Differences in free and total tissue factor pathway inhibitor,
and tissue factor in peripheral artery disease
compared to healthy controls
Andrew D. Blann
a,*, Jean Amiral
b, Charles N. McCollum
c, Gregory Y.H. Lip
aaHaemostasis,Thrombosis and Vascular Biology Unit,Uni
6ersity,Department of Medicine,City Hospital,Birmingham B18 7QH, UK
bSerbio (Diagnostica Stago),125 rue Loius Roche,92635Genne
6illiers,Cedex, France
cDepartment of Surgery,Uni
6ersity Hospital of South Manchester,Nell Lane,Didsbury,Manchester M20 8LR, UK
Received 20 July 1999; received in revised form 8 October 1999; accepted 25 October 1999
Abstract
Tissue factor (TF) is one of the major initiators of coagulation and raised plasma levels have been found in various cardiovascular diseases. TF activity is, however, regulated by tissue factor pathway inhibitor (TFPI), and alteration in levels of TF and/or TFPI may thus relate to thrombogenesis and atherogenesis. To investigate possible abnormalities in TF and free TFPI (i.e. unbound to TF) and total TFPI among patients with peripheral artery disease (PAD), we studied 42 patients (mean age 57, 35 men) with objectively proven (by ABPI/Doppler) disease and 42 age- and sex- matched healthy controls. TF, free TFPI and total TFPI were measured in citrated plasma by ELISA. TF was higher in the patients with PAD compared to controls (2759122 pg/ml versus 158960,PB0.0001) but levels of total TFPI were lower in the patients (43910 ng/ml versus 50915,P=0.021). There was no significant difference in levels of free TFPI between patients and controls (7.291.5 ng/ml in controls, 7.591.6 among patients, P=0.39). Within the control patients, levels of free and total TFPI were significantly correlated (Spearman r=0.51,P=0.001) but in the patients with PAD this correlation was poor (r=0.21,P=0.178). We suggest that reduced levels of total TFPI and raised levels of TF may contribute to the process of atherogenesis and the increased risk of thrombosis among patients with cardiovascular disease. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Endothelium; Peripheral vascular disease; Tissue factor; Tissue factor pathway inhibitor; von Willebrand factor
www.elsevier.com/locate/atherosclerosis
1. Introduction
One of the most frequent and serious complications associated with cardiovascular disease is thromboem-bolism, possibly arising from increased platelet activa-tion and/or a loss of control of the regulaactiva-tion of coagulation and fibrinolysis. The latter may be the product of increased levels of procoagulants such as plasminogen activator inhibitor, von Willebrand factor (vWf) and tissue factor, or reduced levels of anticoagu-lants such as tissue factor pathway inhibitor (TFPI) and membrane bound thrombomodulin. Several of these molecules are products of the endothelium (i.e. may be markers of this tissue, but not necessarily specific
mark-ers), underlining the importance of this cell in haemostasis [1,2].
Tissue factor, a 44 kDa glycoprotein demonstrable in vitro on endothelial cells, smooth muscle cells and monocyte cell membranes, is both a receptor and essen-tial co-factor for factor VII and VIIa. This complex subsequently activates factor X to factor Xa, and as such is the primary cellular trigger of the coagulation cascade. A soluble form of tissue factor may also be found in the plasma, which can be induced by a variety of chemical stimuli including endotoxin, thrombin and inflammatory cytokines, and raised levels are present in pro-coagulant states such as ischaemic heart disease [1,3 – 5]. TFPI, with a mass of 35 – 42 kDa, also arising from the endothelium and monocytes, and possibly from megakaryocytes and smooth muscle cells, circu-lates as a lipoprotein-bound form (95%) and a free form (5%), and acts in vivo to constrain the procoagu-* Corresponding author. Tel./fax: +44-121-507-5076.
E-mail address:[email protected] (A.D. Blann).
lant tendency of tissue factor [6,7]. Increased levels of plasma TFPI have been reported in subjects with dia-betes or cancer and in ischaemic heart disease, and can follow the therapeutic use (i.e. injection) of heparin [4,6 – 9]. The presence of TFPI in atherosclerotic carotid plaque, and the associated reduced tissue factor activity in those plaques, underlines the possible role for these molecules in this disease [7].
Endothelial cell injury is believed to be a major factor in many of the clinical disturbances related to atherosclerosis, and can be monitored by measuring levels of plasma markers of endothelial damage or dysfunction, such as vWf [1,2,10]. Increased levels of this marker are an established feature of cardiovascular disease, and may be related to low-grade inflammation and/or cardiovascular risk factors such as smoking, diabetes, hypercholesterolaemia and hypertension, and indicate a poor prognosis [10,11].
As changes in levels of tissue factor and TFPI have been reported in coronary artery disease [4,5], we hy-pothesised that levels of the free and/or total forms of TFPI (both forms of which have only recently been possible to measure by ELISA) would also be altered in patients with peripheral artery disease. In addition, we measured vWf levels, to determine whether or not tissue factor and/or free or total TFPI were significantly related to endothelial cell injury, as has been suggested [12]. This is pertinent as the processes of thrombogene-sis and atherogenethrombogene-sis are intimately related.
2. Subjects and methods
2.1. Subjects
The patients with atherosclerosis had angiographi-cally and/or Doppler/ultrasound-proven atherosclerosis of the carotid (stenosis\75%) and/or iliac or femoral arteries (stenosis\75% and ankle-brachial pressure in-dexB0.85) and were recruited consecutively from a dedicated peripheral vascular disease clinic. No patients had surgery or an acute cardiovascular/cerebrovascular event in the previous three months. Although, the presence of peripheral artery disease was confirmed by rigorous standards, our patients were initially recruited by symptoms and clinical assessment alone. Thus, we cannot be certain that the patients were absolutely free of coronary heart disease or, indeed, unsymptomatic carotid disease when presenting with iliac/femoral dis-ease, or vice versa.
Normal healthy control subjects were recruited from those attending for endoscopy, hernia repair or for minor operations, and from healthy hospital staff. All controls were asymptomatic for any cardiovascular or cerebrovascular disease. Exclusion criteria for all sub-jects was diabetes, venous ulceration, serological
evi-dence of hepatitis B virus or HIV infection, malig-nancy, abdominal aortic aneurysm, acute or chronic liver and kidney disease, connective tissue disease, or treatment with warfarin, antibiotics, vasopressin, im-munosuppressive or cytotoxic drugs. No subject was on a lipid lowering therapy or taking uni- or multivitamin tablets or cod liver oil, but seven patients were taking aspirin and five were taking anti-hypertensive treat-ments. Systolic and diastolic blood pressure was recorded in each subject following a minimum of 5 min rest. Subjects were asked if they were regularly smoking cigarettes. The approval of the Ethics Committee of South Manchester Health Authority and informed con-sent from each subject was obtained in accordance with the Declaration of Helsinki [13].
2.2. Methods
Fasting venous blood was obtained following non-traumatic venepuncture: one aliquot was allowed to clot at room temperature, another was taken for ery-throcyte sedimentation rate, and two were taken into 0.11 M sodium citrate. In the pathology laboratory, which participates in the United Kingdom External Quality Assurance Scheme and is accredited by the Association of Clinical Pathologists, serum was analysed by standard enzymatic or precipitation tech-niques for total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol and glucose. If the glu-cose level was \9 mmol/l, or the erythrocyte sedimen-tation rate was \20 mm/h, the subject was excluded. Low-density lipoprotein (LDL) cholesterol was esti-mated (where possible) by the Friedewald formula. Fibrinogen in citrated plasma was estimated according to a functional assay (Clauss) on a KC10 Coagulometer with thrombin from Baxter Diagnostics (Thetford, UK).
kits [4] as the latter measures TFPI complexed to FXa (and most likely also in complexes with tissue factor and FVIIa) as well as free and TFPI bound to phos-pholipid. The Stago kit does not measure TFPI in FXa complexes but measures free TFPI and TFPI bound to phospholipids. Other workers (e.g. [14]) have used a functional assay for TFPI.
2.3. Data analysis and statistics
Our power calculation was based on previous work [4], that compared 55 patients with 48 controls, and our hypothesis that tissue factor would be raised by approx-imately one half of a standard deviation, required 40 patients and 40 controls for PB0.05 with a power of 80% [15]. Categorical data (sex, smoking) was analysed by the x2-test. Following application of the Shapiro – Wilk W test to determine distribution, non-categorical data distributed normally was analysed by t-test, and data distributed non-normally by the Mann – Whitney U-test. Correlations were sought with Spearman’s rank method. The data are presented as mean and standard deviation, median and full range, or as percent (%) incidence. All statistical analyses were performed on a
Minitab release 12 package on Windows 98. A proba-bility of 0.05 was considered as statistically significant.
3. Results
3.1. Cross-sectional data
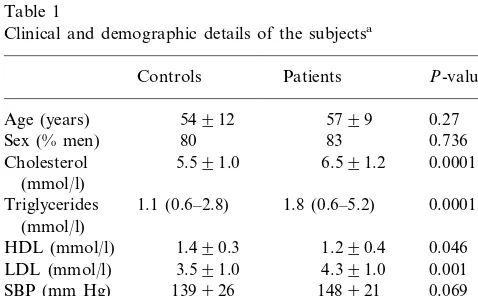
Clinical, demographic and risk factor profiles of the two groups are presented in Table 1. When compared to the controls, the patients with peripheral artery disease had higher levels of lipids and fibrinogen, and more patients than controls were smokers. vWf, tissue factor and free and total TFPI levels are shown in Table 2. The cases had higher levels of vWf and tissue factor, but lower levels of total TFPI than the controls. There was no difference in levels of free TFPI.
Smoking did not significantly influence levels of tis-sue factor, free TFPI or total TFPI. In the controls, tissue factor was 148940 pg/ml in the nine smokers and was 163965 pg/ml in the 32 non-smokers (P= 0.39). In the patients, levels were 2599103 pg/ml in the 18 smokers and 2879136 pg/ml in the 25 non-smokers (P=0.45). Similarly, free TFPI was 73916 ng/ml in smokers versus 72915 ng/ml (P=0.087) in the non-smoking controls and was 87921 ng/ml versus 73911 ng/ml (P=0.36) in the patients. Total TFPI was 509 11 ng/ml versus 49915 ng/ml in controls (P=0.94) and was 43911 ng/ml versus 42911 ng/ml (P=0.65) in the patients.
3.2. Correlations between indices
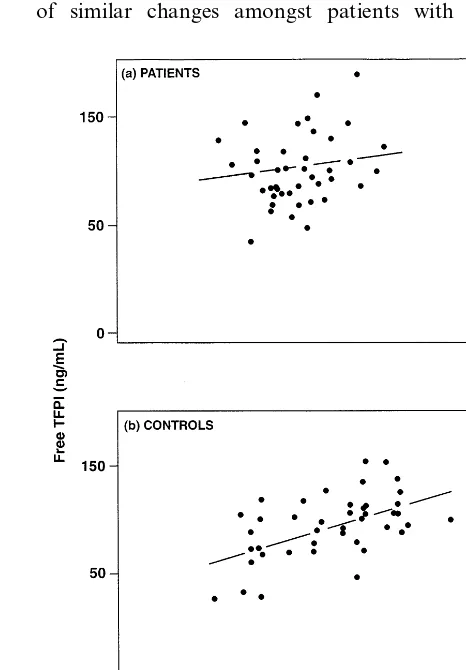
Within the patients with peripheral artery disease, there were highly significant correlations between vWf and tissue factor (r=0.451, P=0.002, Fig. 1a). How-ever, amongst controls, the same correlation was not significant (r= −0.136, P=0.389, Fig. 1b). Con-versely, the correlation between free and total TFPI in the patients was not significant (r=0.209, P=0.178, Fig. 2a), but in the controls this correlation was signifi-cant (r=0.507, P=0.001, Fig. 2b). At best, correla-tions between vWf and both free TFPI (controls r=0.181, P=0.251; patients r=0.383,P=0.011) and total TFPI (controls r=0.334, P=0.031; patients r= 0.122, P=0.476) were weak.
There was no correlation between levels of total TFPI and tissue factor in the patients (r= −0.054, P=0.729) or the controls (r= −0.198, P=0.209) but in the combined groups the correlation was weakly significant (r= −0.225, P=0.039). There were no sig-nificant correlations between any of the indices and the degree of carotid stenosis in the 18 subjects with disease in this locus, or with the ankle-brachial pressure index in those 24 patients with atherosclerosis of the iliac/ femoral disease (data not shown).
Table 1
Clinical and demographic details of the subjectsa
Patients
aData are mean and standard deviation, median and range or
percentage incidence. Univariate P-values were derived from the t-test, the Mann–Whitney U-test, or thex2-test.
Table 2
von Willebrand factor, tissue factor, and free and total TFPI in the subjectsa
aData are mean and standard deviation. UnivariateP-values were
Fig. 1. (a) Correlation between levels of von Willebrand factor and tissue factor in the patients (r=0.451, P=0.002) Correlation be-tween levels of von Willebrand factor and tissue factor in the controls (r= −0.136,P=0.389)
rides, HDL and LDL) and group (control/patient) found that both group and triglycerides were indepen-dent influences. With free TFPI as depenindepen-dent variable, no influences were detected, whilst with total TFPI as dependent variable, only group was an influence.
Our subjects were a small group of patients with diverse symptomatic and objectively-proven atheroscle-rotic disease. As we have neither hypothesised nor requited according to a defined power calculation re-garding sub-groups (i.e. those with primarily carotid disease versus those with primarily iliac/femoral dis-ease), further analyses are inappropriate.
4. Discussion
In this small, preliminary study, we found higher levels of tissue factor in relatively young patients with peripheral artery disease compared to controls. There-fore our findings may not necessarily be extrapolated to all subjects with atherosclerosis, especially the elderly. Increased tissue factor levels are consistent with reports of similar changes amongst patients with coronary
Fig. 2. (a) Correlation between levels of free TFPI and total TFPI in the patients (r=0.209,P=0.178); (b) Correlation between levels of free TFPI and total TFPI in the controls (r=0.507,P=0.001). 3.3. Correlations between indices and lipids
triglyce-artery disease [4,5]. In the present study, however, levels of free TFPI did not differ between the groups whilst levels of total TFPI were lower in the patients relative to the controls, although this difference was weak, especially when allowing for multiple comparisons. We believe it unlikely that the changes we have reported are due to different rates of degradation or excretion, espe-cially as renal impairment was an exclusion criterion. As TFPI probably contributes significantly to the anti-coagulant properties of the endothelium, low levels may be particularly important for the outcome of vascular injury [6]. The high vWf levels were expected and are common in cardiovascular disease [10].
Our finding of lower levels of total TFPI in patients with peripheral artery disease compared with controls contrasts with a previous reports of increased levels of TFPI activity in coronary artery disease [4,14]. Nota-bly, we measured free and total TFPI using a new ELISA method from a different manufacturer, which could perhaps explain these differences: the technology to differentiate free from total TFPI has only recently been developed [19]. These differences could be ac-counted for by the fact that the method used in this study quantifies total bioavailable TFPI whilst the pre-vious study [4] used a method that quantified both bioavailable and FXa complexed (i.e. ‘used’) TFPI. An alternative possibility, however, is that the pathophysi-ology of TFPI and tissue factor in peripheral artery disease may differ from that seen among patients with coronary artery disease. High TFPI levels have also been reported in patients with malignancy and septi-caemia [6,9], although low TFPI levels are present in some patients with liver disease, disseminated intravas-cular coagulation, and in some patients with arterial and venous thrombosis [16,20 – 22].
The increase in tissue factor and reduced levels of total TFPI may be related, in that the lower TFPI may perhaps permit the measurable increase in tissue factor levels. Caplice and colleagues has made similar sugges-tions from observasugges-tions of atherosclerotic plaque, i.e. that increased levels of TFPI attenuates tissue factor activity [7]. Both these findings may therefore explain, at least in part, the increased tendency to thrombosis in patients with atherosclerosis. The processes of throm-bogenesis and atherogenesis are also closely integrated. It is uncertain however, whether or not the low levels of TFPI may be the primary pathophysiological process, with a consequent increase (and lack of homeostatic control) of tissue factor. Conversely, the increased lev-els of tissue factor may be the primary abnormality, which is inadequately regulated by normal or lower levels of TFPI.
These perspectives were examined in the present study by exploring the relationship of tissue factor and its inhibitor (TFPI) with vWf, a marker of endothelial cell injury [1,10,23]. In the (presumably healthy)
con-trols, there was no significant correlation between tissue factor and vWf, but there was a significant correlation between these two indices amongst the patients with frank atherosclerosis. Therefore raised tissue factor lev-els in peripheral artery disease may, like raised vWf, be the result of damage to the endothelium by factor(s) unknown; this hypothesis has been postulated using an alternative marker of endothelial cell damage, soluble thrombomodulin [12,24]. Regrettably our small study was not powered to explore if smoking and hyperlipi-daemia represented just such injurious factors. A caveat to the concept of tissue factor as an endothelial marker is the demonstration in vitro that it may also be ob-tained from monocytes and smooth muscle cells [25,26]. However, whether or not these contribute to plasma levels in vivo is unclear. Despite this, tissue factor may be a better discriminator of atherosclerotic vascular disease as levels were marginally higher in this disease compared to levels of vWf. The relationship between the endothelium and free or total TFPI is less clear as correlations with vWf were weak. This argues against the hypothesis that free and/or total TFPI were influ-enced by the same factors which influence vWf, and suggests that free or total TFPI may be poor markers of endothelial cell damage. However, one reason for this discrepancy may be that TFPI is synthesised by different cells (endothelial and other) than is vWf [6,27]. Free and total TFPI were correlated strongly amongst controls, but this was not significant amongst the patients with peripheral artery disease, with no difference in levels of free TFPI between patients and controls. The reasons for this are unclear but may relate to the relationships between TFPI and lipids [6,16,17,20,26]. Only in the normolipaemic controls did levels of total TFPI correlate with LDL, and inversely with HDL cholesterol (thereby confirming Sandset’s data [17,18]). Perhaps the high lipid levels in the pa-tients with peripheral artery disease were sequestering a greater proportion of (bound) TFPI, permitting less TFPI to be available to inhibit tissue factor. Although, both free cholesterol and oxidised LDL induce an increase in tissue factor activity (but not antigen or TFPI) from human monocyte-derived macrophages in vitro, the clinical relevance of this is unclear [28]. Thus, hyperlipidaemia may be an underlying reason for the loss of haemostasis, especially as lipoproteins may actu-ally provide an alternative stimulus and template for the initiation of the coagulation cascade [29].
antibod-ies to tissue factor and recombinant TFPI have been described [26]. As hyperlipidaemia probably contributes to endothelial cell damage [30 – 32] and increasing atherogenesis, tissue factor and TFPI changes may provide a further link and possible mechanisms to explain the relationship between vascular risk factors and atherothrombotic disease.
Acknowledgements
We thank Barry Woodhams for constructive comments.
References
[1] Pearson JD. The control of production and release of haemo-static factors in the endothelial cell. Balliere’s Clin Haematol 1993;6:629 – 51.
[2] Ross R. Atherosclerosis — an inflammatory disease. New Engl J Med 1999;340:115 – 26.
[3] Nemerson Y. Tissue factor: then and now. Thromb Haemost 1995;7:180 – 4.
[4] Falciani M, Gori AM, Fedi S, Chiarugi L, et al. Elevated tissue factor and tissue factor pathway inhibitor circulating levels in ischaemic heart disease patients. Thromb Haemost 1998;79:495 – 9.
[5] Soejima H, Ogawa H, Yasue H, Suefuji H, Kaikita K, Tsuji I, Kumeda K, Aoyama N. Effects of enalapril on tissue factor in patients with uncomplicated acute myocardial infarction. Am J Cardiol 1996;78:336 – 40.
[6] Lindahl AK. Tissue factor pathway inhibitor: from unknown coagulation inhibitor to major antithrombotic principle. Cardio-vasc Res 1997;33:286 – 91.
[7] Caplice NM, Mueske CS, Kleppe LS, Simari RD. Presence of tissue factor pathway inhibitor in human atherosclerotic plaques is associated with reduced tissue factor activity. Circulation 1998;98:1051 – 7.
[8] Leurs PB, van Oerle R, Hamulyak K, Wolffenbuttel BHR. Tissue factor pathway inhibitor activity in patients with IDDM. Diabetes 1995;44:80 – 4.
[9] Iversen N, Lindahl AK, Abildgaard U. Elevated TFPI in malig-nant disease: relation to cancer type and hypercoagulation. Br J Haematol 1998;102:889 – 95.
[10] Blann AD, Lip GYH. The endothelium in cardiovascular dis-ease: functions, assessment and implications. Blood Coagul Fibrinolysis 1998;9:297 – 306.
[11] Thompson SG, Kienast J, Pyke SDM, Haverkate F, van de Loo JCW, et al, for the ECAT group. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris, New Engl J Med 1995;332:635 – 641.
[12] Koyama T, Ohdama S, Aoki N. Plasma tissue factor reflects endothelial cell injury rather than upregulation of tissue factor expression. Thromb Haemost 1997;78:973 Letter.
[13] World Medical Association Inc. World Medical Association Declaration of Helsinki. Cardiovasc Res 1997:35;2 – 3.
[14] Sandset PM, Sirnes PA, Abilgaard U. Factor VII and extrinsic pathway inhibitor in acute coronary disease. Br J Haematol 1989;72:391 – 6.
[15] Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall, 1991.
[16] Novotny WF, Girard TJ, Miletich JP, Broze GJ. Purification and characterisation of the lipoprotein-associated coagulation inhibitor from human plasma. J Biol Chem 1989;264:18832 – 7. [17] Hansen JB, Huseby NE, Sandset PM, Svensson B, Lyngmo V,
Nordoy A. Tissue factor pathway inhibitor and lipoproteins. Evidence for association with and regulation by LDL inhuman plasma. Arterioscler Thromb 1994;14:223 – 9.
[18] Sandset PM, Lund H, Norseth J, Abildgaard U, Ose L. Treat-ment with hydroxy methylglutaryl coenzyme A reductase in-hibitors in hypercholesterolaemia induces changes in thecomponents of the extrinsic coagulation system. Arterioscler Thromb 1991;11:138 – 45.
[19] Bridey F, Lacombe C, Sustendal L, Moatti D, Combe F, Mam-mes O, de Prost D. Development of a method to separate lipoprotein-bound and lipoprotein depleted tissue factor path-way inhibitor. Measurement of free tissue factor pathpath-way in-hibitor activity. Blood Coagul Fibrinolysis 1998;9:637 – 43. [20] Sandset PM, Bendz B. Tissue factor pathway inhibitor: clinical
deficiency states. Thromb Haemost 1997;78:467 – 70.
[21] Oksuzoglu G, Haznedaroglu IC, Buyukasik Y, Simsek H, Soylu AR, Ozdemir O, Ozcebe O, Dundar S, Kirazil S. Tissue factor pathway inhibitor (TFPI) in chronic liver disease with and without portal vein thrombosis. Thromb Haemost 1997;78:411. [22] Ariens RAS, Alberio G, Moia M, Mannucci PM. Low levels of heparin-releasable tissue factor pathway inhibitor in young pa-tients with thrombosis. Thromb Haemost 1999;81:203 – 7. [23] Pearson JD. Markers of endothelial perturbation and damage.
Br J Rheumatol 1993;32:651 – 2.
[24] Boffa MC. Considering cellular thrombomodulin distribution and its modulating factors can facilitate the use of plasma thrombomodulin as a reliable endothelial marker. Haemostasis 1996;26(Suppl. 4):233 – 43.
[25] Drake TA, Ruf W, Morrissey JH, Edgington TS. Functional tissue factor activity is entirely cell surface expressed on lipo-polysaccharide-stimulated human blood monocytes and a consti-tutively tissue factor-producing cell line. J Cell Biol 1989;109:389 – 95.
[26] Penn MS, Patel CV, Cui MZ, DiCorleto PE, Chisholm GM. LDL increases inactive tissue factor on vascular smooth muscle cell surfaces. Hydrogen peroxide activates latent cell surface tissue factor. Circulation 1999;99:1753 – 9.
[27] Bajaj MS, Bajaj SP. Tissue factor pathway inhibitor: potential therapeutic applications. Thromb Haemost 1997;78:471 – 5. [28] Petit L, Lesnik P, Dachet C, Moreau M, Chapman MJ. Tissue
factor pathway inhibitor is expressed by human monocyte-derived macrophages: relationship to tissue factor induction by cholesterol and oxidised LDL. Arterioscler Thromb Vasc Biol 1999;19:309 – 15.
[29] Mitropoulos KA. Lipid-thrombosis interface. Br Med Bull 1994;50:813 – 32.
[30] Henry PD. Hyperlipidaemic endothelial injury and angiogenesis. Basic Res Cardiol 1994;89(Suppl. 1):107 – 14.
[31] Blann AD, Miller JP, McColum CN. Von Willebrand factor and soluble E-selectin in the prediction of cardiovascular disease progression in hyperlipidaemia. Atherosclerosis 1997;132:151 – 6. [32] Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol lowering therapy on the coronary endothelium in patients with coronary artery disease. New Engl J Med 1995;332:481 – 7.