www.elsevier.com / locate / bres
Research report
Responses of adrenal sympathetic preganglionic neurons to stimulation
of cardiopulmonary receptors
*
Wei-Hua Cao, Shaun F. Morrison
Department of Physiology, Northwestern University Medical School, Chicago, IL 60611, USA
Accepted 12 September 2000
Abstract
The current study examined whether or not the activation of Bezold-Jarisch reflex with administration of phenylbiguanide (PBG, 100
mg / kg) into right atrium elicits differential responses in the two populations of adrenal sympathetic preganglionic neurons (SPNs) regulating the release of epinephrine (EPI ADR SPNs) and norepinephrine (NE ADR SPNs), respectively, from adrenal medullary chromaffin cells. Extracellular activity of 48 adrenal SPNs in the intermediolateral cell column (IML) were recorded in urethane / chloralose-anesthetized rats. Twenty-three EPI ADR SPNs and 25 NE ADR SPNs were antidromically activated by stimulation of left adrenal nerve and orthodromically activated by rostral ventrolateral medulla (RVLM) stimulation. At a mean arterial pressure (MAP) of 99.662.8 mmHg, the mean spontaneous discharge rates of EPI ADR SPNs and NE ADR SPNs were 6.260.5 and 4.360.5 spikes / s, respectively. Intra-atrial PBG markedly inhibited 96% of EPI ADR SPNs (by 3.860.4 spikes / s; n522) and 76% of NE ADR SPNs (by 2.960.5 spikes / s; n519) with hypotensive responses (DMAP533.265.3 and 26.465.0 mmHg, respectively). The remaining SPNs were weakly excited or unaffected. We conclude that both groups of SPNs regulating catecholamine release are primarily inhibited by stimulation of cardiopulmonary receptors and that these responses parallel the sympathoinhibitory and hypotensive components of the Bezold-Jarisch reflex. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Cardiovascular regulation
Keywords: Phenylbiguanide; Epinephrine; Bezold-Jarisch reflex; Sympathoinhibition; Intermediolateral cell column; Rostral ventrolateral medulla
1. Introduction subsequently involve inhibitory neurons in the caudal ventrolateral medulla (CVLM), projecting to the rostral Activation of cardiopulmonary, chemosensitive vagal ventrolateral medulla (RVLM) [7,25,27,28]. While these afferent C-fibers ending in the lungs and heart, by injection data suggest a pathway similar to that of the baroreceptor of chemical substances such as veratrum alkaloids, reflex [25,27,28], the central integration mechanisms un-serotonin, phenylbiguanide (PBG) or capsaicin, causes a derlying the Bezold-Jarisch reflex are less well understood. profound bradycardia, hypotension and apnoea [14,24]. The demonstration that stimulation of chemosensitive These responses, first described by von Bezold and Hirt in cardiac vagal afferents by phenyldiguanide decreased renal 1867 and involving receptors characterized by Jarisch and sympathetic nerve activity (SNA), but increased adrenal Zotterman in 1948 [11], are referred to as the Bezold- SNA [9], suggests that the sympathetic outflow to the Jarisch reflex [6,13,14,24]. Bezold-Jarisch reflex responses adrenal gland may be an exception to the general finding are mediated by neurons in the nucleus tractus solitarii that Bezold-Jarisch reflex responses are sympatho-(NTS) that receive input from vagal primary afferents, inhibitory. To test this possibility and to determine if the originating in the heart and lungs [3,12,15,16], and may phenyldiguanide-evoked increase arose from pre- or post-ganglionic axons in the adrenal nerve, we recorded the responses of adrenal SPNs to activation of the
Bezold-*Corresponding author. Tel.: 11-312-503-5024; fax: 1
1-312-503-Jarisch reflex with right atrial administration of PBG.
5101.
E-mail address: [email protected] (S.F. Morrison). Recently, adrenal sympathetic preganglionic neurons
(SPNs) have been divided into two physiologically distinct effect. The position of the atrial catheter was verified after populations: EPI ADR SPNs, which putatively regulate the each experiment.
activity of adrenal epinephrine-secreting chromaffin cells, and NE ADR SPNs that control adrenal
norepinephrine-2.3. Recording and identification of adrenal sympathetic secreting chromaffin cells [17]. In the present study, we
preganglionic neurons
distinguished between these two groups of adrenal SPNs to ascertain if PBG might evoke differential responses in
The extracellular action potentials of adrenal SPNs were those regulating adrenal epinephrine secretion and those
recorded with glass pipettes containing an electropho-controlling norepinephrine release. The results suggest that
retically sharpened 7-mm-diameter carbon filament. The the cardiopulmonary receptors activated by intra-atrial
reference electrode was inserted into muscle lateral to the PBG evoke a uniform inhibition of adrenal SPNs. A
vertebral column or lateral to the occipital bone. Spinal preliminary report of these results has been published [4].
penetrations were made along the dorsal root entry zone, and adrenal SPNs were located 0.7–1.1 mm below the dorsal surface. Neuronal signals were amplified, filtered
2. Materials and methods
(bandpass frequencies 300–3000 Hz), monitored on an oscilloscope and recorded on VCR tape. As previously 2.1. General procedures
described [17], adrenal SPNs were antidromically iden-tified with stimuli applied to the left adrenal nerve. Three Experiments were performed on 19 male
Sprague–Daw-criteria were used to establish the antidromic nature of the ley rats (300–500 g) anesthetized with urethane (800
response of spinal neurons to adrenal nerve stimulation: mg / kg, i.p.) and chloralose (70 mg / kg, i.v.). The trachea
(1) constant onset latency, (2) high following frequency, and femoral vein were cannulated for artificial ventilation
and (3) collision with spontaneous action potentials or and drug administration, respectively. Arterial pressure
those evoked by medullary stimuli. To perform a time-(AP) was measured from the abdominal aorta by a
controlled collision test, pulses coincident with neuronal polyethylene catheter inserted through the femoral artery,
action potentials were obtained from a window dis-and heart rate (HR) was determined from the AP signal
criminator and used to trigger a stimulator at a specified with a biotach. The animals were paralyzed with
d-delay. tubocurarine chloride (initially 0.6 mg / rat, i.v., thereafter,
To distinguish between the two physiologically distinct 0.2 mg / h, i.v.) and then artificially ventilated with 100%
groups of rat adrenal SPNs [17], the following were O at a minute volume of 140–180 ml. Respiratory rate2 determined for antidromically activated adrenal SPNs: (1)
was adjusted to maintain end-tidal CO between 3.5 and2 their response to RVLM stimulation, (2) the relationship of
4.5%. Body temperature was maintained at 378C with a
their spontaneous activity to the cardiac cycle, (3) their thermostatically controlled heating lamp. Rats were placed
different sensitivities to baroreflex, and (4) their responses prone in a stereotaxic apparatus and spinal investigation
to the glucopenia induced with intravenous 2-deoxy-glu-unit with the bite bar 11 mm below the interaural line and
cose. a spinal clamp on the T10 and T11 vertebral processes. An
occipital craniotomy and T –T7 9 laminectomy were
per-formed. Bilateral pneumothoraces reduced respiratory 2.4. Neural stimulation pump-related movements of tissue near the recording and
stimulating electrodes. To orthodromically activate adrenal SPNs by stimulation
of their premotor input, paired-pulse (6 ms interpulse
2.2. Bezold-Jarisch reflex activation interval, 50–300mA, 1 ms duration, 0.25 Hz) stimuli were
delivered to the RVLM (coordinates: 2.6 mm rostral, 1.9 PBG was selected because it directly activates chemical- mm lateral and 2.3 mm ventral to the calamus scriptorius) ly sensitive cardiopulmonary vagal afferents through through a monopolar tungsten electrode (50 mm exposed serotonin (5-HT ) membrane receptors. PBG is more3 tip). The RVLM contains spinally projecting, sympathetic specific and has fewer systemic effects than other physio- premotor neurons that regulate adrenal medullary function logical activators of cardiopulmonary receptors [2]. A [22,29]. Histological examination of the locations of polyethylene catheter (PE 50) was inserted into the right marker lesions made through the stimulating electrode external jugular vein and advanced until its tip reached the indicated that they were consistently within 400mm of the entrance of the right atrium. This right atrial catheter was caudal pole of the facial nerve nucleus.
gland. The anode was clipped to the muscle near the to RVLM stimulation and their differential sensitivity to
adrenal nerve. the baroreceptor reflex. In the upper trace of Fig. 1A, the
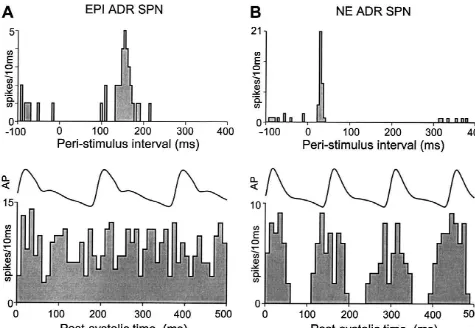
To activate the baroreceptor reflex, the left aortic peristimulus time interval histogram for an EPI ADR SPN depressor nerve (ADN) was identified in the neck and the responding to paired-pulse stimulation of the RVLM shows central cut end was placed on a pair of platinum hook the characteristic early inhibition of spontaneous discharge electrodes spaced 2 mm apart. Electrode stimuli were triad during the first 50–80 ms following RVLM stimulation, square-wave pulses (7 ms interpulse interval, 10mA, 1 ms, followed by a prominent excitation at a longer latency 0.25 Hz). Natural stimulation of arterial baroreceptors (mean response latency, 150 ms). In contrast, the RVLM consisted of the rise in arterial pressure following an stimulus-evoked responses of NE ADR SPNs (Fig. 1B, intravenous bolus of phenylephrine (50–100 mg / ml) in a upper trace) consisted of an early, marked increase in
volume of 0.1 ml of saline over 1–2 s. discharge probability (mean response latency, 25 ms),
which was often followed by a period of reduced firing lasting 100–200 ms. The average mean response latency
2.5. Data analysis for the 23 EPI ADR SPNs in this study was 14164.3 ms
(range, 87 to 172 ms), which was significantly (P,0.001) The action potentials of adrenal SPNs and the arterial longer than that of the 25 NE ADR SPNs (average, 3061.9 blood pressure were digitized at 22 kHz and recorded on ms; range, 17–53 ms).
VCR tape, along with trigger pulses coincident with As illustrated in the lower traces of Fig. 1, the sponta-stimulus delivery. Computer-aided data analysis consisted neous discharge probability of EPI ADR SPNs usually of peristimulus and perisystolic time interval histograms of exhibited no modulation over the time course of the the discharges of adrenal SPNs. Results were expressed as cardiac cycle, indicating little or no influence of the means6S.E.M. Significant differences between population baroreceptor reflex on the neurons that determine the means were assessed with Student’s t-test (P,0.05). spontaneous activity of EPI ADR SPNs. In contrast, the
perisystolic time interval histogram of the spontaneous activity of an NE ADR SPN (Fig. 1B, lower trace) illustrates a pronounced cardiac cycle-related modulation
3. Results
of the unit’s discharge probability. Nineteen of the 20 EPI ADR SPNs recorded under conditions in which the mean In 19 rats, 48 neurons in the IML region of thoracic
AP was greater than 80 mmHg (i.e., above the expected spinal segments, T –T , were antidromically activated6 8
threshold pressure for activation of baroreceptors) had no with electrical stimuli applied to the left adrenal nerve. The
discernible modulation of their spontaneous discharge by responses of individual adrenal SPNs consisted of single
the baroreceptor reflex. In contrast, 14 of the18 NE ADR action potentials with constant onset latencies that collided
SPNs recorded at AP greater than 80 mmHg exhibited a with spontaneous action potentials. The evoked responses
discharge pattern with a marked reduction in discharge in adrenal SPNs followed paired adrenal nerve stimuli
probability during a consistent portion of the cardiac cycle. separated by 4 ms or less. The mean onset latency of the
Similarly, 13 of 15 NE ADR SPNs that were tested adrenal SPN responses to adrenal nerve stimulation was
showed a complete inhibition of their spontaneous dis-3461.6 ms (range, 13 to 59 ms), corresponding to an
charge during the rise in AP caused by intravenous approximate conduction velocity of 1.2 m / s (average
injection (10mg) of phenylephrine (data not shown). Only estimated distance from the adrenal nerve stimulation site
four of 14 tested EPI ADR SPNs, by contrast, were to the spinal recording site, 33 mm). At mean arterial
completely inhibited during similarly evoked pressor re-pressures between 70 and 135 mmHg (mean, 10062.8
sponses. Electrical stimulation of the baroreceptor afferents mmHg), the mean spontaneous discharge rates of adrenal
in the ipsilateral aortic depressor nerve (ADN) with a burst SPNs ranged from 0.9 to 10.8 spikes / s (mean, 5.960.5
of three pulses produced a transient inhibition in 19 out of spikes / s).
20 tested NE ADR SPNs, but a baroreceptor afferent stimulus-evoked inhibitory response was detected in only 3.1. Identification of two distinct groups of adrenal two of the 20 EPI ADR SPNs that were tested.
sympathetic preganglionic neurons The mean antidromic onset latencies of the EPI ADR SPNs (3161.1 ms; n523; range, 16 to 52 ms) were not Based on the criteria described above and detailed in different (P.0.) from those of NE ADR SPNs (3762.6 [17], the 48 spontaneously active adrenal SPNs were ms; n525; range, 13 to 59 ms). The mean spontaneous divided into two groups: 23 (48%) EPI ADR SPNs and 25 discharge frequency of EPI ADR SPNs (6.260.5 Hz;
(52%) NE ADR SPNs. range, 3.1 to 10.8 Hz; mean AP, 10264.0 mmHg) was
Fig. 1. Responses to stimulation of the rostral ventrolateral medulla (RVLM) and cardiac-related activity patterns of an EPI ADR SPN and an NE ADR SPN. (A) Upper trace: peri-stimulus time histogram of the discharge of an EPI ADR SPN during paired-pulse stimulation of the RVLM (200mA, 0.25 Hz, 30 stimuli). Bin width is 5 ms. Note the early inhibition lasting about 100 ms and the late excitation of this EPI ADR SPN. Lower trace: post-systolic average of arterial pressure (AP) and time interval histogram of the spontaneous discharge of an EPI ADR SPN. Note the absence of baroreceptor modulation of EPI ADR SPN discharge. Histogram based on 100 sweeps and bin width is 10 ms. (B) Similar traces as in (A) for an NE ADR SPN. Note the short-latency (25 ms) excitation of the NE ADR SPN and the strong cardiac-related modulation of its discharge (lower histogram based on 300 sweeps).
3.2. Effects of activation of Bezold-Jarisch reflex on spontaneous discharge rates were reduced from 6.260.5
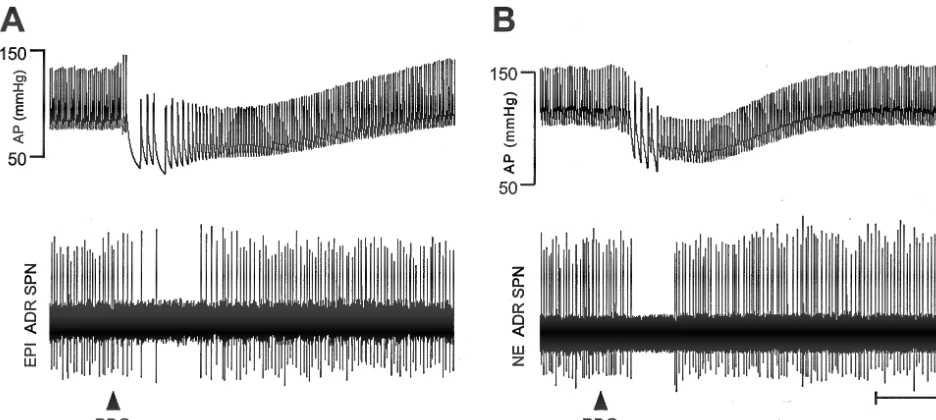
adrenal SPNs Hz to a minimum of 2.560.4 Hz (Fig. 4), accompanied by a depressor response from a mean AP of 10364.0 to Activation of the Bezold-Jarisch reflex by administration 7064.5 mmHg (Fig. 4). Atrial injection of PBG decreased of PBG (100 mg / kg) into the right atrium produced a the mean discharge frequency of 19 (76%) of 25 NE ADR consistent and characteristic cardiopulmonary chemoreflex, SPNs (Figs. 2, 3) from 4.360.5 to 1.260.4 Hz (Fig. 4). In consisting of the prompt bradycardia (Dheart rate5 these experiments, the mean AP decreased from 9964.2 to
2272638 bpm) and hypotensive response (Dmean AP5 7264.9 mmHg (Fig. 4). Neither the Bezold-Jarisch
reflex-22963.2 mmHg from a baseline of 10062.8 mmHg; evoked decreases in firing rate nor the mean depressor
n548). This hypotensive response started 2–5 s after the responses evoked by the PBG injections were significantly injection of PBG, reached peak effects 5 s later, and different between EPI ADR SPNs and NE ADR SPNs. The completely returned to the control level 10–20 s after the other six NE ADR SPNs increased their discharge rate by peak (Fig. 2). Control injections (50ml) of saline into the 2.760.5 Hz, during a fall in AP of22369.1 mmHg. right atrium did not elicit any changes in heart rate or AP.
Bilateral cervical vagotomy abolished the response to the
right atrial injection of PBG. 4. Discussion
Forty-one (85%) of the 48 adrenal SPNs were inhibited
Further-Fig. 2. Arterial pressure (AP) and adrenal SPN responses to Bezold-Jarisch reflex activation. (A) Fall in AP and inhibition of the spontaneous discharge of an EPI ADR SPN evoked by intra-atrial injection of phenylbiguanide (PBG, 100mg / kg, filled triangle). Also note the strong bradycardia evoked by PBG. (B) Similar traces for an NE ADR SPN response to injection of PBG. Calibration: horizontal55 s, vertical550mV.
more, this result is independent of the function of the response to baroreceptor stimulation. While our data do not adrenal SPNs, in that atrial injection of PBG caused a 60% directly address the potential for common elements in the reduction in the discharge rate of EPI ADR SPNs, which Bezold-Jarisch reflex and the baroreceptor reflex circuits, regulate adrenal medullary epinephrine release and a 72% the finding that EPI ADR SPNs and NE ADR SPNs are reduction in the firing of NE ADR SPNs, which determine inhibited to a similar degree by atrial injection of PBG, but adrenal medullary norepinephrine secretion. From these differ markedly in the amplitude of their inhibitory re-data, we conclude that Bezold-Jarisch reflex activation sponses to stimulation of the baroreceptors, suggests that, inhibits the preganglionic component of adrenal nerve at least for the regulation of EPI ADR SPN discharge, the activity as it does the renal [1,21,26] and lumbar [20] two reflexes are not mediated by the same medullary
sympathetic outflows. pathway.
Anatomical data [22,29] and indirect physiological Intrapericardial injection of phenyldiguanide has been results [19] indicate that the RVLM is a source of the shown to increase adrenal sympathetic nerve activity but sympathetic premotor input to adrenal SPNs. A high decrease renal sympathetic nerve activity, leading to the percentage of barosensitive, bulbospinal RVLM neurons, conclusion that stimulation of chemosensitive cardiac which maintain tonic sympathetic tone, are inhibited by vagal afferents produces differential sympathetic nerve intrajugular injections of PBG in anesthetized rats [28] and responses [9]. The results of the present study, however, cats [25]. These observations are consistent with the indicate that the preganglionic component of adrenal possibility that the intra-atrial PBG-evoked inhibition of sympathetic nerve activity could not have been the princi-adrenal SPNs seen in our experiments resulted from the ple source of the reported adrenal sympathoexcitatory disfacilitation of these SPNs due to inhibition of their tonic response to phenyldiguanide. The adrenal nerve, located excitatory inputs from RVLM sympathetic premotor neu- between the suprarenal ganglion and the adrenal gland in
rons. the rat, contains the axons of: (a) the sympathetic
Fig. 4. Peak changes in arterial pressure (AP) and in adrenal SPN (AdrSPN) discharge frequency in response to Bezold-Jarisch reflex activation. The maximum fall in AP evoked by right atrial injection of phenylbiguanide (100mg / kg) during recordings of EPI ADR SPNs (open bars) and that during recordings of NE ADR SPNs (solid bars) were not significantly different (n.s.). The maximum reductions in the discharge frequencies of EPI ADR SPNs (open bars) and of NE ADR SPNs (solid bars) were also not significantly different.
atrium, right ventricle, and lungs [28]. This difference may not have been a significant factor, however, since reflex cardiovascular and respiratory responses to PBG (60mg / kg, i.v.) in the conscious rabbit are not mediated by pulmonary receptors because all responses were blocked by acute denervation of cardiac nerves with intrapericardial procaine [1].
In summary, both EPI ADR SPNs and NE ADR SPNs, which respectively regulate the secretions of epinephrine
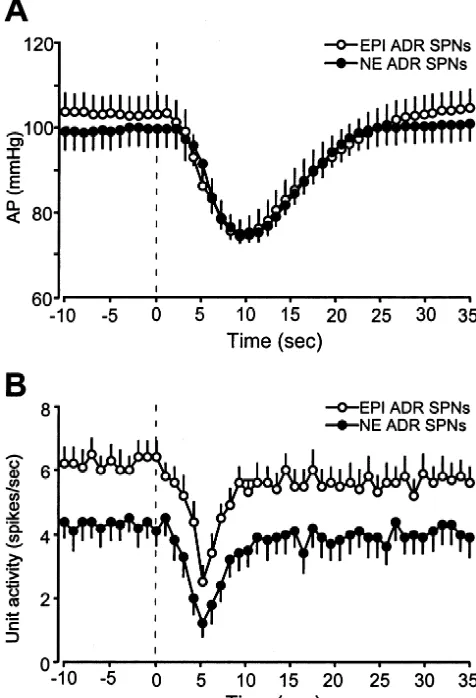
Fig. 3. Time courses of the mean arterial pressure (AP) and the adrenal
SPN discharge frequency during Bezold-Jarisch reflex activation. (A) and norepinephrine from different populations of adrenal
Average mean AP before and following right atrial injection of medullary chromaffin cells, were similarly inhibited by phenylbiguanide (PBG, 100mg / kg) at time 0. Open circles (bar indicates
activation of the Bezold-Jarisch reflex following right
S.E.M.) indicate data during recordings of EPI ADR SPNs (n522) and
intra-atrial application of PBG. These results indicate that,
filled circles indicate data during recordings of NE ADR SPNs (n519).
in addition to sympathoinhibition and hypotension,
activa-(B) Average mean discharge rate of EPI ADR SPNs (open circles, bar
indicates S.E.M.) and NE ADR SPNs (filled circles) before and following tion of the Bezold-Jarisch reflex induces a reduction in
right atrial injection of phenylbiguanide. Note comparable PBG-evoked adrenal catecholamine secretion mediated via the inhibition inhibitions of both groups of adrenal SPNs. Data are presented at 1-s
of adrenal SPNs. Although the afferents mediating the
intervals.
Bezold-Jarisch and the baroreceptor reflexes are distinctly separate, the widespread sympathoinhibitory responses blood flow [10], may account for more than 50% of the evoked by both reflexes suggests that they may share some activity on adrenal nerves [5]. An additional contribution common central components. However, the relative
insen-to the adrenal sympathoexcitatory response to sitivity to baroreceptor inputs exhibited by EPI ADR
phenyldiguanide may have arisen from the vagal efferents SPNs, coupled with a potent, PBG-evoked inhibition
within the adrenal nerve [18]. similar to that of other sympathetic outflows, is consistent
A notable difference between the present experiments with a model in which the inputs from cardiac and those of Higuchi et al. [9] is that whereas we used chemoreceptor afferents in the vagus nerve, but not arterial right intra-atrial injection of PBG to activate the Bezold- baroreceptor information, modulates the segregated path-Jarisch reflex in carotid sinus nerve intact rats, they used way controlling adrenal epinephrine secretion.
intrapericardial injection of PDG in sinoaortic barorecep-tor-denervated rats. Intrapericardial administration of
chemical substances has been reported to stimulate cardiac Acknowledgements
receptors more selectively [23,30] than administration of
[16] T.M. Lee, J.S. Kuo, C.Y. Chai, Central integrating mechanism of the References
Bezold-Jarisch and baroceptor reflexes, Am. J. Physiol. 222 (1972) 713–720.
[1] L.B. Bell, K.P. O’Hagan, P.S. Clifford, Cardiac but not pulmonary [17] S.F. Morrison, W.-H. Cao, Different adrenal sympathetic pregan-receptors mediate depressor response to IV phenyl biguanide in glionic neurons regulate epinephrine and norepinephrine secretion., conscious rabbits, Am. J. Physiol. 264 (1993) R1050–R1057. Am. J. Physiol. 279 (2000) R1763–R1775.
[2] V. Bishop, S. DiCarlo, Role of vagal afferents: experimental, in: M. [18] A. Niijima, Electrophysiological study on the vagal innervation of Levy, P. Schwartz (Eds.), Vagal Control of the Heart, Atmonk, New the adrenal gland in the rat, J. Auton. Nerv. Syst. 41 (1992) 87–92. York, 1994, pp. 325–344, Ch. 21. [19] C.A. Ross, D.A. Ruggiero, D.H. Park, T.H. Joh, A.F. Sved, J. [3] A.C. Bonham, J.P. Joad, Neurones in commissural nucleus tractus Fernandez-Pardal, J.M. Saavedra, D.J. Reis, Tonic vasomotor con-solitarii required for full expression of the pulmonary C fibre reflex trol by the rostral ventrolateral medulla: effect of electrical or in rat, J. Physiol. (Lond.) 441 (1991) 95–112. chemical stimulation of the area containing C1 adrenaline neurons [4] W.-H. Cao, S.F. Morrison, Responses of adrenal sympathetic on arterial pressure, heart rate, and plasma catecholamines and
preganglionic neurons (SPNs) to stimulation of cardiopulmonary vasopressin, J. Neurosci. 4 (1984) 474–494.
receptors, Soc. Neurosci. Abstr. 24 (1998) 622. [20] T.J. Scislo, S.E. DiCarlo, Gender difference in cardiopulmonary [5] S. Carlsson, J.O. Skarphedinsson, E. Jennische, M. Delle, P. Thoren, reflex inhibition of sympathetic nerve activity, Am. J. Physiol. 267
Neurophysiological evidence for and characterization of the post- (1994) H1537–1543.
ganglionic innervation of the adrenal gland in the rat, Acta Physiol. [21] K.E. Scrogin, R. Veelken, A.K. Johnson, Central methysergide Scand. 140 (1990) 491–499. prevents renal sympathoinhibition and bradycardia during hypoten-[6] G. Dawes, J. Mott, J. Widdicombe, Respiratory and cardiovascular sive hemorrhage, Am. J. Physiol. 274 (1998) H43–51.
reflexes from the heart and lungs, J. Physiol. 115 (1951) 258–291. [22] A.M. Strack, W.B. Sawyer, K.B. Platt, A.D. Loewy, CNS cell groups [7] Z.J. Gieroba, L. MacKenzie, J.O. Willoughby, W.W. Blessing, Fos- regulating the sympathetic outflow to adrenal gland as revealed by determined distribution of neurons activated during the Bezold- transneuronal cell body labeling with pseudorabies virus, Brain Res. Jarisch reflex in the medulla oblongata in conscious rabbits and rats, 491 (1989) 274–296.
Brain Res. 683 (1995) 43–50. [23] M.D. Thames, Impaired responses of sympathetic nerves to cardiac [8] R. Hainsworth, Reflexes from the heart, Physiol. Rev. 71 (1991) receptor stimulation in hypertension, Hypertension 9 (1987) 478–
617–658. 484.
[9] S. Higuchi, D.A. Morgan, A.L. Mark, Contrasting reflex effects of [24] P. Thoren, E. Noresson, S.E. Ricksten, Cardiac receptors with chemosensitive and mechanosensitive vagal afferents, Hypertension non-medullated vagal afferents in the rat, Acta Physiol. Scand. 105
11 (1988) 674–679. (1979) 295–303.
[10] M.A. Holzwarth, L.A. Cunningham, N. Kleitman, The role of [25] C. Vayssettes-Courchay, F. Bouysset, M. Laubie, T.J. Verbeuren, adrenal nerves in the regulation of adrenocortical functions, Ann. Central integration of the Bezold-Jarisch reflex in the cat, Brain Res. NY Acad. Sci. 512 (1987) 449–464. 744 (1997) 272–278.
[11] A. Jarisch, Y. Zotterman, Depressor reflex from the heart, Acta [26] R. Veelken, L.L. Sawin, G.F. DiBona, Epicardial serotonin receptors Physiol. Scand. 16 (1948) 31–351. in circulatory control in conscious Sprague–Dawley rats, Am. J. [12] M. Kalia, M.M. Mesulam, Brain stem projections of sensory and Physiol. 258 (1990) H466–472.
motor components of the vagus complex in the cat: I. The cervical [27] A.J. Verberne, P.M. Beart, W.J. Louis, Excitatory amino acid vagus and nodose ganglion, J. Comp. Neurol. 193 (1980) 435–465. receptors in the caudal ventrolateral medulla mediate a vagal [13] W. Karczewski, J.G. Widdicombe, The role of the vagus nerves in cardiopulmonary reflex in the rat, Exp. Brain Res. 78 (1989)
the respiratory and circulatory responses to intravenous histamine 185–192.
and phenyl diguanide in rabbits, J. Physiol. (Lond.) 201 (1969) [28] A.J. Verberne, P.G. Guyenet, Medullary pathway of the Bezold-271–291. Jarisch reflex in the rat, Am. J. Physiol. 263 (1992) R1195–1202. [14] O. Krayer, The history of the Bezold-Jarisch effect, Naunyn- [29] A. Zagon, A.D. Smith, Monosynaptic projections from the rostral
Schmiedeberg’s Arch. Exp. Pathol. Pharmacol. 240 (1961) 361– ventrolateral medulla oblongata to identified sympathetic
pregan-368. glionic neurons, Neuroscience 54 (1993) 729–743.