Atherosclerosis 154 (2001) 137 – 140
Blood endothelin-1 levels before and after carotid endoarterectomy
for atherosclerotic stenosis
Giuliana Properzi
a,*, Felice Francavilla
a, Cesare Bellini
a, Piera D’Abrizio
a,
Bambina Mangiacotti
b, Claudio Ferri
a, Carlo Spartera
b, Anna Santucci
a,
Sandro Francavilla
aaDepartment of Internal Medicine,School of Medicine,Uni
6ersity of L’Aquila,Via S.Sisto,67100L’Aquila,Italy
bDepartment of Surgery,School of Medicine,Uni
6ersity of L’Aquila,L’Aquila,Italy
Received 2 February 1999; received in revised form 17 February 2000; accepted 25 February 2000
Abstract
Background: Elevated plasma levels of endothelin-1 (ET-1) have been reported in advanced atherosclerosis. Further in vivo demonstration of cause-effect relationship between atherosclerotic lesion and high levels of ET-1 needs to be carried out. The aim of this study was to determine whether circulating levels of ET-1 are influenced by removing haemodynamically significant atherosclerotic stenosis in selected patients with mono or bilateral carotid atherosclerotic stenosis. Methods: Cubital venous ET-1-immunoreactive (IR) levels were measured in 20 patients: 11 (mean age9S.D. 63.195.36 years; range 53 – 70 years) were affected by monolateral, and nine patients (mean age9S.D. 64.799.8 years; range 52 – 78 years) by bilateral extracranial carotid artery atherosclerotic stenosis. ET-1-IR levels were evaluated before and 7 days after monolateral surgical endoarterectomy. Pre-surgery levels of ET-1-IR were compared with those obtained from 18 healthy younger volunteers (mean age9S.D. 27.892.7 years; range 20 – 50 years). Findings: The mean cubital venous levels of ET-1-IR in the atherosclerotic patients before endoarterectomy (mean9S.D. 4.5093.35 pg/ml; range 1.28 – 10.66 pg/ml) were significantly higher than those observed in healthy subjects (mean9S.D. 0.64190.137 pg/ml; range 0.36 – 1.02 pg/ml) (P=0.000). The mean ET-1-IR level decreased significantly after endoarterectomy in the group of patients with monolateral stenosis (pre-surgery: mean9S.D. 4.3593.11 pg/ml; range 1.28 – 10.66 pg/ml; post-surgery: mean9S.D. 3.0592.94 pg/ml, range 0.28 – 8.86 pg/ml) (P=0.005), but not in patients with bilateral extracranial carotid stenosis submitted to monolateral endoarterectomy (pre-surgery: mean9S.D. 4.7793.79 pg/ml; range 2.18 – 10.3 pg/ml; post-surgery: mean9S.D. 4.6093.70 pg/ml; range 2.20 – 11.10 pg/ml).Interpretation: The removal of a haemodynamically significant atherosclerotic vascular stenosis is associated with a decrease in the circulating ET-1-IR levels 7 days after surgery when haemodynamically significant atherosclerotic lesions are absent. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Endothelin-1; Atherosclerosis; Carotid stenosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
Endothelin-1 (ET-1) is a 21-amino acid peptide pro-duced by endothelial cells (EC) [1]. Released predomi-nantly in a polar fashion directed toward the underlying intimal smooth muscle cells (VSMC) [2], ET-1 binds to specific receptors and causes vasocon-striction by increasing the cytosol free calcium concen-tration, due to phospholipase C activation [3]. The
demonstration of a promitogenic effect on VSMC through the activation of c-fos and c-myc gene expres-sion [4 – 6], and the induction of fibroblast proliferation and collagen synthesis [7], is supported by a possible involvement of ET-1 in atherogenesis. Moreover, sev-eral potential contributors to atherogenesis, such as transforming growth factor-1 [8], insulin-like growth factor-1 [9 – 11], as well as atherogenic substances accu-mulating inside the plaque such as oxidized LDL [12], stimulate ET-1 release by EC. Recently, enhanced ex-pression of endothelin-A receptors (ET-A) has been shown in the tunica media of porcine saphenous
vein-* Corresponding author. Tel.: +39-0862-432856; fax: + 39-0862-432858.
G.Properzi et al./Atherosclerosis154 (2001) 137 – 140 138
carotid artery grafts [13] and in carotid arteries of rat after balloon injury. This suggests a significant role of ET-1 in injury-induced-VSMC proliferation and neointima formation [14]. Taken together, these in vitro observations suggest that ET-1, released in excess by endothelial cells during atherosclerosis, might con-tribute to the development of atherosclerotic lesions. Increased circulating ET-1 levels were reported in patients with advanced atherosclerosis [15 – 18] and a positive correlation has been found between levels of ET-1 and thickness of the intima-media complex in the common and internal carotid artery [17]; moreover, the increased venous levels of ET-1 are suggested to predict an increased cerebrovascular morbidity in patients with internal carotid stenosis [18]. The mentioned studies in men affected by atherosclerotic diseases agree with a possible role of ET-1 in the progression of carotid atherosclerosis.
While increased circulating ET-1 levels are associated with advanced clinical atherosclerosis [16 – 18], it is not known whether circulating levels of ET-1 in patients affected by isolated carotid artery stenosis are modified by removing a vascular atherosclerotic stenosis. Re-cently Cacoub et al. [16] reported a local decrease of ET-1 in internal jugular vein and only a small decrease in external and internal carotid arteries after carotid cross-clamping, during human carotid revasculariza-tion. To investigate the effect of endoarterectomy on cubital vein blood levels of the peptide, we measured plasmatic levels of ET-1-IR in a selected group of patients, affected by extracranial carotid artery atherosclerotic vascular stenosis, before and after surgi-cal endoarterectomy.
2. Materials and methods
2.1. Study patients
Informed consent was obtained from each subject enrolled. A total of 20 patients (16 males and four females), aged 63.997.5 years (mean9S.D.; range 52 – 78 years) undergoing endoarterectomy for symp-tomatic extracranial carotid stenosis due to atheroscle-rosis, were studied. No patients were affected by any other symptomatic vascular stenosis. Echo-colour-Doppler, NMR angiography and carotid angiography were performed to localise the vascular lesion and the degree of carotid stenosis, ranging between 70 and 90%, was recorded. Any significant stenosis in other vascular areas was excluded by non-invasive diagnostic proce-dures [19]. Patients were divided into two groups: the first group consisted of 11 patients (mean age9S.D. 63.195.36 years; range 53 – 70 years) affected by monolateral carotid stenosis while the second group consisted of nine patients (mean age9S.D. 64.799.8
years; range 52 – 78 years) affected by bilateral carotid stenosis.
Patients were evaluated for occurrence of hyperten-sion, coronary disease, peripheral vasculopathy, neu-rovascular disease, diabetes, and hyperlipemia. Moderate hypertension and/or diabetes were diagnosed only in a minority of cases equally distributed within the two groups (3/11 in group I and 2/9 in group II). Cardiovascular medications were discontinued at least 12 h before blood collection for the measurement of plasma ET-1.
2.2. Test procedures
ET-1-IR levels were evaluated in the cubital venous blood before surgery and 7 days after monoen-doarterectomy. Blood samples were drawn from the patients after overnight bed rest. Blood was collected in tubes containing chilled potassium EDTA (1 mg/ml of blood) at 0°C. The blood was separated at 3000×gfor 10 min at 4°C and plasma was stored at −20°C until assay. A total of 1 ml of plasma was passed through C18 octyloecylsilane columns (Amersham, UK), previ-ously activated with 0.1% trifluoroacetic acid. Each eluate was then analysed by reverse-phase high-pressure liquid chromatography over 70 min, using a linear gradient of 15 – 75% acetonitrile/0.1% trifluoroacetic acid in water. Fractions were collected each minute and dried under vacuum by a centrifugal evaporator system (Gyrovap, Hove, London, UK) and then reconstituted in 1 ml buffer (50 mmol/l phosphate buffer, pH 7.4 containing 0.9% sodium chloride, 0.05% nitrosamine and 0.5% bovine serum albumin). The chromatographic separation of plasma eluates indicated a single peak of immunoreactive ET, perfectly corresponding to the elu-tion posielu-tion of human ET-1 standard and 125I E-1. ET-1-IR was then assayed on reconstituted fraction by a sensitive radioimmunoassay (Peninsula Laboratories, Belmont, CA). Human ET-1 (Peptide Institute, Osaka, Japan) was used as standard. Interassay and intra-assay variation was B10%. Mean recovery of our methods for human ET-1 standard was 85%. Cross reactivity of ET-1 antibody with ET-2 and ET-3 was B7%, accord-ing to the supplier. Circulataccord-ing levels of ET-1-IR col-lected before surgery in the two groups were compared to those obtained from 18 healthy volunteers (13 males and five females) with a mean age9S.D. of 27.892.7 years; range 20 – 50 years) after overnight bed rest.
2.3. Statistics
ra-G.Properzi et al./Atherosclerosis154 (2001) 137 – 140 139
Fig. 1. Pre-endoarterectomy circulating ET-1-IR levels in patients with atherosclerotic carotid stenosis (2) and in healthy subjects (). (P=0.000, Mann – Whitney U-test). ET-1-IR, endothelin-1-like im-munoreactivity.
No correlations were observed between pre-en-doarterectomy plasma ET-1-IR levels and age (r= −
0.42; P=0.06), or the degree of stenosis (r= −0.16; P=0.52, Spearman rank order correlation test).
4. Discussion
In the present study, pre-endoarterectomy serum lev-els of ET-1-IR, observed in patients with atheroscle-rotic extracranial carotid stenosis and no other haemodynamically significant atherosclerotic lesions, decreased significantly 7 days after surgical endoarterectomy.
So far there is no agreement on the effect of the revascularization procedures on the ET-1 circulating levels. An ET-1 increase has been reported immediately after performing percutaneous transluminal coronary angioplasty [20], after balloon vascular dilatation [21], in patients undergoing coronary bypass after induction of cardiopulmonary bypass [22] and during infrarenal aortic clamping and nifedipine infusion [23]. In con-trast, other authors reported a decrease in the levels of ET-1 in internal jugular vein after carotid cross-clamp-ing durcross-clamp-ing human carotid revascularization procedures [17], or in patients after coronary revascularization [24]. In the present study, ET-1 was measured 7 days after endoarterectomy, to avoid the potential effect of surgery stress on ET-1 release. The reduction in circu-lating levels of ET-1-IR following endoarterectomy could be due to the improvement of the haemodynamic consequences related to the stenosis [25 – 28]. No signifi-cant decrease in plasma levels of ET-1 was found in patients with bilateral carotid stenosis submitted to monolateral endoarterectomy. We speculate that the persistence of a haemodynamically significant carotid stenosis after monolateral endoarterectomy could pre-vent the decrease of ET-1 circulating levels.
Patients with monolateral stenosis showed a signifi-cant reduction of ET-1 levels after endoarterectomy, although they were still significantly higher than in healthy subjects. This could be due to the older age of patients. A different possibility is that the carotid steno-sis was associated with other, although not documented by non-invasive diagnostic procedures, atherosclerotic changes. Previous observations suggested that the func-tional balance between endothelium-mediated vasodila-tion and vasoconstricvasodila-tion is deranged in early stage atherosclerosis, leading to a reduced release of endothe-lium-derived relaxing and antimitogenic factor (EDRF) and to an increased release of the vasoconstrictor and mitogenic peptide endothelin-1 [28,29]. Since ET-1 may participate, as a mitogen or as a comitogen peptide in the atherogenic process [4 – 7], we speculate that en-doarterectomy, by removing the haemodynamic shear stress, a major contributing factor to the release of dioimmunoassay, age and degree of stenosis. Statistical
analysis was performed with Complete Statistical Sys-tem for personal computers (CSS/pc) release 2.1, ver-sion B 640, 1988 (Stat Soft, Chicago, IL).
3. Results
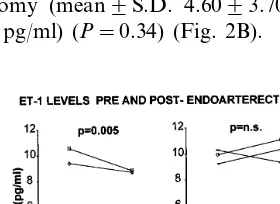
Atherosclerotic patients before surgery had circulat-ing cubital venous plasma levels of ET-1-IR signifi-cantly higher than healthy subjects (mean9S.D. 4.5093.35 pg/ml; range 1.28 – 10.6 vs. 0.6490.137 pg/
ml; range 0.36 – 1.02 pg/ml) (Fig. 1). Pre-endoarterec-tomy mean levels of ET-IR were not different in patients with monolateral and bilateral stenosis (mean9S.D. 4.3593.11 pg/m; range 1.28 – 10.66; and 4.7793.79 pg/ml; range 2.18 – 10.3 pg/ml, respectively). However, 7 days after monolateral endoarterectomy, a significant decrease of the mean venous ET-1-IR level was found in the group of patients with monolateral stenosis (mean9S.D. 3.0592.94 pg/ml; range 0.82 – 8.86 pg/ml) (P=0.005) (Fig. 2A). In contrast no signifi-cant difference, compared with pre-endoarterectomy ET-1 levels, was observed in the group of patients with bilateral stenosis, who underwent monolateral en-doarterectomy (mean9S.D. 4.6093.70 pg/ml; range 2.20 – 11.1 pg/ml) (P=0.34) (Fig. 2B).
G.Properzi et al./Atherosclerosis154 (2001) 137 – 140 140
endothelin-1 [24 – 26], may contribute to reducing the progression of atherosclerotic disease.
Acknowledgements
This study was supported by the Ministero dell’Uni-versita` e della Ricerca Scientifica, Italy.
References
[1] Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1998;332:11 – 5. [2] Wagner OF, Christ G, Woyta J, Vierhapper H, Parzer S,
Nowotny PJ, et al. Polar secretion of endothelin-1 cultured endothelial cells. J Biol Chem 1992;267:16066 – 8.
[3] Resink TJ, Scott-Burden T, Buhler FR. Endothelin stimulates phospholipase C in cultured vascular smooth muscle cells. Biochem Biophys Res Commun 1988;157:1360 – 8.
[4] Komuro I, Kurihara H, Sugijama T, Takaku F, Yazaki Y. Endothelin-1 stimulates c-fos and c-myc expression and prolifer-ation of vascular smooth muscle cells. FEBS Lett 1988;238:249 – 52.
[5] Hirata Y, Takagi Y, Fukuda Y, Marumo F. Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atheroscle-rosis 1989;78:225 – 8.
[6] Bobik A, Grooms A, Mitchell A, Grinpukel S. Growth factor activity of endothelin on vascular smooth muscle. Am J Physiol 1990;258:C408 – 15.
[7] Kahaleh MB. Endothelin, an endothelial-dependent vasocon-strictor in scleroderma: enhanced production and profibrotic action. Arthritis Rheum 1991;31:978 – 83.
[8] Kuriara H, Yoshizumi M, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, et al. Trasforming growth factor-b stimulates expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun 1989;159:1435 – 40.
[9] Hu R, Levin ER, Pedrama A, Frank HJI. Insulin stimulates production and secretion of endothelin from bovine endothelial cells. Diabetes 1993;42:351 – 8.
[10] Hattori Y, Kasay K, Makamura T, Emoto T, Shimoda S. Effects of glucose and insulin on immunoreactive endothelin-1 release from cultured porcine aortic endothelial cells. Metabolism 1991;40(2):165 – 9.
[11] Ferri C, Piccoli A, Pittoni V, Bellini C, Valesini G, Santucci A. Insulin stimulates endothelin-1 secretion from cultured human endothelial cells and modulates its circulating levels in vivo. J Clin Endocrinol Metab 1995;80(3):829 – 35.
[12] Boulanger CM, Tanner FC, Bea ML, Hahn AWA, Werner A, Luscher TF. Oxidized low-density lipoproteins induce mRNA expression and release of endothelin from the human and porcine endothelium. Circ Res 1992;70:1991 – 7.
[13] Dashwood MR, Jeremy JY, Mehta D, Izzat MB, Timm M, Bryan AJ, et al. Endothelin-1 and endothelin receptor in porcine saphenous vein-carotid artery grafts. Cardiovasc Pharmacol 1998;31(suppl 1):5328 – 30.
[14] Viswanathan M, De Oliveira AM, J’Ohren O, Savedra JM. Increased endothelin ET(A) receptors in rat carotid arteries after balloon injury. Pepides 1997;18:247 – 55.
[15] Lerman A, Edwards BS, Hallet JW, Heublein DM, Sondberg SM, Burnett JC Jr. Circulating and tissue endothelin immunore-activity in advanced atherosclerosis. New Engl J Med 1991;325:997 – 1001.
[16] Cacoub P, Koskas F, Timsit S, Maistre G, Gatel A, Piette JC, et al. Decrease in internal jugular endothelin levels after carotid cross-clamping during human carotid revascularization proce-dures. Ann Vasc Surg 1996;10(3):239 – 43.
[17] Minami S, Yamano Sawai N, Nomura K, Fukui R, et al. Relationship between carotid atherosclerosis and plasma en-dothelin-1 concentration in senile patients with hypertension Nippon Ronen Igakkai Zasshi 1997;34(12):1009 – 16.
[18] Kugler CF, Funk H, Vlajic P, Platt D-J. The relationship between endothelin-1, event-related P3000 potentials, and prog-nosis in cerebral arteriosclerosis. Am Geriatr Soc 1997;45(4):427 – 34.
[19] Crouse JR, Thompson CJ. An evaluation of methods for imag-ing and quantifyimag-ing coronary and carotid lumen stenosis and atherosclerosis. Circulation 1993;87(suppl II):17 – 33.
[20] Emori T, Hirata Y, Aizawa T, Ano K, Shichiri M, Marumo F. Plasma endothelin levels in patients with coronary artery disease undergoing percutaneous coronary angioplasty. Circulation 1998;80(suppl II):586.
[21] Ino T, Ohkubo M, Shimazaki S, Akimoto K, Nishimoto K, Iwahara M, et al. Plasma endothelin concentration: relation with vascular resistance and comparison before and after balloon dilatation procedures. Eur J Pediatr 1992;151:416 – 9.
[22] St Rammos K, Koullias GJ, Hatzibougias JD, Argyrakis NP, Panagopoulos PG. Plasma endothelin-1 levels in adult patients undergoing coronary revascularization. Cardiovasc Surg 1996;4(6):808 – 12.
[23] Antonucci F, Bertolissi M, Calo L. Plasma endothelin and renal function during infrarenal aortic cross clamping and nifedipine infusion. Lancet 1990;336:1449 (letter).
[24] Yoshizumi M, Kurihara H, Sugyama T, Takaku F, Yanagisawa M, Masaki T, et al. Hemodynamic shear stress stimulates en-dothelin-production by cultured endothelial cells. Biochem Bio-phys Res Commun 1989;161:859 – 64.
[25] Sharefkin JB, Diamond SL, Eskin SG, McIntire LV, Dieffen-bach CW. Fluid flow decreases pre-proendothelin mRNA levels and suppresses endothelin-1 peptide release in cultured human endothelial cells. J Vasc Surg 1991;14:1 – 9.
[26] Zarins CK, Giddens DP, Bharadvai BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 1993;53(4):502 – 14.
[27] Ferri C, De Marzio P, Desideri G, Baldoncini R, Bellini C, Morelli S, et al. Plasma endothelin-1 levels during transient acute myocardial ischaemia in men: effects of coronary revasculariza-tion. Eur J Clin Invest 1997;27(6):526 – 32.
[28] Lerman A, Burnett JC, Jr. Intact and altered endothelium in regulation of vasomotion. Circulation 1992;86(suppl III):2 – 19. [29] Lerman A, Webster MWI, Chesebro JH, Edwards WD, Wei
CM, Fuster V, et al. Circulating and tissue endothelin im-munoreactivity in hypercholesterolemic pigs. Circulation 1993;88:2923 – 28.