www.elsevier.com / locate / bres
Short communication
Retinal dopaminergic neurons (A17) expressing neuromedin K
receptor (NK ): a double immunocytochemical study in the rat
3a ,
*
b a a a cLiang-Wei Chen
, Li-Chun Wei , Hui-Ling Liu , Li Duan , Gong Ju , Ying-Sheng Chan
a
Institute of Neuroscience, The Fourth Military Medical University, Xi’an, 710032, People’s Republic of China b
Department of Radiotherapy, Xijing Hospital, The Fourth Military Medical University, Xi’an, 710032, People’s Republic of China c
Department of Physiology, The University of Hong Kong, Hong Kong, People’s Republic of China
Accepted 12 September 2000
Abstract
By using a double immunofluorescence method we examined the distribution of dopaminergic neurons (A17) expressing neuromedin K receptor (NKR, NK ) in the rat retina. The distribution of NKR-like immunoreactive (-LI) neurons partially overlapped that of tyrosine3 hydroxylase (TH)-LI neurons in the inner retina of section and flat-mount preparation. Neurons showing both TH- and NKR-like immunoreactivities were found in the retina (A17): 100% of these TH-LI neurons displayed NKR-like immunoreactivity, and they constituted about 3.5% of total NKR-LI neurons. The majority of double-labeled neurons with TH- and NKR-like immunoreactivities were distributed in the proximal inner nuclear layer and the upper part of inner plexiform layer of the retina, and characterized with appearance of amacrine cells. The present study has provided morphological evidence for direct physiological modulation of dopaminergic neurons by tachykinins through NKR in the rat retina (A17). 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Peptide receptor structure and function
Keywords: Neuromedin K receptor; Dopaminergic neuron; Co-localization; Immunocytochemistry; Retina; Rat
It is well documented that dopaminergic neurons are information processing. The A17 dopamine neurons sub-widely distributed in the mammalian brain, which include serve their biological functions through influencing the the retrorubral field (A8), substantia nigra (A9), the ventral activity of retinal amacrine, bipolar and ganglion neurons tegmental area and nucleus raphe linealis (A10), caudal [2,8,12,44–47].
hypothalamic periventricular nucleus (A11), arcuate nu- The mammalian tachykinin family, including substance cleus (A12), zona incerta (A13), rostral hypothalamic P (SP), substance K (SK; neurokinin A) and neuromedin K periventricular nucleus (A14, A15), olfactory bulb (A16) (NK; neurokinin B), is well known to act on neurokinin [12]. In addition, the dopamine-containing neurons (A17) receptors subtypes to induce their physiological effects, are also found in inner retinas of the rat, rabbit, cat, e.g. neurotransmission, neuromodulation and growth regu-guinea-pig, bovine, monkey and humans lation in the peripheral and central nervous system [5,16,19,25,27,28,33,34,36,40,41,44,46,47]. Majority of [1,14,17,18,22,24,31,32,35,38,43]. Recent evidence has A17 dopamine neurons are morphological amacrine cells shown that dopaminergic neurons may be regulated by and distribute in inner nuclear layer or inner plexiform tachykinins in the mammals [8,17]. Morphological studies layer of the retina. Previous studies have shown that have found that tachykinins (SP or NK)-containing neu-A8–A16 dopamine neurons are involved in motor control, rons are abundantly distributed in retina cardiovascular, respiratory and olfactory activity, while [3,7,10,11,20,22,23,29,37]. Electrophysiological or phar-retinal A17 dopamine neurons play important role in visual macological evidence has indicated that tachykinins or tachykinin-related peptides are involved in the visual information processing and has modulating influence on
*Corresponding author. Fax:186-29-324-6270.
E-mail address: [email protected] (L.-W. Chen). retinal dopamine neurons [11,13,15,29,42,48]. For
ple, tachykinin (SP) has potent effects on the excitatory scopy (BX-60). Blue or green filter was utilized to activity of retinal neurons [13,15], and elicits visualize DTAF-labeled neurons or TRITC-labeled
neu-3
[ H]dopamine release in the rat retina [42]. Thus, it is rons, respectively.
possible that dopamine neurons in retina may express For the control experiments, the primary antibody was tachykinin receptors. On the other hand, in situ hybridiza- replaced with normal mouse serum (for TH immuno-tion and immunohistochemical studies indicted that both cytochemistry) or normal rabbit serum (for NKR immuno-substance P receptor (SPR, NK ) and neuromedin K1 cytochemistry): No tyrosine hydroxylase-like immuno-receptor (NKR, NK ) are abundantly distributed in the3
retinas of the mammals, whereas neurokinin A receptor (NK ) is hardly detected [4,6,21,26,39]. Though the2
existence of SPR in a population of dopamine neurons was reported [6], it is still unknown whether retinal dopamine neurons express NKR. Hence, in the present study we examined the distributions of dopamine neurons expressing NKR in retina (A17) of the adult rat.
Eight adult male rats (Sprague–Dawley) weighing 220– 250 g were used in the present study. All animal experi-ment protocols conformed the National Institute of Health guide for the care and use of Laboratory animals (NIH Publications No. 80-23), and have been approved by the Committee of Animal Use for Research and Education of the People’s Republic of China. The animals were anes-thetized with an over-dose of sodium pentobarbital (100 mg / kg, i.p.), and then perfused transcardially with a volume of 100 ml of saline. The eyeballs were removed, and the anterior segments were cut off, and posterior eyecups containing the retinas were picked up. Then the eyecups of four rats were immersed in 4% paraformal-dehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 30 min at 48C. After fixation, the retinas were placed overnight in 0.1 M PB containing 30% sucrose at 48C. Dissected retinas were serially cut perpendicular to the vitreal surface at 10
mm with a cryostat, and mounted onto gelatin-coated slides. The remaining eyecups were performed for flat-mount retina preparations. Retinas was gently separated from the eyecups with a writing brush and immersed in 4% paraformaldehyde in 0.1 M PB for 30 min, and followed by wash in phosphate buffered saline (PBS, pH 7.4).
The sections and flat-mount retinas were incubated for 48 h at 48C with a mixture of mouse anti-tyrosine hydroxylase (TH) IgG (sigma; 1:1000 dilution) and 0.5
mg / ml of affinity-purified rabbit anti-NKR IgG in 0.01 M PBS (pH 7.4) containing 1% normal donkey serum and 0.1% Triton X-100. The rabbit anti-NKR serum was generated against a fusion protein containing NKR 388-452, and characterized by Western blotting [14]. Sub-sequently, the sections were rinsed in 0.01 M PBS (pH 7.4), and then incubated for 24 h at 48C with a mixture of
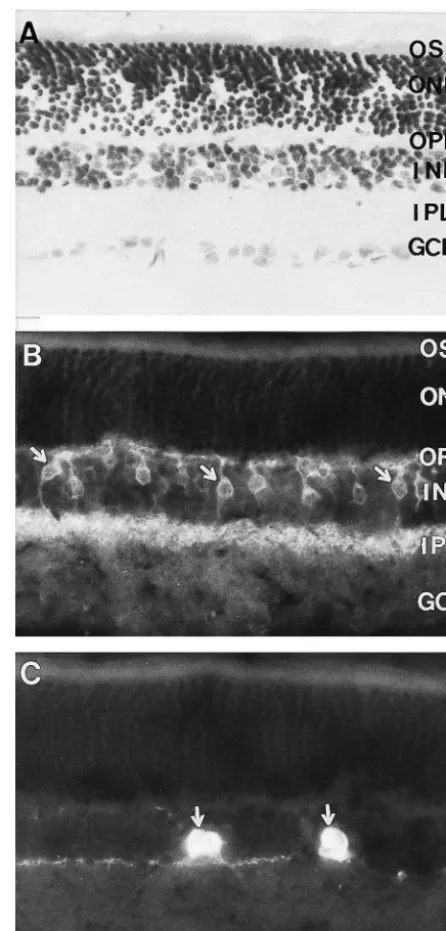
dichorotriazinylamino-fluorescein (DTAF)-conjugated Fig. 1. Photomicrographs showing the laminar structure and localization donkey anti-mouse IgG (Chemicon, 1:100 dilution) and of TH-or NKR-like immunoreactivities in rat retina. (A) The
photo-tetramethyl rhodamine isothiocyanate (TRITC)-conjugated receptor outer segment (OS), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL) and
donkey anti-rabbit IgG (Chemicon, 1:100 dilution). Afer
ganglion cell layer (GCL) are seen in the Nissl-stained section. (B)
being washed, the sections were mounted on gelatin-coated
NKR-like immunoreactivity is seen in retina, and NKR-LI bipolar cells
glass slides, and coverslipped in 0.01 M PBS (pH 7.4) are indicted by arrows. (C) TH-like immunoreactivity is shown in retina, containing 50% glycerin and 2.5% triethylenediamine, and and TH-LI amcrine cells in proximal INL are indicated by arrows. Scale
reactive (TH-LI), or NKR-like immunoreactive (NKR-LI) immunoreactivities were abundantly distributed in INL, neurons were found in the control sections. OPL and upper part of IPL, whereas TH- or NKR-like The laminar structures of retina, consisting of the immunoreactivities are hardly detected in OS, ONL and photoreceptor outer segment (OS), outer nuclear layer GCL of retina (Fig. 1B,C). The distribution of TH-LI or (ONL), outer plexiform layer (OPL), inner nuclear layer NKR-LI neurons was well consistent with that of previous (INL), inner plexiform layer (IPL) and ganglion cell layer reports [4,19,27,28,33,39–41]. Generally, NKR-LI neurons (GCL), were clearly identified in the Nissl-stained sections were more widely and densely distributed that TH-LI (Fig. 1A). neurons in retina. Majority of NKR-LI neurons, character-Both TH-LI and NKR-LI neurons were found in the ized with morphology of bipolar and amacrine cells, were dually immunostained sections. The TH- or NKR-like distributed in the INL and IPL. Dense NKR-LI neuronal
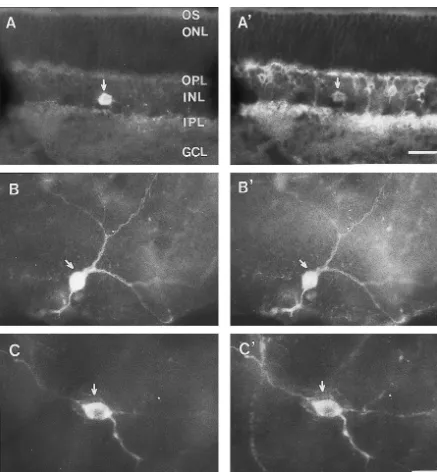
processes were seen in the OPL and upper part of IPL soon as the day of birth, while the large stellate cells (Fig. 1B). TH-LI neurons, with the morphological appear- appeared by postnatal day 7 and gradually reached adult-ance of amacrine cellls, were scattered in the proximal INL retina level afterwards. Peripheral crescents containing a and IPL. TH-LI neuronal processes were sparsely distribut- high density of small amacrine cells which disappear ed in OPL and the upper part of IPL as well (Fig. 1C). proportionately as the number of large amacrine cells TH-LI neurons partially overlapped with NKR-LI neu- increases [5,28]. Several arguments are put forward to rons in the section or flat-mount of retina. These neurons support this hypothesis or conclusion that small unipolar showing both TH- and NKR-like immunoreactivies were amacrine cells are transformed into large stellate amacrine scattered in the proximal INL and the upper part of IPL cells as they mature [5,19,28,41].
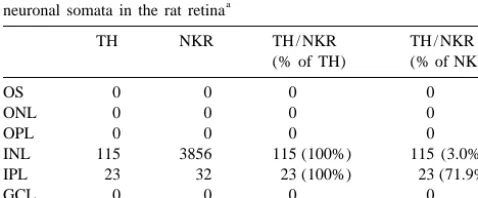
(Fig. 2A and A9, B and B9, C and C9). It was found that all Our previous study has confirmed that the A8–A10 (100%) these TH-LI neurons expressed NKR-like im- dopaminergic neurons expressed NKR in the midbrain [8], munoreactivity. On the other hand, these double-labeled while A5-7 nordrenalinergic neurons express SPR in the neurons constitute about 3.5% of total NKR-LI neurons locus coeruleus complex of the rat [9]. Casini et al. has (Table 1). Furthermore, all NKR-LI amacrine cells dis- found that a population (71–100%) of dopamine neurons played TH-like immunoreactivity. Majority of the double- shows SPR-like immunoreactivity in the rat ratina [6], labeled neuron somata were oval or piriform in shape and suggesting that large percentage of retinal dopaminergic medium or large-sized with their diameters ranged from 10 neurons receive the modulation of substance P. Our present to 15 mm. In addition, a few of small-sized double-labeled study has further revealed that all the retinal A17 dopa-neuron somata were also identified and occasionally mine neurons express NKR in the adult rat. On the basis of observed, but they displayed faint TH-LI immunoreactivi- these findings, it can be speculated that A17 dopaminergic ty. No double-labeled neuronal somata, however, were neurons may be directly modulated by tachykinins in the found in OS, OPL, ONL and GCL of the rat retina. rat retina via NKR in addition to SPR.
The distribution of neurons containing TH and NKR in Evidence has also indicated that the dopamine neurons the retinas has been examined in mammals in the previous are modulated by tachykinins in retina, and most studies studies [4,5,16,19,25,28,33,34,36,39–41,44,46]. The pres- on this subject concentrated on SP. For example, SP- and ent results were well consistent with these reports, and SPR-containing neurons were densely distributed in retinas further indicated that all the dopaminergic neurons in A17 [3,4,6,10,11,21,23,37,39]. The endogenous SP was found expressed NKR. in amacrine cells, while SPR was not detected in these It is well known that majority of retinal dopamine SP-containing amacrine cells. SPR-LI amacrine cells, neurons are morphologic amacrine cells and play important however, may receive synapses from SP-containing amac-role in visual information processing rine cells [11,20,22,45]. Electophysiological and pharma-[2,5,16,19,25,28,34,36,44,46,47]. It is necessary to point cological studies have showed that SP affects the excitat-out that the majority of TH-LI dopamine neurons are ory activity of retinal neurons and elicits dopamine release medium or large-sized amacrine cells characterized with an in retina [13,15,42,48]. Our present double-immunofluores-appearance of stellate cells, while small-sized amacrine cence study has revealed that A17 dopaminergic neurons cells are rarely seen in the present study, and this observa- express NKR. Few studies have dealt with the NK-NKR in tion is consistent with previous studies [5,19,28,41]. the physiological function of retinal dopaminergic system. Evidence has suggested that small amacrine cells pref- Evidence suggested that tachykinins stimulate both phos-erentially distribute in the developmental retinas. For phatidylinositol hydrolysis and cyclic AMP intracellular instance, it is found that small unipolar cells appeared as signal cascades in transfected Chinese Hamster ovary cells via three types of tachykinin receptors [17,30]. It is also well known that tachykinin receptors (SPR and NKR)
Table 1
share a high degree of sequence homology and signal
Comparison of TH single-, NKR single-, and TH / NKR double-labeled a
neuronal somata in the rat retina transduction mechanism [17,18,24]. Therefore, it is
pre-sumably that ligand cross-interaction might occur between
TH NKR TH / NKR TH / NKR
(% of TH) (% of NKR) tachykinin (SP and NK) and tachykinin receptors (SPR and NKR) in the retina. Further studies are still needed to
OS 0 0 0 0
elucidate the tachykinin receptors (NKR and SPR)
interact-ONL 0 0 0 0
OPL 0 0 0 0 ing with dopaminergic neurons and their role in visual INL 115 3856 115 (100%) 115 (3.0%) physiology and pathophysiology of the mammalian retina.
IPL 23 32 23 (100%) 23 (71.9%)
GCL 0 0 0 0
Total 138 3888 138 (100%) 138 (3.5%)
Acknowledgements
a
The neurons were counted on 10 sections from a representative rat. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; IPL,
The authors are grateful to Dr Shigemoto, Department
inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform
[19] S. Kato, T. Nakamura, K. Negishi, Postnatal development of
Kyoto University, Japan and Dr Y.-Q. Ding for their gift of
dopaminergic cells in the rat retina, J. Comp. Neurol. 191 (1980)
antiserum to NKR. The authors are also grateful to Dr
27–36.
J.-W. Zhao for whole-mount retina preparations. This work [20] H. Kolb, E. Fernandez, J. Ammermuller, N. Cuenca, Substance P: a was supported by a grant from the National Natural neurotransmitter of amacrine and ganglion cells in the vertebrate
Science Foundation of China (30040012). retina, Histol. Histopathol. 10 (1995) 947–968.
[21] A. Kondoh, T. Houtani, T. Ueyama, K. Baba, M. Ikeda, K. Yamagishi, H. Miki, M. Uyama, S. Nakanishi, T. Sugimoto, In situ hybridization analysis of substance P receptor in the rat retina, Exp.
References Brain Res. 112 (1996) 181–186.
[22] M.Y. Lee, M.H. Chun, S.H. Han, S.J. Oh, J.W. Chung, Light- and electron-microscopic study of substance P-immunoreactive neurons [1] H. Arai, P.C. Emson, Regional distribution of neuropeptide K and
in the guinea pig retina, Cell Tissue Res. 281 (1995) 261–271. other tachykinins (neurokinin A, neurokinin B and substance P) in
[23] H.B. Li, K.F. So, W. Cheuk, Substance P-immunoreactive neurons in rat central nervous system, Brain Res. 399 (1986) 240–249.
hamster retinas, Vis. Neurosci. 16 (1999) 475–481. [2] B. Bjelke, M. Goldstein, B. Tinner, C. Andersson, S.R. Sesack, H.W.
[24] H. Liu, J.L. Brown, L. Jasmin, J.E. Maggio, S.R. Vigna, P.W. Steinbusch, J.Y. Lew, X. He, S. Watson, B. Tengroth, K. Fuxe,
Mantyh, A.I. Bausbaum, Synaptic relationship between substance P Dopaminergic transmission in the rat retina: evidence for volume
and the substance P receptor: light and electron microscopic transmission, J. Chem. Neuroanat. 12 (1996) 37–50.
characterization of the mismatch between neuropeptide and their [3] N.C. Brecha, C. Sternini, K. Anderson, J.E. Krause, Expression and
receptors, Proc. Natl. Acad. Sci. USA 91 (1994) 1009–1013. cellular localization of substance P/ neurokinin A and neurokinin B
[25] C.M. Main, M. Wilhelm, R. Gabriel, Colocalization of GABA-mRNAs in the rat retina, Vis. Neurosci. 3 (1989) 527–535.
immunoreactivity in neuropeptide- and monoamine-containing [4] G. Casini, N.C. Brecha, L. Bosco, D.W. Rickman, Developmental
amacrine cells in the retina of Bufo marinus, Arch. Histol. Cytol. 56 expression of neurokinin-1 and neurokinin-3 receptors in the rat
(1993) 161–166. retina, J. Comp. Neurol. 421 (2000) 275–287.
[26] P.W. Mantyh, T. Gates, C.R. Mantyh, J.E. Maggio, Autoradiographic [5] G. Casini, N.C. Brecha, Postnatal development of tyrosine
hydroxy-localization and characterization of tachykinin receptor binding sites lase immunoreactive amacrine cells in the rabbit retina: I.
Mor-in the rat braMor-in and peripheral tissues, J. Neurosci. 9 (1989) phological characterization, J. Comp. Neurol. 326 (1992) 283–301.
258–279. [6] G. Casini, D.W. Rickman, C. Sternini, N.C. Brecha, Neurokinin 1
[27] E. Martin-Martinelli, C. Savy, J. Nguyen-Legros, Morphometry and receptor expression in the rat retina, J. Comp. Neurol. 389 (1997)
distribution of displaced dopaminergic cells in rat retina, Brain Res. 496–507.
Bull. 34 (1994) 467–482. [7] G. Casini, L. Trasarti, L. Andolfi, P. Bagnoli, Morphologic
matura-[28] E. Martin-Martinelli, A. Simon, A. Vigny, J. Nguyen-Legros, tion of tachykinin peptide-expressing cells in the postnatal rabbit
Postnatal development of tyrosine-hydroxylase-immunoreactive retina, Brain Res. Dev. Brain Res. 99 (1997) 131–141.
cells in the rat retina. Morphology and distribution, Dev. Neurosci. [8] L.-W. Chen, Z.-L. Guan, Y.-Q. Ding, Mesencephalic dopaminergic
11 (1989) 11–25. neurons expressing neuromedin K receptor (NK3): a double
im-[29] T.J. Millar, I.W. Chubb, Substance P in the chick retina: effects of munocytochemical study in the rat, Brain Res. 780 (1998) 150–154.
light and dark, Brain Res. 307 (1984) 303–309. [9] L.-W. Chen, L.-C. Wei, H.-L. Liu, Z.-R. Rao, Noradrenergic neurons
[30] Y. Nakajima, K. Tsuchida, M. Negishi, S. Ito, S. Nakanishi, Direct expressing substance P receptor (NK1) in the locus coeruleus
linkage of three tachykinin receptors to stimulation of both phospha-complex: a double immunofluorescence study in the rat, Brain Res.
tidylinositol hydrolysis and cyclic AMP cascades in transfected 873 (2000) 155–159.
Chinese hamster ovary cells, J. Biol. Chem. 267 (1992) 2437–2442. [10] N. Cuenca, J. De Juan, H. Kolb, Substance P-immunoreactive
[31] S. Nakanishi, Mammalian tachykinin receptors, Annu. Rev. Neuro-neurons in the human retina, J. Comp. Neurol. 356 (1995) 491–504.
sci. 14 (1991) 123–136. [11] N. Cuenca, H. Kolb, Circuitry and role of substance
P-immuno-[32] Y. Nakaya, T. Kaneko, R. Shigemoto, S. Nakanishi, N. Mizuno, reactive neurons in the primate retina, J. Comp. Neurol. 393 (1998)
Immunohistochemical localization of substance P receptor in the 439–456.
central nervous system of the adult rat, J. Comp. Neurol. 347 (1994) ¨
[12] A. Dahlstrom, K. Fuxe, Evidence for existence of monoamine
249–274. containing neurons in the central nervous system I. Demonstration
[33] J. Nguyen-Legros, B. Berger, A. Vigny, C. Alvarez, TH-like of monoamines in cell bodies of the brain stem neurons, Acta
immunoreactive interplexform cells in the rat retina, Neurosci. Lett. Physiol. Scand. 232 (1964) 1–55.
27 (1981) 255–259. [13] E. Dick, R.F. Miller, Peptides influence retinal ganglion cells,
[34] J. Nguyen-Legros, C. Botteri, L.H. Phuc, A. Vigny, M. Gay, Neurosci. Lett. 26 (1981) 131–135.
Morphology of primate’s dopaminergic amacrine cells as revealed [14] Y.-Q. Ding, R. Shigemoto, M. Takada, H. Ohishi, S. Nakanishi, N.
by TH-like immunoreactivity on retinal flat-mounts, Brain Res. 295 Mizuno, Localization of the neuromedin K receptor (NK3) in the
(1984) 145–153. central nervous system of the rat, J. Comp. Neurol. 364 (1996)
[35] J. Nilsson, A.M. von Euler, C.J. Dalsgaard, Stimulation of connec-290–310.
tive tissue cell growth by substance P and substance K, Nature 315 [15] M.B.A. Djamgoz, J.E.G. Downling, D.J. Prince, Physiology of
(1985) 61–63. neuroactive peptide in the vertebrate retina, Biochem. Soc. Trans. 11
[36] S.J. Oh, I.B. Kim, E.J. Lee, K.Y. Kim, H.I. Kim, M.H. Chun, (1983) 686–689.
Immunocytological localization of dopamine in the guinea pig [16] J.M. Frederick, M.E. Rayborn, A.M. Laties, D.M. Lam, J.G.
retina, Cell Tissue Res. 298 (1999) 561–565. Hollyfield, Dopaminergic neurons in the human retina, J. Comp.
[37] N.N. Osborne, Substance P in the bovine retina: localization, Neurol. 210 (1982) 65–79.
identification, release, uptake and receptor analysis, J. Physiol. [17] C.J. Helke, J.E. Kraus, P.W. Mantyh, R. Couture, M.J. Bannon,
(Lond.) 349 (1984) 83–93. Diversity in mammalian tachykinin peptidergic neurons: multiple
[38] M. Otsuka, K. Yoshika, Neurotransmitter functions of mamamlian peptides, receptors, and regulatory mechanisms, Fed. Proc. Fed. Am.
tachykinins, Physiol. Rev. 73 (1993) 229–308. Soc. Exp. Biol. 4 (1990) 1606–1614.
[39] H. Oyamada, K. Takatsuji, E. Senba, P.W. Mantyh, M. Tohyama, [18] A.M. Khawaja, D.F. Rogers, Tachykinin: receptor to effector, Int. J.
expression in the rat retina, Brain Res. Dev. Brain Res. 117 (1999) tribution and quantitation of the mRNAs for three tachykinin
59–70. receptors, Eur. J. Biochem. 193 (1990) 751–757.
[40] C. Savy, F. Moussafi, J. Durand, J. Yelnik, A. Simon, J. Nguyen- [44] C. Versaux-Botteri, E. Martin-Martinelli, J. Nyuyen-Legros, M. Legros, Distribution and spatial geometry of dopamine interplex- Geffard, A. Vigny, L. Denoroy, Regional specialization of the retina: iform cells in the retina II. External arborizations in the adult rat and catecholamine-containing amacrine cell characterization and dis-monkey, J. Comp. Neurol. 355 (1995) 392–404. tribution, J. Comp. Neurol. 243 (1986) 422–433.
[41] C. Savy, J. Yelnik, E. Martin-Martinelli, I. Karpouzas, J. Nguyen- [45] H. Wassle, B.B. Boycott, Functional architecture of the mammalian¨ Legros, Distribution and spatial geometry of dopamine interplex- retina, Physiol. Rev. 71 (1991) 447–480.
iform cells in the rat retina: I. Developing retina, J. Comp. Neurol. [46] H. Wassle, M.H. Chun, Dopaminergic and indolamine-accumulating¨ 289 (1989) 99–110. amacrine cells express GABA-like immunoreactivity in the cat [42] D. Tsang, Effects of substance P on dopamine release in rat retina, retina, J. Neurosci. 7 (1988) 3383–3393.
in: O.L. Kon, M.C.M. Chung, P.L.H. Hwang, S.F. Leong, K.H. [47] L.L. Wright, D.I. Vaney, The fountain amacrine cells of the rabbit Loke, P. Thiayagarjah, P.H.T. Wong (Eds.), Contemporary Themes retina, Vis. Neurosci. 16 (1999) 1145–1156.
in Biochemistry, Cambridge University Press, Cambridge, MA, [48] R.A. Zalutsky, R.F. Miller, The physiology of substance P in the 1986, pp. 588–589. rabbit retina, J. Neurosci. 10 (1990) 394–402.