 . Although cooling rate was found to interact with glycerol concentration, the cooling rates from 10 to 1008Crmin had a smaller effect on the survival of spermatozoa than
Ž .
glycerol concentration Visser and Salamon, 1974c; Fiser and Fairfull, 1984 .
The shape of the freezing curve may also be important for straw freezing. Instead of a linear decrease in temperature, it is better to cool the semen according to a parabola-shaped curve, which can be achieved by suspending the straws 4–6 cm above the liquid nitrogen surface. Post-thaw recovery of motility of spermatozoa frozen in straws and minitubes on the surface of dry ice was poorer than of pelleted spermatozoa, but this
Ž .
was not reflected in the fertility rate Maxwell et al., 1995a .
Pellet-freezing is generally done by placing semen droplets into depressions on dry
Ž .
ice or on a polymer plate cooled toy808C toy958C in the former Soviet Union . The velocity of cooling can be regulated by the volume of the semen pellet and the temperature of the freezing agent. Nevertheless, pellet volumes from 0.03 to 0.86 ml, with different surface to volume ratios, have been frozen on dry ice without a reduction
Ž .
in post-thaw motility, although the cooling rates varied 95 to 308Crmin for pellet sizes
Ž .
and also within the pellet Lightfoot and Salamon, 1969b; Visser and Salamon, 1974b . Increased cooling rates obtained by pelleting on a stainless steel plate or on dry ice
Ž .
cooled in liquid nitrogen y1008C to y1608C had no effect on recovery of sperm
Ž .
motility Salamon, 1970 and lambing for semen pellet-frozen at y798C or y1408C
Ž .
was not different Salamon, 1971 . Dropping the semen directly into liquid nitrogen resulted in poor recovery of motility, or all spermatozoa died. The reason for this may be that liquid nitrogen is not a good medium for very fast freezing. Since it is a liquid at its boiling point, it has a calefactory effect which delays the thermal contact with the
Ž . Ž .
Ž .
nitrogen, or vials with semen 1.5–2.5 ml were immersed into liquid nitrogen for 5–7 s, then elevated and held in liquid nitrogen vapour for a few minutes before re-immersion into liquid nitrogen, post-thaw motility of pelleted spermatozoa was nearly similar to
Ž .
that of sperm pelleted onto dry ice Visser and Salamon, 1974c , and after insemination
Ž .
with the semen thawed in vials satisfactory pregnancy rates 51–69% were claimed
ŽKasymov and Ashimov, 1975 ..
3.4. Thawing of semen
In the freeze–thaw procedure, the warming phase is just as important to the survival of spermatozoa as the cooling phase. Spermatozoa that have survived cooling to
y1968C still face the challenge of warming and thawing, and thus must traverse twice
Ž .
the critical temperature zone y158C toy608C .
Both rate of cooling and thawing exert an effect on the survival of spermatozoa
ŽMazur, 1985 . The effect depends on whether the rate of cooling has been sufficiently.
high to induce intracellular freezing, or low enough to produce cell dehydration. In the former case, fast thawing is required to prevent recrystallisation of any intracellular ice present in the spermatozoa. Spermatozoa thawed at a fast rate may also be exposed for a
Ž .
shorter time to the concentrated solute and cryoprotectant glycerol , and the restoration of the intracellular and extracellular equilibrium is more rapid than for slow thawing.
There was no agreement among early investigators on the optimum temperature for thawing of semen frozen in ampoules. Some found that ram spermatozoa cooled slowly in ampoules by ‘‘the bull method’’ revived better when thawed at 40–438C, than slowly ‘‘by reversing the cooling procedure’’. Other workers thawed the ampoules in ice water,
Ž .
in water at 2–58C, 6–88C or at room temperature 18–208C with conflicting results regarding recovery of spermatozoa. In the majority of reports, ampoule–frozen ram semen was thawed in a water bath at 378C.
Ram semen frozen in straws has been thawed by most investigators at 38–428C.
Ž .
Some workers reported that thawing at high temperatures 60–758C was comparable to that at 38–428C in terms of post-thaw motility, acrosome integrity and fertility of spermatozoa.
Ž .
Pellet-frozen ram semen may be thawed either in a solution wet thawing or in dry
Ž .
tubes dry thawing . In the former case, not only the presence, but also the composition of the thawing solution influenced the recovery of sperm motility, and the effect
Ž
depended on the composition of the sugar–citrate–yolk freezing diluent Lightfoot and
.
Salamon, 1969b . Inositol–citrate, glucose–citrate and fructose solutions were the best
Ž .
and of equal value for thawing at 378C semen pellets frozen in raffinose–citrate–yolk diluent. A relationship between composition of freezing diluent and of thawing solution
Ž
was also found when the tris-based freezing media were used Salamon and Visser,
.
1972 . Further, there were interactions between the composition of freezing diluents and
Ž .
method of dry or wet thawing.
without the risk of overheating the semen, interrupted thawing or the use of special thawing devices have been recommended. In the former case the semen pellet, placed in
Ž .
warm thawing solution 60–808C , was transferred to a water bath at 35–408C when
Ž .
about two-thirds of the pellet has melted Smirnov et al., 1978 . The thawing devices assure a continuous and fast separation of the liquid portion from the remaining solid pellet, and thus prevent further heating of the thawed semen portion. The different types
Ž .
of thawing device have been described by Salamon and Maxwell 1995a .
3.5. Causes of low fertility after cerÕical insemination
Satisfactory sperm survival rates were obtained with a number of diluents and the complex technology of freezing ram semen appears to be satisfactorily developed. Nevertheless, final judgement on the technologies elaborated should be tempered, because lambing results similar to those for fresh semen have only rarely been obtained after cervical insemination. The low fertility after cervical insemination with frozen– thawed ram semen could be due to a variety of factors.
3.5.1. Damage to spermatozoa during freeze–thawing
Ž .
Although a relatively high proportion 40–60% of ram spermatozoa preserve their motility after freeze–thawing, only about 20–30% remain biologically undamaged. A spermatozoon may be motile, but damaged, in which case it is doubtful if such a cell will fertilise the egg. Motility and structure of the spermatozoa are affected to different extents, and it is not known whether the changes occur simultaneously or are caused at different stages of the freeze–thaw procedure.
Ž .
The basic cryogenic damage to spermatozoa may be ultrastructural physical , biochemical or functional. Ultrastructural damage occurs to the plasma and acrosome membranes, the acrosome, the mitochondrial sheath and the axoneme. Ultrastructural damage generally is more severe for ram than bull spermatozoa. After both slow and fast freezing of ram semen, motility is better preserved than the morphological integrity of spermatozoa. The plasma and acrosome membranes are more sensitive than the nucleus
Ž .
and locomotor mid-piece part of the sperm cell. The outer membrane of the acrosome is more vulnerable than its inner part and internal membrane. It has been observed that the mitochondrial architecture is altered by freeze–thawing, but the tail filament and fibrils showed no detectable change after freeze–thawing.
Ultrastructural damage during freeze–thawing is accompanied by biochemical changes or loss of their vital contents. Some of the measured losses and changes include the
Ž .
release of glutamic oxaloacetic transaminase GOT , losses of lipoproteins and amino acids, decrease in phosphatase activity, decrease in loosely bound cholesterol protein, increase in sodium and decrease in potassium content, inactivation of hyaluronidase and acrosin enzymes, loss of prostaglandins, reduction of ATP and ADP synthesis, decrease
Ž .
in acrosomal proteolytic activity reviewed by Salamon and Maxwell, 1995b . Denatura-tion of DNA is also possible, as modificaDenatura-tions in the chromatin structure of bull, boar,
Ž
cat, human and recently of ram spermatozoa have been observed Gillan and Maxwell,
.
These ultrastructural and biochemical cryogenic changes to spermatozoa could be responsible for a decrease in their functional integrity, survival in vivo, and fertilising capacity.
3.5.2. Transport, Õiability and fertilising ability of spermatozoa in the female genital
tract
Ž
A number of investigators have examined these aspects reviewed by Salamon and
.
Maxwell, 1995b and found that there is inadequate transport and reduced viability of frozen–thawed spermatozoa in the genital tract of the ewe, which are the major causes of low fertility after cervical insemination. After cervical insemination, frozen–thawed spermatozoa were expelled faster than fresh spermatozoa from the female reproductive
Ž .
tract Gillan and Maxwell, 1999 . However, when the thawed semen was deposited into
Ž .
the uterus or oviducts, high 85–95% egg fertilisation rates have been obtained, showing that the presumably biologically intact spermatozoa maintained their fertilising capacity after freeze–thawing.
3.5.3. Capacitation
Ž .
It has been advocated Watson, 1995; Maxwell and Watson, 1996 that some aspects
Ž .
of the cooling–freezing–thawing cycle similar to liquid storage may advance the maturation of sperm membranes, and increase the proportion of capacitated and acro-some reacted spermatozoa. Changes to the membranes of spermatozoa may not affect motility, but shorten the lifespan of the cells and render them less fertile or incapable of fertilisation. Ageing of capacitated spermatozoa in the reproductive tract after cervical insemination may further aggravate the situation. If such spermatozoa are not exposed to oocytes within a short time after insemination, fertilisation will not occur, as the cells
Ž
may prematurely die in the lower part of the reproductive tract. In vitro studies Gillan
.
and Maxwell, 1999 showed that frozen–thawed spermatozoa are released earlier than fresh spermatozoa after binding to oviduct cells, confirming their physiological readiness
Ž
to participate in fertilisation. The membrane status of spermatozoa intact, capacitated
. Ž
and acrosome reacted can be assessed by functional assay zona-free hamster egg
w x .
penetration: Garde et al., 1993; and chlortetracycline CTC staining: Gillan et al., 1997 .
Ž .
Where hamsters are not available like Australia, due to quarantine restrictions , in vitro
Ž .
matured zona-intact sheep oocytes may be used for penetrationrfertilization assays
ŽGillan and Maxwell, 1999 ..
3.5.4. Embryonic mortality
Spermatozoa weakened or not mortally injured during freeze–thawing, and those which after thawing and insemination are aged in the female tract may initiate embryos
Ž .
that are not viable and perish at an early stage. Maxwell et al. 1996a found that after in vitro fertilisation of in vitro matured sheep oocytes, the stage of fertilisation may be more advanced for oocytes inseminated with frozen–thawed than fresh spermatozoa. The fast cleavage observed also after cervical insemination with liquid stored semen
ŽLopyrin and Rabocev, 1968 , can be considered to presage embryonic mortality. There.
Ž .
is some evidence Gillan et al., 1997 that ewes inseminated with frozen–thawed
Žcapacitated spermatozoa into the uterine horns, and at a time close to ovulation are.
3.5.5. Other causes of low fertility
Late oestrus is not favourable for transport and survival of spermatozoa and fertilisa-tion is generally lower for short than long oestrus. Fertility may be reduced if eCG is not used in combination with a progestagen sponge for synchronisation and induction of
Ž . Ž .
oestrus, and fertility is also low when oestrus occurs early 36 h rather than late 48 h after progestagen sponge removal. The optimum time of insemination is between 48 and 58 h after sponge removal.
3.6. Methods and attempts to improÕe fertility after cerÕical insemination
Since the low fertility obtained after cervical insemination with frozen–thawed semen is primarily due to failure of establishment of an adequate cervical population of functionally intact spermatozoa, which is associated with impaired sperm transport in the ewe’s genital tract, several methods and substances have been used in attempts to improve the situation.
3.6.1. Increased concentration of spermatozoa
An obvious method was the reconcentration of the thawed semen by centrifugation in order to deposit high numbers of spermatozoa at insemination. This resulted in an increased number of sperm cells throughout the tract up to the oviduct, improved egg
Ž
fertilisation and lambing Lightfoot and Salamon, 1970b; Salamon and Lightfoot, 1970;
.
Colas and Guerin, 1981 . Reconcentration of thawed semen by filtration through a
´
Ž .
milliporous membrane Volkov, 1974 and insemination of high numbers of
spermato-Ž
zoa in increased volumes have also been used to increase fertility Colas and Brice,
.
1976; Colas, 1975 . However, the above methods were of low efficiency, as only a few ewes could be inseminated with one frozen–thawed ejaculate. Dilution of semen at a
Ž .
low rate 1.5-fold before freezing in order to obtain inseminates with a high concentra-tion of thawed spermatozoa yielded poor lambing.
3.6.2. Use of relaxin, oxytocin, prostaglandins, other substances and methods
Injection of ewes with relaxin, oxytocin and prostaglandins had two possible aims: to relax the cervix, which would permit deep cervical or uterine deposition of frozen– thawed semen, or to increase the contractility of the genital tract and thus improve the transport of spermatozoa.
Relaxin injected 12 h before insemination had no relaxation effect on the cervix and
Ž .
did not affect the mean depth of insemination Salamon and Lightfoot, 1970 . Oxytocin stimulated the contractile activity of the cervix and uterus in vivo and in vitro, dilated the cervix and improved sperm transport after mating, but not after insemination with frozen–thawed semen in which case lambing was also depressed.
Introduction of the muscle relaxant cocaine into the cervix, or electrical stimulation
Ž3.7 V. of the cervix before insemination to induce uterine contraction , had noŽ .
Ž . w Ž
beneficial effect on lambing Varnavskij and Turbin, 1974 . Injection of Partusisten a
.
tocolytic substance 20 min before insemination improved the penetrability of the cervix
Ž .
Ž .
Prostaglandin PG E , PGE1 2 and PGF2a stimulated the contractile activity of the cervix and uterus in vitro and in vivo, and supplementation of semen with PGE and1
PGF2a before freezing also improved the transport of spermatozoa in the genital tract. PG improved the sperm motility and lambing when added to thawed semen, but not when used before freezing. Injection of ewes with PGF2a before insemination with thawed semen also improved the lambing results, and hylase and buscolysin injected
Ž
intramuscularly increased the penetrability of the cervix reviewed by Salamon and
.
Maxwell, 1995b .
Treatment of frozen–thawed semen with metabolic stimulants such as methylxan-thines caffeine and pentoxifylline, proteinase, kallikrein calcium, cyclic adenosine-3X,
X Ž .
5 -monophosphate cAMP , and 2-deoxyadenosine, which in a number of other species stimulated sperm motility, had no such effect on ram spermatozoa, except for pentoxi-fylline. However, as the increase in motility by the latter compound was not reflected in
Ž .
improved fertility after intrauterine insemination Maxwell et al., 1995b , it is doubtful whether pentoxifylline would improve the fertility in the case of cervical insemination.
3.6.3. Double insemination
Double insemination is a generally accepted method of increasing fertility. The magnitude of the response may depend on the sperm concentration in the inseminate and the timing of insemination in relation to the stage of oestrus. Little advantage was gained from the second insemination when the first had been performed at about the middle of
Ž .
oestrus Salamon, 1972 . It should also be noted that in studies on double insemination, the number of motile frozen–thawed spermatozoa deposited by two inseminations was twice that deposited by single insemination, and in such a case the benefit of double
Ž
insemination can be accounted for by the increased number of spermatozoa Visser and
.
Salamon, 1974a . When equal total numbers of motile spermatozoa were deposited by
Ž .
single and double inseminations within the optimum time range 12–28 h after detection of oestrus, the lambing results were similar, and the number of motile spermatozoa inseminated was more important than whether single or double
insemina-Ž .
tions were performed Salamon, 1977 . Regarding the time interval between insemina-tions, a better lambing result can be expected when the first, performed on the morning of detection of oestrus, is followed by the second 8–10 h rather than 24 h later.
3.6.4. Depth of insemination
Penetration of the ewe’s cervix is difficult due to its peculiar structure, and for deposition of thawed semen deep into the cervical canal different techniques and devices
Ž
have been adopted. The cervical traction method which consisted of pulling the
.
entrance of the cervix into the vagina by forceps and the cervical traction combined
Ž .
with digital manipulation of the cervix through the rectum Andersen et al., 1973 allowed semen deposition to a depth of 2–5 cm, and the latter method even uterine deposition of semen. Both methods improved the fertility, but were stressful to the animal. Deep cervical semen deposition was easier to achieve by special inseminating
Ž .
pipettes and devices described by Salamon and Maxwell, 1995b . The lambing results
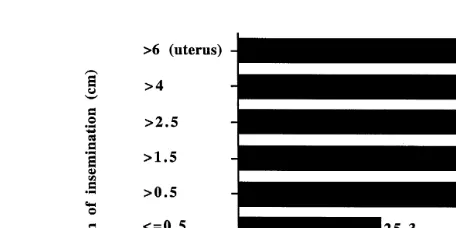
Ž .
Fig. 1. Effect of depth of insemination on fertility of frozen–thawed ram semen introduced through the cervix
Žweighted means from 26 publications; 9716 ewes ..
relatively consistent between workers inseminating to approximately the same depth. Deep cervical insemination allowed a decrease in the number of motile spermatozoa to a dose of 20 or 40 million, which after single insemination gave 50% and 53% lambing respectively, compared with 51% for control insemination with 80–100 million motile
Ž .
spermatozoa Stojanov, 1980 .
3.6.5. Intrauterine insemination
The problem of the cervical barrier in the ewe can be overcome by deposition of
Ž .
frozen–thawed semen into the uterus via the cervix transcervical insemination or directly into the uterus by laparotomy or laparoscopy.
Success of penetration transcervically into the uterus varied between investigators from 39% to 62%. By using the ‘‘Guelph system’’ which involved traction of the cervix,
Ž .
87% successful penetration has been claimed Buckrell et al., 1992 . The transcervical insemination methods, although offering success for intrauterine insemination, vary regarding the repeatability of penetration, are time consuming and rather stressful to the animal. In an experiment conducted in Australia, the pregnancy rate at day 70 for ewes
Ž .
inseminated by laparoscopy 48% was higher than for ewes inseminated by
transcervi-Ž . Ž . Ž .
cal intrauterine 32% or cervical 9% methods Windsor et al., 1994 .
Surgical insemination by laparotomy was used by early investigators to examine the fertilising capacity of frozen–thawed spermatozoa. Since the introduction of
laparo-Ž .
scopic semen deposition Killeen and Caffery, 1982 , this method has been generally
Ž
adopted for intrauterine insemination in most sheep producing countries Evans, 1991;
.
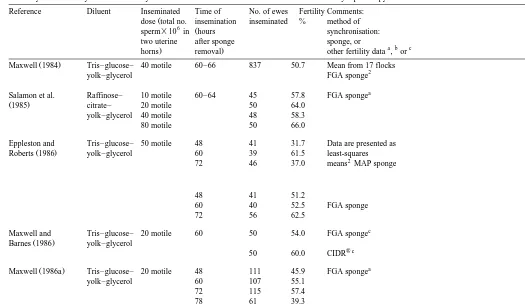
Haresign, 1992 , as it offers reliable and predictable lambing results. Noteworthy reports are summarised in Table 1.
Laparoscopic insemination has been mainly performed at a synchronised oestrus and to a lesser extent in natural oestrus. In the case of synchronised oestrus, the main variables examined have been type of synchronising device, season of synchronisation, time of insemination, dose of inseminate and site of uterine deposition.
3.6.5.1. Type of synchronising deÕice. Laparoscopic insemination yielded better lambing
Ž .
in ewes synchronised with fluorogestone acetate FGA; 30 mg Chronogest than with
Ž .
()
S.
Salamon,
W.M.C.
Maxwell
r
Animal
Reproduction
Science
62
2000
77
–
111
Table 1
Summary of noteworthy results on fertility of frozen–thawed semen after intrauterine insemination by laparoscopy Reference Diluent Inseminated Time of No. of ewes Fertility Comments:
Ž
dose total no. insemination inseminated % method of 6 Ž
sperm=10 in hours synchronisation:
two uterine after sponge sponge, or
a b c
. .
horns removal other fertility data , or
Ž .
Maxwell 1984 Tris–glucose– 40 motile 60–66 837 50.7 Mean from 17 flocks
2
yolk–glycerol FGA sponge
a
Salamon et al. Raffinose– 10 motile 60–64 45 57.8 FGA sponge
Ž1985. citrate– 20 motile 50 64.0
yolk–glycerol 40 motile 48 58.3
80 motile 50 66.0
Eppleston and Tris–glucose– 50 motile 48 41 31.7 Data are presented as
Ž .
Roberts 1986 yolk–glycerol 60 39 61.5 least-squares
2
72 46 37.0 means MAP sponge
48 41 51.2
60 40 52.5 FGA sponge
72 56 62.5
c
Maxwell and Tris–glucose– 20 motile 60 50 54.0 FGA sponge
Ž .
Barnes 1986 yolk–glycerol
wc
50 60.0 CIDR
a
Ž .
Maxwell 1986a Tris–glucose– 20 motile 48 111 45.9 FGA sponge
yolk–glycerol 60 107 55.1
72 115 57.4
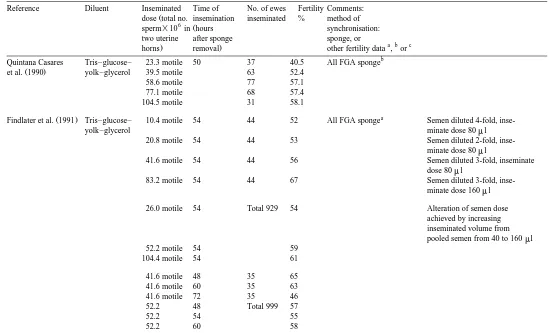
()
S.
Salamon,
W.M.C.
Maxwell
r
Animal
Reproduction
Science
62
2000
77
–
111
99
yolk–glycerol 5 motile 164 25.0
10 motile 85 38.8
20 motile 96 53.1
25 motile 87 56.3
50 motile 103 62.1
a
20 motile 75 44.9 FGA sponge Insem. in ipsilateral
a
69 76.8 FGA sponge horn only.
Insem. in both uterine horns.
Findlater and Tris–glucose– NA 48 122 64 Type of sponge used
a
Ž .
Haresign 1987 yolk–glycerol 60 63 not stated
72 44
d e d e b
Ž .
Hunton et al. 1987 Tris–glucose– NS 59–63 61r60 49 r53 FGA sponge
d e d e
yolk–glycerol 38 r38 66 r47
d e d e 38 r39 66 r49
a
Ž .
Nehring et al. 1989 Milk–fructose– 50 56–60 20 50.0 FGA sponge
yolk
b
Ž . Ž .
Walker et al. 1989 Tris–glucose– 40 48–52 or 330 57.3 FGA sponge No GnRH control
yolk–glycerol 56–60 86 32.6 GnRH 24 h after removal
326 57.7 GnRH 36 h after removal
Gourley and Tris–glucose– 20 55–60 294 60.2 Insemination in breeding
Ž .
Riese 1990 yolk–glycerol season
30 63.3 FGA sponge Insemination in
non-and norgesto- breeding season b
met implant
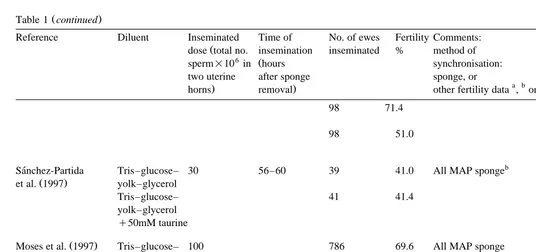
()
S.
Salamon,
W.M.C.
Maxwell
r
Animal
Reproduction
Science
62
2000
77
–
111
Ž .
Table 1 continued
Reference Diluent Inseminated Time of No. of ewes Fertility Comments:
Ž
dose total no. insemination inseminated % method of 6 Ž
sperm=10 in hours synchronisation:
two uterine after sponge sponge, or
a b c
. .
horns removal other fertility data , or
b
Quintana Casares Tris–glucose– 23.3 motile 50 37 40.5 All FGA sponge
Ž .
et al. 1990 yolk–glycerol 39.5 motile 63 52.4
58.6 motile 77 57.1
77.1 motile 68 57.4
104.5 motile 31 58.1
a
Ž .
Findlater et al. 1991 Tris–glucose– 10.4 motile 54 44 52 All FGA sponge Semen diluted 4-fold,
inse-yolk–glycerol minate dose 80ml
20.8 motile 54 44 53 Semen diluted 2-fold,
inse-minate dose 80ml
41.6 motile 54 44 56 Semen diluted 3-fold, inseminate
dose 80ml
83.2 motile 54 44 67 Semen diluted 3-fold,
inse-minate dose 160ml
26.0 motile 54 Total 929 54 Alteration of semen dose
achieved by increasing inseminated volume from pooled semen from 40 to 160ml
52.2 motile 54 59
104.4 motile 54 61
41.6 motile 48 35 65
41.6 motile 60 35 63
41.6 motile 72 35 46
52.2 48 Total 999 57
52.2 54 55