Intima-media thickness after pravastatin stabilizes also in patients
with moderate to no reduction in LDL-cholesterol levels: the
carotid atherosclerosis Italian ultrasound study
Damiano Baldassarre
a, Fabrizio Veglia
b, Cecilia Gobbi
b, Giuseppe Gallus
c,
Alessandro Ventura
d, Gaetano Crepaldi
e, Maurizio Fisicaro
f, Silvana Rimondi
g,
Giorgio Ricci
h, Mario Mancini
i, M. Gene Bond
j, Stefano Collatina
k,
Cesare R. Sirtori
a,*
aE. Grossi Paoletti Center,Institute of Pharmacological Sciences,Uni6ersity of Milan,Via Balzaretti 9,20133 Milan, Italy bUnit of Biostatistics,Institute H. San Raffaele,Milan, Italy
cInstitute of Medical Statistics and Biometrics,Uni
6ersity of Milan,Milan, Italy
dII Institute of Clinical Medicine,Uni
6ersity of Perugia,Perugia, Italy
eInstitute of Internal Medicine,Uni
6ersity of Padua,Padua, Italy
fInstitute of Clinical Medicine,Uni
6ersity of Trieste,Trieste, Italy
gInstitute of Internal Medicine,Uni
6ersity of Bologna,Bologna, Italy
hInstitute of Systematic Medical Therapy,Uni6ersity of Rome‘La Sapienza’,Rome, Italy iInstitute of Internal Medicine,Uni6ersity of Naples,Naples, Italy
jDi6ision of Vascular Ultrasound Research,Wake Forest Uni6ersity School of Medicine,Winston-Salem,NC, USA kBristol-Myers Squibb S.P.A., Rome, Italy
Received 4 May 1999; received in revised form 24 September 1999; accepted 13 October 1999
Abstract
The Carotid Atherosclerosis Italian Ultrasound study (CAIUS), a multicenter, double-blind clinical trial, performed in 305 asymptomatic, moderately hypercholesterolemic patients, clearly demonstrated beneficial effects of pravastatin on the carotid intima-media thickness (IMT) progression. The database of the CAIUS study was examined in order to investigate the presence of a relationship, if any, between the activity of pravastatin on IMT progression rate and its hypocholesterolemic effect. Quantitative B-mode ultrasound imaging was used to quantify the individual mean maximum IMT progression rate in 3 years. In the overall group of patients (placebo and pravastatin) covariance analysis showed that while the variable ‘treatment’ (0=placebo, 1=pravastatin) was significantly related to the reduction of IMT progression (F=6.6, P=0.01), the IMT progression did not correlate with the extent of LDL-C lowering (F=0.00, P=0.98). To further investigate this issue, the pravastatin treated group was stratified into quartiles of LDL-C reduction. In contrast to what was observed in the placebo group, in which a positive mean IMT progression rate was observed, independent of the extent of LDL-C reduction, no IMT progression www.elsevier.com/locate/atherosclerosis
For the CAIUS Research group. The CAIUS research group: Sirtori CR, Baldassarre D, Pazzucconi F, Poli A, Werba JP; (E. Grossi Paoletti Center, University of Milan, Italy). Crepaldi G, Calabro` A, Abruzzese E; (Institute of Internal Medicine, University of Padua, Italy). Feruglio FS, Da Col P, Fisicaro M, Tonizzo M; (Institute of Clinical Medicine, University of Trieste, Italy). Descovich G†, Rimondi S, Finazzo L, Mussoni C; (Institute of Internal Medicine, University of Bologna, Italy). Ventura A, Susta A, Brunetti G, Vedovelli MG; (II Institute of Clinical Medicine, University of Perugia, Italy). Ricci G, Bucci A, Scarno AG, Giovannelli C; (Institute of Systematic Medical Therapy, University of Rome ‘La Sapienza’, Italy). Mancini M, Rubba P, Faccenda F, Jannuzzi A; (Institute of Internal Medicine, University of Naples, Italy). Bond MG, Mercuri M, Safrit AW, Phillips R, Holley B, Pignatelli MR, Tang R, Wilmoth SK; (Wake Forest University School of Medicine, Division of Vascular Ultrasound Research. Winston-Salem, NC, USA). Veglia F, Gobbi C; (Unit of Biostatistics, Institute H. San Raffaele, Milan, Italy). Gallus G; (Institute of Medical Statistics and Biometrics, University of Milan, Italy). Collatina S, Ricci M; (Medical Department, Bristol-Myers Squibb, Rome, Italy).
* Corresponding author. Tel.: +39-2-20488311; fax: +39-2-29404961.
E-mail address:[email protected] (C.R. Sirtori)
was observed in any subgroup treated with pravastatin. No significant difference was found among quartiles and no trend could be identified. In conclusion, the effect of pravastatin treatment on carotid IMT progression rate is beneficial; however the CAIUS study demonstrated that lowering LDL-C by itself, does not explain the variability of beneficial changes in IMT. © 2151 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Pravastatin; Carotid atherosclerosis; Ultrasound; IMT progression; Long-term therapy
1. Introduction
Cholesterol-lowering with, e.g. statins, has resulted in an impressive reduction of cardiovascular events in a large number of primary and secondary prevention studies [1 – 3]. The reduction in events has not been, however, constantly proportional to the plasma lipid changes induced by the studied drugs [4]. Some au-thors suggest that the relative reductions in the final endpoints, i.e. cardiovascular death or myocardial in-farction, after drug treatment are similar at widely different cholesterol lowering responses [3,5]; whereas other maintain that the degree of cholesterol reduction is proportional to the positive effect on the cardiocular endpoints [6,7]. A poor correlation between vas-cular changes and baseline or on-trial lipid levels has also emerged from angiographic studies evaluating the progression of lesions at the coronary artery level [8] or, more recently, at the carotid wall level, as exam-ined by ultrasound methodologies [9]. In addition, the inhibited progression/enhanced regression observed in coronary artery studies appears to be more closely related to the starting LDL cholesterolemia, rather than to the cholesterol lowering effect induced by the drug [8,10]. Very recent animal investigations have, on the other hand, suggested that pravastatin may exert, in primates kept at equal cholesterol levels, additional, cholesterol independent benefits, i.e. improving coro-nary distensibility and reducing the extension of arte-rial macrophage-rich plaques, more prone to rupture [11].
An opportunity to evaluate a statin’s vascular effect, independent of changes in cholesterolemia, is provided by a post-hoc analysis of the data from the CAIUS study. The CAIUS study was designed to test the efficacy of the HMG-CoA reductase inhibitor pravas-tatin on carotid atherosclerosis progression. In line with other similar trials [12 – 14], it showed a fa-vourable effect of treatment on the carotid intima-me-dia thickness (IMT) progression [15]. In the present study the database of the CAIUS study was examined, in order to investigate the presence of a relationship, if any, between the beneficial activity of pravastatin on IMT progression rate and its hypocholesterolemic ef-fect.
2. Methods
2.1. Study design
CAIUS was a multicenter, parallel group, ran-domised, placebo-controlled, double blind clinical trial on 305 asymptomatic, moderately hypercholes-terolemic patients (LDL cholesterol levels between 3.88 and 6.47 mmol/l and triglycerides level B2.82 mmol/l) of both sexes (50% males, 50% females), 45 – 65 years, with at least one carotid artery lesion de-tected by quantitative B-mode ultrasound imaging. The rationale, study design and main results have been previously reported [15,16]. The primary outcome of the study was the difference between treatment groups, in the slope of progression of carotid artery mean maximum intima-media thickness (MM-IMT), a global summary measure widely used in epidemiologi-cal studies and intervention cliniepidemiologi-cal trials. The MM-IMT is computed as the mean of the single maximum IMTs detected in up to 12 standard carotid artery walls including near and far walls of the distal com-mon carotid, the bifurcation, and the proximal inter-nal carotid artery.
Patients were screened in seven Lipid Clinics of Academic Medical Centers (Universities of Milan, Padua, Trieste, Bologna, Perugia, Rome and Naples). At each visit, fasting lipid profiles and other labora-tory values were determined using standard procedures approved by the European Atherosclerosis Society [17]. Quality control for plasma total cholesterol (TC), triglycerides (TG) and HDL-cholesterol (HDL-C) measurements were established by using calibrated sera for cholesterol and triglyceride measurements. LDL-cholesterol (LDL-C) levels were determined us-ing Friedewald’s formula [18].
an independent Ultrasound Center (NGB) reviewed these recorded exams, where final ultrasonographic eligi-bility was established. Individuals meeting all the clini-cal, laboratory and ultrasonographic criteria, and who signed an approved informed consent, were instructed and counselled to adhere to a low-fat diet, meeting the recommendations of the European Atherosclerosis Soci-ety [17]. After a 6-week run-in period and an additional evaluation of lipid profiles, patients were randomised to either pravastatin (40-mg qd) or a corresponding placebo. Patient randomisation to one of the two treat-ment arms was performed at an independent Statistical Analysis Center. Before starting treatment, in order to establish baseline values, patients underwent two com-plete quantitative B-mode ultrasound imaging examina-tions, and a comprehensive set of laboratory tests and clinical evaluations.
After the baseline visits, all patients were seen every 3 months at their respective referring Clinical Centers. Drug safety was assessed at every visit and included ALT, AST, gGT, and CPK. Treatment compliance, determined by pill counts, was considered adequate when the pill intake exceeded 80% of the total.
The primary goal of the present post-hoc analysis was to investigate whether the slope of progression of MM-IMT correlated with the extent of LDL-C lowering. Moreover, in order to investigate whether this relation-ship could be pointed out at least as a trend, statistical analysis was also performed after stratification of pa-tients treated with the active drug into quartiles of LDL-C response, all being compared to patients in the placebo group. The slope of progression of MM-IMT measurements of common carotid (CC-IMT), bifurca-tion (Bif-IMT) and common carotid plus bifurcabifurca-tion (CC+Bif-IMT) of the same subgroups were secondary goals.
2.2. B-mode ultrasound imaging protocol
Quantitative ultrasound examinations were per-formed every 6 months using a previously described ultrasonographic protocol [15,16]. The ultrasound exam was performed using an 8 MHz annular array ultra-sound imaging system (2000 II s.a., Bioultra-sound, Indiana-polis, IN). The protocol requires video recording of a complete circumferential examination of both carotid arteries in order to image near and far walls of the distal 1.0 cm of the common, the bifurcation, and the proximal 1.0 cm of the internal carotid arteries. The video-recorded examinations are interpreted centrally by read-ers masked to patients’ information. To avoid mixed operators bias, the patients’ initial evaluation and subse-quent follow-up visits were assigned to a specific sonog-rapher/reader pair. To define intra- or inter-operator reproducibility, the same or a different operator on a 50/50 basis repeated all baseline, 18 months and final
examinations. This procedure increased the precision of the IMT slope estimates, and established the intra- and inter-operator reproducibility. Data on cross sectional and longitudinal quality control have been previously reported [15] and can also be provided, on request, in a specific separate technical report.
2.3. Statistical analysis
The primary goal of the present study required a statistical analysis procedure to determine the efficacy of pravastatin when compared to placebo, in changing the carotid MM-IMT progression slope over a three years period. The individual extent of LDL-C lowering was calculated as differences between baseline and the mean of all on trial measurements obtained between the 6th and 36th months of the trial. Each patient’s carotid MM-IMT progression slope was estimated by weighted linear regression of up to ten serial measurements versus time. Weights were proportional to the number of visualised arterial walls used to compute the carotid MM-IMTs. The analytical model assumes a linear pro-gression of carotid IMT. The mean carotid MM-IMT progressions of the subgroups were compared by analysis of covariance (ANCOVA). Progression rates were weighted proportionally to the inverse of the estimated variance of progression slope. Baseline carotid MM-IMTs and clinical centers were used as covariates. All statistical tests were two-sided with a significance level for the outcome measurement set at PB0.05.
3. Results
3.1. Distribution of lipid responses in treated patients
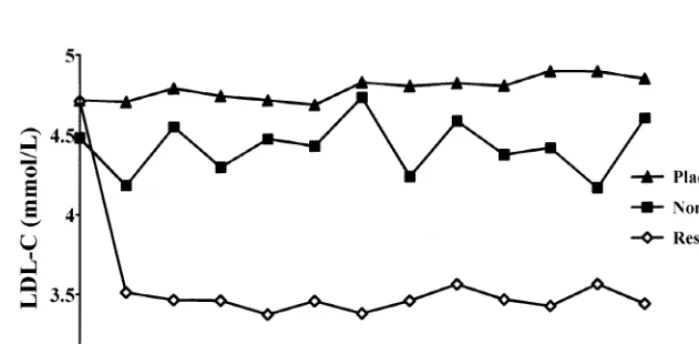
Data relative to LDL-C changes in the patients who reached the final third year examination are shown in Fig. 1.
In the placebo group, 133 patients reached the end of the follow up period and among these the individual LDL-C changes, calculated as differences between base-line and the mean of all on trial measurements, ranged from −24.9 to +32.4% with a mean value of +1.8%. Of the 151 patients treated with pravastatin, 142 had the final third year visit, but six were excluded because of inadequate compliance, as assessed by pill counting. Of the 136 patients included in the analysis, 116 (76.8%) could be effectively considered as ‘responders’ (LDL-C reduction greater than 10%) whereas 20 (13.2%) showed an unsatisfactory response to therapy, with an LDL-C reduction lower than 10%. The individual response in terms of LDL-C reduction, ranged from −47.3 to 2.1% with a mean value of 22.5%.
allowing the study of a possible relationship between LDL-C changes and progression of carotid athero-sclerosis.
3.2. Co6ariance analysis: IMT progression 6ersus
treatment and 6s LDL-C response
In order to investigate whether the effect of pravas-tatin treatment on the reduction of IMT progression correlated with the hypocholesterolemic effect, a covari-ance analysis was performed by pooling data of the placebo and pravastatin groups. This analysis showed that, while the reduction of IMT progression rate corre-lated with treatment (F=6.6, P=0.01), it did not correlate with the extent of LDL-C lowering (F=0.00, P=0.98). Regression analysis performed in the pravas-tatin treated group only, confirmed the lack of
correla-tion between percentage of LDL-C reduccorrela-tion and IMT progression rate (r=0.06, slope= −0.0001 mm-year/ DLDL-C, P=0.54) (Fig. 2).
3.3. IMT progression and quartiles of LDL cholesterol response
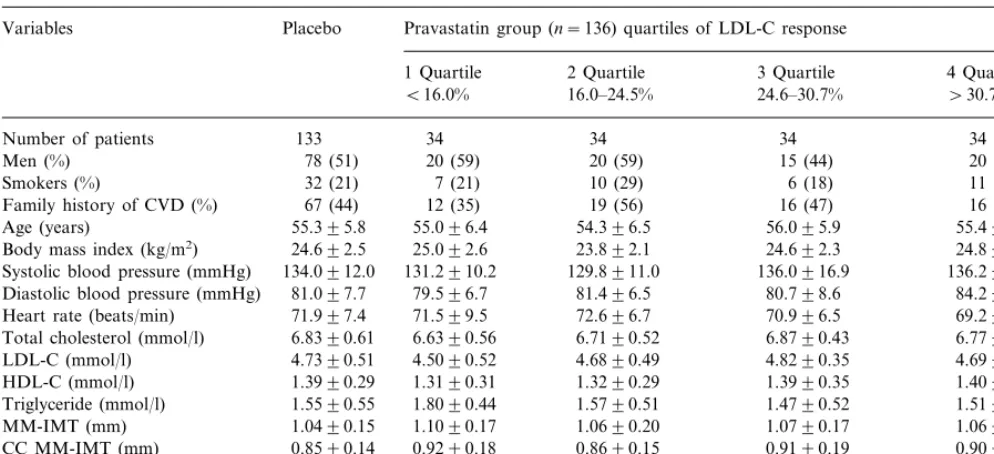
To further investigate this issue, patients under active treatment were divided into quartiles according to their individual LDL-C response. The baseline characteristics of these subgroups as well as of placebo are shown in Table 1. No differences in any demographic or clinical characteristics or conventional risk factors were found between pravastatin subgroups and placebo as well as among quartiles. The subgroups were also similar in terms of baseline ultrasonographic measurements. In Table 2 the effects of treatment on serum lipids are
Fig. 1. LDL-C by follow-up visits in the groups of patients studied. Data are unadjusted.
Table 1
Baseline characteristics in the placebo group and in pravastatin group stratified by quartiles of LDL-C responsea
Pravastatin group (n=136) quartiles of LDL-C response
Variables Placebo
1 Quartile 2 Quartile 3 Quartile 4 Quartile
\30.7%
B16.0% 16.0–24.5% 24.6–30.7%
Number of patients 133 34 34 34 34
20 (59) 20 (59)
78 (51) 15 (44)
Men (%) 20 (59)
7 (21) 10 (29)
Smokers (%) 32 (21) 6 (18) 11 (32)
12 (35) 19 (56)
67 (44) 16 (47)
Family history of CVD (%) 16 (47)
55.096.4
Age (years) 55.395.8 54.396.5 56.095.9 55.495.6
25.092.6 23.892.1
24.692.5 24.692.3
Body mass index (kg/m2) 24.893.0
134.0912.0
Systolic blood pressure (mmHg) 131.2910.2 129.8911.0 136.0916.9 136.2912.6 81.097.7
Diastolic blood pressure (mmHg) 79.596.7 81.496.5 80.798.6 84.297.5 71.599.5 72.696.7
71.997.4 70.996.5
Heart rate (beats/min) 69.298.0
6.8390.61
Total cholesterol (mmol/l) 6.6390.56 6.7190.52 6.8790.43 6.7790.66 4.5090.52 4.6890.49
4.7390.51 4.8290.35
LDL-C (mmol/l) 4.6990.54
1.3190.31 1.3290.29
HDL-C (mmol/l) 1.3990.29 1.3990.35 1.4090.26
1.8090.44 1.5790.51
1.5590.55 1.4790.52
Triglyceride (mmol/l) 1.5190.59
1.1090.17 1.0690.20 1.0790.17 1.0690.16
MM-IMT (mm) 1.0490.15
0.9290.18 0.8690.15
0.8590.14 0.9190.19
CC MM-IMT (mm) 0.9090.23
1.3290.23
Bif. MM-IMT (mm) 1.3390.27 1.3890.27 1.3190.19 1.3490.25
1.0890.15
CC+Bif. MM-IMT (mm) 1.1390.17 1.1190.19 1.1190.16 1.1190.19
aContinuous variables are means9SD. Group differences were calculated by ANOVA for the continuous variables, and by Fisher’s Exact test (2 tails) for discrete variables. AllP=not significant. LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; CC, common carotid; bif., bifurcation; MM-IMT, mean max-intima-media thickness.
Table 2
Effects of the treatments on serum lipids (D versus baseline) in the placebo group and in pravastatin group stratified by quartiles of LDL-C responsea
Pravastatin Pravastatin group stratified into quartiles of LDL-C response
Placebo p†for trend
1st quartile 2nd quartile 3rd quartile 4th
24.6–30.7% quartile\30.7% 16.0–24.5%
B16.0%
−0.9990.06** −0.4790.08 −0.9790.15 −1.4790.05 −1.8590.06
TC (mmol/l) −0.0790.05 §
0.0590.05 −1.0290.05** −0.3190.07 −0.9590.03 −1.3090.02
LDL-C −1.6890.04 §
(mmol/l)
0.0190.02 0.0890.02** 0.0990.03 0.0390.03
HDL-C 0.0790.03 0.0990.03 0.72
(mmol/l)
−0.1090.04* −0.1690.08 −0.0190.06
TG (mmol/l) 0.0990.04* −0.1590.07 −0.1590.07 0.71
aData expressed as mean (9SEM), represent the differences between baseline values and the mean of all on trial measurements between 6 and 36 months.
**PB0.0001;
*P=0.004 versus baseline.
†Evaluated by linear regression analysis. §Trend results from stratification.
reported. The values represent the differences between baseline and means of all on trial measurements ob-tained between the 6th and 36th months of the trial. In the placebo group only TG levels increased slightly, but significantly, during follow-up, whereas in pravastatin treated patients aside from the expected reduction of TC and LDL-C a significant reduction of plasma levels of TG and a significant increase of HDL-C levels were recorded. No trend could be identified between the
effect of pravastatin on LDL-C levels and its effects on TG and HDL-C levels (Table 2).
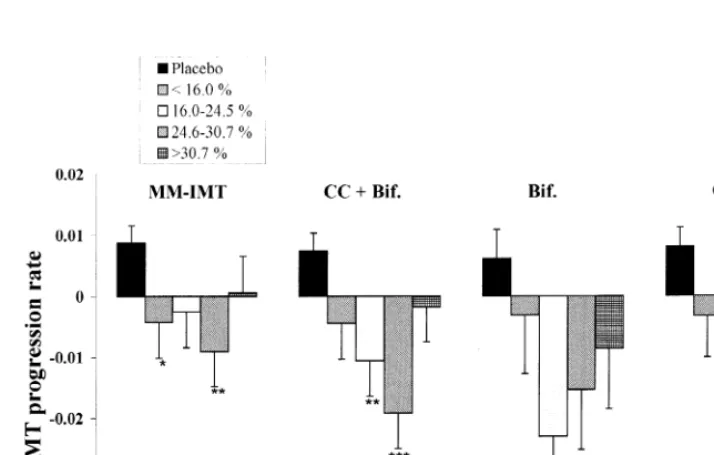
that the pravastatin effect on the reduction of IMT progression does not appear to be directly associated with the changes in plasma lipids. The majority of the other ultrasonographic outcome measures (CC+ Bif-IMT, Bif-IMT, CC-IMT) provided results of similar magnitude and generally in the same direction as observed for the MM-IMT (Fig. 3).
No trend was finally observed after stratification of pravastatin treated patients into quartiles of triglyceride or HDL-C changes (Fig. 4).
4. Discussion
The mechanism(s) by which statins act at the vascu-lar level have, as yet, not been fully elucidated. Since LDL are potent atherogenic factors which induce carotid thickening [19 – 22], it appears likely that any effective lipid lowering treatment should show an effect on arterial disease. A number of angiographic trials have generally confirmed the possibility to affect the evolution of coronary atherosclerosis by using either
Fig. 3. Carotid IMT changes in the placebo group and in the pravastatin treated group stratified into quartiles of LDL-C lowering. Differences were analysed using data weighted for gender, baseline intima-media thickness and clinical center. MM-IMT, mean maximum-intima media thickness; CC, common carotid; Bif, bifurcation; CC+Bif., MM-IMT of CC and Bif analysed together. Values are means9SEM.
hypolipidemic drugs [23,24] or alternative methods for blood cholesterol lowering, such as partial ileal bypass surgery [25]. In addition, this hypothesis is also sup-ported by studies focused on the carotid artery, which demonstrate that other lipid lowering drugs, different from the HMG-CoA reductase inhibitors, can effec-tively reduce carotid IMT progression [26]. Thus, as a general hypothesis, it may be inferred that pravastatin should influence carotid atherosclerosis progression through its hypocholesterolemic activity.
The results of the CAIUS study show that the effect of pravastatin treatment on carotid IMT progression rate is beneficial; however, they also demonstrate that lowering LDL-C by itself does not completely explain the variability of beneficial changes in IMT. In fact, after adjustment for treatment effects, the IMT progres-sion does not correlate with the extent of LDL-C changes. Moreover, reduction of IMT progression is detectable in all treatment groups and it is impossible to identify a linear trend between IMT-changes and LDL-C reduction, when patients treated with the active drug are stratified into quartiles of cholesterol reduction.
In contrast to what was observed in the placebo group, in which the mean IMT progression was posi-tive, in no subgroup on active treatment, even in that with the worst response in terms of LDL-C reduction (first quartile), positive mean IMT changes could be detected.
The reported findings derive from a post-hoc analysis and, as such, must be viewed cautiously. The issue of the possible lack of compliance in treated patients is out of question, because drug compliance with short half-life statins may not be monitored (no drug in plasma is found the morning after drug intake) [27] and all data of the large statin trials come from studies that, of necessity, inadequately monitored drug intake. How-ever, it is interesting to note that these findings confirm, and extend to early atherosclerosis, the results of two recently published post-hoc analyses of the CARE and WOSCOPS clinical trials, which suggest that the influ-ence of pravastatin on the event rates (mortality and CV morbidity) may not be completely explained by the reduction in LDL-C [3,5]. A similar post-hoc analysis of the 4S study, in contrast, suggests that the beneficial effects of simvastatin treatment may be determined mainly by the magnitude of LDL-C changes, but no data are offered on the variability of the LDL-choles-terol response to treatment [7]. Interestingly, in the angiographic LCAS study [28], positive coronary di-ameter changes occurred even in patients with very low baseline cholesterolemia, apparently unaffected in simi-lar studies with other statins [29].
In two very recent reports from B-mode ultrasound studies carried out within large preventive studies, the relationship between cholesterolemia response and carotid wall changes was only partly evaluated [9,30].
In the LIPID study, an investigation on a subgroup of 522 patients, out of the approx. 9000 participating in this secondary prevention study with pravastatin, evalu-ated the common carotid wall (not intima-media) thick-ness after 4 years of drug/placebo treatment [9]. The overall results do not differ from those of the present study and there appears to be little difference in tertiles with different baseline LDL-cholesterolemias, but no data are provided on the carotid response vs lipid changes. In a subgroup of 255 patients of the RE-GRESS Study, a coronary angiographic study with pravastatin, no clear correlation between carotid IMT changes after active drug, in a similar direction as in the present study, and coronary diameter changes could be detected; no evaluation was, however, provided on the impact of lipid changes [30].
Concerning the possible mechanisms for the pravas-tatin effects on carotid IMT, aside from the well known linkage between plasma lipid levels and atherosclerotic changes, a number of other mechanisms, particularly for drugs of the statin class, have been postulated [31]. Some of these, generally grouped under the heading of ‘pleiotropic effects’, have been extensively reviewed re-cently [32].
Among lipoprotein related mechanisms, drug treat-ment could have influenced the annual IMT progres-sion rate by reducing TG levels or raising HDL-C [33,34]. In the CAIUS study, treatment effectively in-duced a reduction of TG and an elevation of HDL-C levels but, similar to the case of LDL-C, no significant trend was identified when stratifying patients into quar-tiles of TG-reduction or HDL-C elevation (Fig. 4).
Among the suggested pleiotropic effects, a direct inhibition of arterial smooth muscle cell proliferation has been well supported [35,36]. However, the low efficacy of pravastatin in inhibiting replication of vascu-lar myocytes in vitro and in preventing in-vivo neointi-mal formation in normocholesterolemic rabbits [37] seems to rule out this hypothesis. Finally, pravastatin may exert its antiatherogenic role by directly affecting the rate of lipid deposition in macrophages of the atheromatous plaque [38], as well as by improving defective endothelium-dependent vasodilatation [39,40] and reducing platelet thrombus formation in flowing blood [41,42].
fact, in the WOSCOPS analysis patients not respond-ing to treatment with an LDL-C reduction also had no benefit in terms of disease incidence [5]. However, reduced arterial progression is desirable per se and may provide an early marker of atherosclerosis con-trol. A reasonable question may be whether plasma changes may always accurately reflect body and partic-ularly arterial cholesterol changes. Statins, in fact, while generally exerting a marked plasma cholesterol reduction, modify to a minimal extent total body cholesterol content [43,44]. While plasma changes may not accurately reflect total body and particularly arte-rial cholesterol content, it would be of interest to know in greater detail the possible quantitative aspects of this dissociation, that would allow better clarifica-tion of the relaclarifica-tionship between biochemical and arte-rial benefits.
In conclusion, the effect of pravastatin treatment on carotid IMT progression rate is beneficial; however, the CAIUS study demonstrated that lowering LDL-C by itself does not explain the variability of beneficial changes in IMT. Models evaluating pravastatin activ-ity on atherosclerosis progression in animals not re-sponding in terms of LDL-C reduction [11] could be useful to define such mechanisms. Furthermore, clini-cal trials performed by using statins in normocholes-terolemic patients prone to atherosclerosis development, e.g. with diabetes or hypertension, may allow a better definition of the role of the drug in atherosclerosis-prone patients without LDL-C eleva-tions.
Acknowledgements
Bristol-Myers Squibb S.P.A. (Italy) is gratefully ac-knowledged for kind interest in the study, and for supporting acquisition of the equipment. The study has been financcially supported in part by the Con-siglio Nazionale delle Ricerche of Italy.
References
[1] Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383 – 9.
[2] Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFar-lane PW, McKillop JH, Packard CJ for the West of Scotland Coronary Prevention Study group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. New Engl J Med 1995;333:1301 – 7.
[3] Sacks FM, Moye` LA, Davis BR, Cole TG, Roleau JL, Nash D, Pfeffer MA, Braunwald E. Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the cholesterol and recurrent events trial. Circulation 1998;97:1446 – 52.
[4] Gould AL, Rossouw JE, Santanello NC, Heyse JF, Furberg CD. Cholesterol reduction yields clinical benefit. Impact of statin trials. Circulation 1998;97:946 – 52.
[5] West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 1998;97:1440 – 45.
[6] Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu Ch, Liu Ci, Alaupovic P, Kwong-Fu H, Azen SP. Reduction in carotid arterial wall thickness using lovastatin and dietary therapy. A randomized, controlled clinical trial. Ann Intern Med 1996;124:548 – 56.
[7] Pedersen TR, Olsson AG, Faergeman O, Kjekshus J, Wedel H, Berg K, Wilhelmsen D, Haghfelt T, Thorgeirsson G, Pyorala K, Miettinen T, Christophersen B, Tobert JA, Musliner TA, Cook TJ for the Scandinavian Simvastatin Survival Study group. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998;97:1453 – 60.
[8] Rossouw JE. Lipid-lowering interventions in angiographic trials. Am J Cardiol 1995;76:86C – 92C.
[9] MacMahon S, Sharpe N, Gamble G, Hart H, Scott J, Simes J, White H, on Behalf of the LIPID Trial Research Group. Effects of lowering average or below-average cholesterol levels on the progression of carotid atherosclerosis. Results of the LIPID atherosclerosis substudy. Circulation 1998;97:1784 – 90. [10] Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD,
Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. Cholesterol and Recurrent Events Trial Inves-tigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. New Engl J Med 1996;335:1001 – 9.
[11] Koudy WJ, Sukhova GK, Herrington DM, Libby P. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J Am Coll Cardiol 1998;31:684 – 91. [12] Furberg CD, Adams HP, Applegate WB, Byington RP,
Es-peland MA, Hartwell T, Hunninghake DB, Lefkowitz DS, Prob-stfield J, Riley WA, Young B, for the Asymptomatic Carotid Artery Progression Study (ACAPS) research group. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Circulation 1994;90:1679 – 87.
[13] Crouse III JR, Byington RP, Bond GM, Espeland MA, Craven TE, Sprinkle JW, McGovern ME, Furberg CD. Pravastatin, lipid and atherosclerosis in the carotid arteries (PLAC-II). Am J Cardiol 1995;75:455 – 9.
[14] Salonen R, Nyysso¨nen K, Porkkala E, Rummukainen J, Belder R, Park JS, Salonen JT. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary prevention trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995;92:1758 – 64. [15] Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G,
Feruglio FS, Descovich G, Ricci G, Rubba P, Mancini M, Gallus G, Bianchi G, D’Alo` G, Ventura A. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population. The Carotid Atherosclerosis Italian Ultrasound Study. Am J Med 1996;101:627 – 34.
[16] Sirtori CR, Bianchi G, Bond MG, D’Alo` G, Gallus G, Libera-tore S, Mercuri M, Ventura S, on behalf of The CAIUS research group. Pravastatin intervention trial on carotid artery atheroscle-rosis in patients with mild hypercholesterolemia: the CAIUS Study. Int J Card Imag 1995;11:119 – 24.
[18] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:402 – 99.
[19] Salonen R, Seppanen K, Rauramaa R, Salonen JT. Prevalence of carotid atherosclerosis and serum cholesterol levels in Eastern Finland. Arteriosclerosis 1988;8:788 – 92.
[20] Poli A, Tremoli E, Colombo A, Sirtori M, Pignoli P, Paoletti R. Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients. A new model for the quantitation and follow-up of pre-clinical atherosclerosis in living human subjects. Atherosclerosis 1988;70:253 – 61. [21] Rubens J, Espeland MA, Ryu J, Harpold G, McKinney WM,
Kahl FR, Toole JF, Crouse III JR. Individual variation in susceptibility to extracranial carotid atherosclerosis. Arterioscle-rosis 1988;8:389 – 97.
[22] Wendelhag I, Wiklund O, Wikstrand J. Arterial wall thickness in familial hypercholesterolemia. Ultrasound measurement of in-tima-media thickness in common carotid artery. Arterioscler Thromb 1992;12:70 – 7.
[23] Brown G, Albers JJ, Fisher LD, Schaefer SM, Jiin-Tarng Lin, Kaplan C, Xue-Qiao Zhao, Bisson BD, Fitzpatrik VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. New Engl J Med 1990;323:1289 – 98.
[24] Kane JP, Malloy MJ, Ports TA, Phillips NR, Diehl JC, Havel RJ. Regression of coronary atherosclerosis during treatment of familial hypercholesterolemia with combined drug regimen. J Am Med Assoc 1990;264:3007 – 12.
[25] Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Kamp-bell GS, Pearce MB, Yellin AE, Edmiston WA, Smink RD Jr, Sawin HS Jr, Campos CT, Hansen BJ, Tuna N, Karnegis JN, Sanmarco ME, Amplatz K, Castaneda-Zuniga WR, Hunter DW, Bissett JK, Weber FJ, Stevenson JW, Leon AS, Chalmers TC, POSCH group. Effect of partial ileal bypass surgery on mortality and morbility from coronary heart disease in patient with hypercholesterolemia: report of the Program on the Surgi-cal Control of the Hyperlipidemias (POSCH). New Engl J Med 1990;323:946 – 55.
[26] Blankenhorn DH, Selzer RH, Crawford DW, Barth JD, Chao-ran L, Ci-hua L, Mack WJ, Alaupovic P. Beneficial effects of colestipol-niacin therapy on the common carotid artery. Two-and four-year reduction of intima-media thickness measured by ultrasound. Circulation 1993;88:20 – 8.
[27] Pan HY, De Vault AR, Wang-Iverson D, Ivashkiv E, Swanson BN, Sugerman AA. Comparative pharmacokinetics and pharma-codinamics of pravastatin and lovastatin. J Clin Pharmac 1990;30:1128 – 35.
[28] Herd JA, Ballantyne CM, Farmer JA, Ferguson III JJ, Jones PH, West S, Gould KL, Gotto AM, for the LCAS investigators. Effects of fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations (lipoproteins and coro-nary atherosclerosis study; LCAS). Am J Cardiol 1997;80:278 – 86.
[29] Sacks FM, Pasternak RC, Gibson CN, Rosner B, Stone PH, for the Harvard atherosclerosis Reversibility project (HARP) group. Effect on coronary atherosclerosis of decrease in plasma choles-terol concentrations in normocholescholes-terolaemic patients. Lancet 1994;344:1182 – 6.
[30] De Groot E, Jukema JW, Montauban Van Swijndregt AD, Zwinderman AH, Ackerstaff RGA, van der Steen AFW, Bom N, Lie KI, Bruschke AVG, on Behalf of the REGRESS study
group. B-Mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: a report of the Regression Growth Evaluation Statin Study (REGRESS). J Am Coll Car-diol 1998;31:1561 – 7.
[31] Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet 1996;348:1079 – 82.
[32] Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins. Implications for cardiovascular event reduction. J Am Med Assoc 1998;279:1643 – 50.
[33] Ericsson CG, Hamsten A, Nilsson J, Grip L, Svane B, Faire U. Angiographic assessment of effects of bezafibrate on progression of coronary artery disease in young male postinfarction patients. Lancet 1996;347:849 – 53.
[34] Frick MH, Syva¨nne M, Nieminen MS, Kauma H, Majahalme S, Virtanen V, Kesa¨niemi A, Pasternack A, Taskinen MR, for the Lopid Coronary Angiography Trial (LOCAT) study group. Pre-vention of the angiographic progression of coronary and vein-graft atherosclerosis by gemfibrozil after coronary bypass surgery in men with low levels of HDL cholesterol. Circulation 1997;96:2137 – 43.
[35] Corsini A, Mazzotti M, Raiteri M, Soma MR, Gabbiani G, Fumagalli R, Paoletti R. Relationship between mevalonate path-way and arterial myocyte proliferation: in vitro studies with inhibitors of HMG-CoA reductase. Atherosclerosis 1993;101:117 – 25.
[36] Ne`gre-Aminou P, van Vliet AK, van Erck M, van Thiel GCF, van Leeuwen REW, Cohen LH. Inhibition of proliferation of human smooth muscle cells by various HMG-CoA reductase inhibitors; comparison with other human cell types. Biochim Biophys Acta 1997;1345:259 – 68.
[37] Soma MR, Donetti E, Parolini C, Mazzini G, Ferrari C, Fuma-galli R, Paoletti R. HMG CoA reductase inhibitors. In vivo effects on carotid intimal thickening in normocholesterolemic rabbits. Arterioscler Thromb 1993;13:571 – 8.
[38] Keidar S, Aviram M, Maor I, Oiknine J, Brook JG. Pravastatin inhibits cellular cholesterol synthesis and increases low density lipoprotein receptor activity in macrophages: in vitro and in vivo studies. Br J Clin Pharmacol 1994;38:513 – 9.
[39] Egashira K, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Inou T, Takeshita A. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in pa-tients with hypercholesterolemia. Circulation 1994;89:2519 – 24. [40] O’Driscoll G, Green D, Taylor RR. Simvastatin, an
HMG-Coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 1997;95:1126 – 31.
[41] Lacoste L, Lam JYT, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation 1995;92:3172 – 7.
[42] Lacoste L, Lam JYT. Comparative effect of pravastatin and simvastatin on platelet thrombus formation in hypercholes-terolemic patients. J Am Coll Cardiol 1996;27:413A.
[43] Grundy SM, Bilheimer DW. Inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase by mevinolin in familial hypercoles-terolemia heterozygotes: effects on cholesterol balance. Proc Natl Acad Sci USA 1984;81:2538 – 42.
[44] Goldberg IJ, Holleran S, Ramakrishanan R, Adams M, Palmer RH, Dell RB, Goodman DS. Lack of effect of lovastatin therapy on the parameters of whole-body cholesterol metabolism. J Clin Invest 1990;86:801 – 8.