www.elsevier.com/locate/ibmb
Common functional elements of Drosophila melanogaster seminal
peptides involved in reproduction of Drosophila melanogaster and
Helicoverpa armigera females
Yongliang Fan
a, Ada Rafaeli
b, Pnina Moshitzky
a, Eric Kubli
c, Yves Choffat
c,
Shalom W. Applebaum
a,*aDepartment of Entomology, The Hebrew University, P.O. Box 12, Rehovot 76100, Israel bDepartment of Stored Products, Volcani Center, P.O. Box 6, Bet Dagan 50250, Israel cZoological Institute, University of Zu¨rich-Irchel, Winterthurerstrasse 190, CH-8057, Zu¨rich, Switzerland
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
Sex peptide (SP) and Ductus ejaculatorius peptide (Dup) 99B are synthesized in the retrogonadal complex of adult male
Droso-phila melanogaster, and are transferred in the male seminal fluid to the female genital tract during mating. They have been sequenced
and shown to exhibit a high degree of homology in the C-terminal region. Both affect subsequent mating and oviposition by female
D. melanogaster. SP also increases in vitro juvenile hormone (JH) biosynthesis in excised corpora allata (CA) of D. melanogaster
and Helicoverpa armigera. We herein report that the partial C-terminal peptides SP8–36 and SP21–36of D. melanogaster, and the
truncated N-terminal SP6–20 do not stimulate JH biosynthesis in vitro in CA of both species. Both of these C-terminal peptides
reduce JH-III biosynthesis significantly. Dup99B, with no appreciable homology to SP in the N-terminal region, similarly lacks an effect on JH production by H. armigera CA. In contrast, the N-terminal peptides — SP1–11and SP1–22— do significantly activate
JH biosynthesis of both species in vitro. We conclude that the first five N-terminal amino acid residues at the least, are essential for allatal stimulation in these disparate insect species. We have previously shown that the full-length SP1–36depresses pheromone
biosynthesis in H. armigera in vivo and in vitro. We now show that full-length Dup99B and the C-terminal partial sequence SP8– 36at low concentrations strongly depress (in the range of 90% inhibition) PBAN-stimulated pheromone biosynthesis of H. armigera.
In addition, the N-terminal peptide SP1–22, the shorter N-terminal peptide SP1–11and the truncated N-terminal SP6–20strongly inhibit
pheromone biosynthesis at higher concentrations. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Sex peptide; Dup99B; JH biosynthesis; PBAN; Pheromone production; Calling
1. Introduction
Reproductive maturation in female adult insects is composed of developmental and behavioral components. In many cases, newly-eclosed adult female insects bear
underdeveloped ovaries. Ovarial maturation then
requires yolk protein synthesis and its subsequent uptake into the developing oocytes. Both synthesis and uptake are apparently regulated in Lepidoptera by JH alone. This is concluded from studies of several noctuid moths
* Corresponding author. Tel.: +972-8-9489154; fax: + 972-8-9468586.
E-mail address: [email protected] (S.W. Applebaum).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 5 2 - 7
belonging to the genera Helicoverpa, Heliothis and
Pseudaletia (Ramaswamy et al., 1990; Ramaswamy and
homolog produced by the CA in H. armigera, may play a primer role in the initiation of sex pheromone pro-duction by newly emerged adult females.
During mating, seminal fluid, containing a variety of peptides and proteins derived from the male retrogonadal complex, is transferred to the female genital tract (Chen, 1984, 1991). In D. melanogaster, post-mating responses have been shown hitherto to be induced by three compo-nents: sex peptide (SP), derived from the male accessory glands; Dup99B, derived from the male ejaculatory duct; and a high molecular weight protein, Acp28Aa. SP and Dup99B induce female non-receptivity and ovulation by female D. melanogaster (Chen et al., 1988; Saudan, Hauck and Kubli, unpublished). Acp28Aa stimulates oviposition (Herndon and Wolfner, 1995). SP and Dup99B have been sequenced, synthesized and charac-terized. They exhibit a high degree of homology in the C-terminal region (Chen et al., 1988; Schmidt et al., 1993; Saudan, Hauck and Kubli, unpublished). The sequence of Acp28Aa has been determined. It has a region of sequence similarity to the egg-laying hormone of Aplysia (Herndon and Wolfner, 1995) and is proteo-lytically processed in the female genital tract (Park and Wolfner, 1995). In accessory glands of male H. zea moths, a 57 amino acid peptide has been identified and proposed as a suppressive factor of female sex phero-mone production (Kingan et al., 1995).
We have previously shown that D. melanogaster SP stimulates the biosynthesis of juvenile hormone (JH) in vitro by the corpora allata (CA) of the adult D.
mel-anogaster female and of the adult noctuid moth Helicov-erpa armigera female (Fan et al., 1999a). SP also
acti-vates vitellogenin uptake by maturing oocytes in D.
melanogaster (Soller et al., 1999). Mated females bear
more oocytes of the vitellogenic stage 10 in their ovaries than can be found in virgin females. Injection of SP into virgin females elicits similar oocyte maturation, as does application of the juvenile hormone analogue methop-rene. Taken together, these observations appear to be consistent with a primer effect of SP on allatal matu-ration and of JH-mediated oocyte matumatu-ration.
This present report deals with the functional elements of the D. melanogaster SP and Dup99B, requisite for regulation of allatal activity in these two disparate insect species and for pheromone production and calling behavior in H. armigera.
2. Materials and methods
2.1. Insect culture
Larvae of the noctuid moth H. armigera were reared on an artificial diet (Rafaeli and Soroker, 1989) in the
laboratory under a constant temperature of 26°C, 80%
relative humidity and a 14 h/10 h (light/dark)
non-diapause photoperiod. Pupae were sexed and males and females were allowed to emerge separately. Emerging moths were fed with 10% sugar water. D. melanogaster wild-type Canton-S strain was reared on a standard diet at 25°C. Adults were collected at eclosion, and separated by sex.
2.2. D. melanogaster male retrogonadal synthetic peptides and partial sequences
The partial SP sequences of the D. melanogaster male retrogonadal peptides evaluated in this present study were synthesized in the facilities of the Zoological Insti-tute, University of Zu¨rich-Irchel. They fall into two cat-egories: those with truncated N-terminus, and those with intact N-terminus, but lacking the major part of the C-terminal residues (Fig. 1).
2.3. JH biosynthesis — in vitro radiochemical assay (RCA)
The RCA for JH biosynthesis in vitro of adult virgin female H. armigera CA was adapted from Pratt and Tobe (1974) as described by Fan et al. (1999a,b). Briefly, pairs of excised CA were preincubated for 1 h in meth-ionine-free physiological medium supplemented with
Ficoll 400 and a JH-esterase inhibitor (Hammock et
al., 1984), and incubated for an additional 2 h in medium
supplemented with 5 µCi of L-[3H-methyl]-methionine
(specific activity of 80 Ci/mmol, final concentration in
the medium 1.2µM), in the absence or presence of
syn-thetic D. melanogaster peptides or partial sequences (Fig. 1). At the end of the incubation period, the medium and tissue together were extracted with 150 µl ice-cold
hexane. A 50 µl aliquot of hexane-soluble extract was
evaporated under N2and the radiolabeled methyl moiety
incorporated into total hexane-soluble extract was
coun-ted using a b-counter (LKB).
The RCA for JH biosynthesis in vitro of adult virgin female D. melanogaster CA was modified from Pratt and Tobe (1974) and is based on the incorporation of the
methyl moiety of [3
H-methyl]-methionine into JHB3
(Richard et al., 1989; Altaratz et al., 1991). In this case, preincubation was not performed, and the excised D.
melanogaster CA were incubated for 2–4 h.
2.4. TLC for separation of JHB3
Aliquots of extracts were chromatographed on silica
gel plates (Polygram SIL G/UV254; Macherey-Nagel).
JHB3was separated from JH-III and other JH homologs
by development of the plates in hexane:ethyl acetate (2:1), identified by short UV fluorescence of marker
compounds (Rf=0.37) and counted using a b-counter.
Fig. 1. Sequences of SP, Dup99B and partial SP sequences.
2.5. Reversed phase C18HPLC
Chromatography was performed as previously
described (Fan et al., 1999b). Briefly, evaporated extracts of H. armigera CA incubation media were re-suspended in 40% acetonitrile and chromatographed on
a 5 mm LiChrospher Merck C18 reversed phase HPLC
column (Darmstadt, Germany) (100 A˚ pore, diameter: 4
mm×12.5 cm) using a linear gradient of
acetonitrile/water (40–80%) at a flow rate of 1 ml/min for 40 min. Markers were monitored in the eluant at 220
nm. One ml fractions were collected and 3H-labeled
compounds counted using ab-counter (LKB). Synthetic
JH-I and JH-II were kindly provided by Dr Zdenek Wimmer, of the Institute of Organic Chemistry and Bio-chemistry, Prague, The Czech Republic. JH-III was pur-chased from Sigma. All three non-radioactive JHs were used as standards to identify radioactively-labeled JHs produced in vitro by H. armigera CA. The major JH homolog produced in vitro by H. armigera CA is JH-II (Fan et al., 1999b). Results are herein reported as femto-mol JH-II produced per hour by pairs of female moth CA.
2.6. In vitro bioassay of pheromone production
A radiochemical bioassay was used to monitor de novo pheromone production according to the method of Rafaeli and Gileadi (1995). Briefly, intersegmental membranes (hereafter referred to as pheromone glands) between the eighth and ninth abdominal segments were removed. After 1 h preincubation in Pipes buffered incu-bation medium, pheromone glands were dried on tissue
paper and then transferred individually to 10 µl
incu-bation medium containing 0.25 µCi [1-14C]-acetate (56
mCi/mmole, NEN, Boston, USA) in the presence or absence of Hez-PBAN (Peninsula Labs., Belmont, CA, USA) and in the presence or absence of synthetic D.
melanogaster regulatory peptides or partial sequences
(Fig. 1). Incubations were performed for 3 h at room temperature. In order to measure the incorporation of
[1-14C]-acetate, glands were extracted in 200µl hexane for
0.5 h at room temperature. Radioactivity in a 100 µl
aliquot of the upper hexane phase was determined using
ab-counter. Based on TLC and GC separations, we have
previously shown that the total incorporation levels depict relative levels of incorporation into the phero-mone component where the majority of the label was found to co-elute with the main pheromone component of H. armigera, Z11-hexadecenal (Rafaeli and Gileadi, 1995).
2.7. In vivo pheromone production and calling behavior
In vivo sex pheromone production by female moths was determined in 2-day-old females (D2) which were decapitated during the photophase of D2 and sub-sequently maintained for an additional 24 h, after which they were injected with either physiological saline (control) or 5 pmol/moth PBAN in saline. Full length SP, SP partial sequences, or Dup99B were injected 1 h later. Ovipositor tips were removed 2 h after injection and extracted for 30 min in hexane, containing 25 ng tridecanyl acetate (Sigma) as internal standard. The
hex-ane extract was concentrated to 1–2 µl final volume
under a slow stream of N2 and chromatographed on a
Carlo Erba GC-6000 gas chromatographic system (Italy) using a 30 m SE-54 fused silica capillary column (internal diameter 0.32 mm) (Alltech, USA). The results were analyzed using a Barspec Chrom-A-Set (Israel). The following conditions were used: initial temperature of 120°C was held for 0.4 min then increased to 270°C at 10°C/min, and kept for 15 min at the final tempera-ture. The detector temperature was held at 300°C,
col-umn inlet at 280°C. Helium was used as a carrier at a
1980), was quantified using the internal standard quanti-fication methods as described previously (Soroker and Rafaeli, 1989; Rafaeli, 1994).
“Calling” in H. armigera was defined as the extrusion of the ovipositor and exposure of pheromone-producing abdominal integumental pheromone gland and was monitored by observations in a dark room, using a dim red light as reported previously (Rafaeli and Soroker, 1989).
2.8. Statistics
Differences between treatments were compared by ANOVA.
3. Results
3.1. Effect of Dup99B, SP and partial sequences on JH biosynthesis in vitro
Full-length D. melanogaster SP1–36 stimulates JH
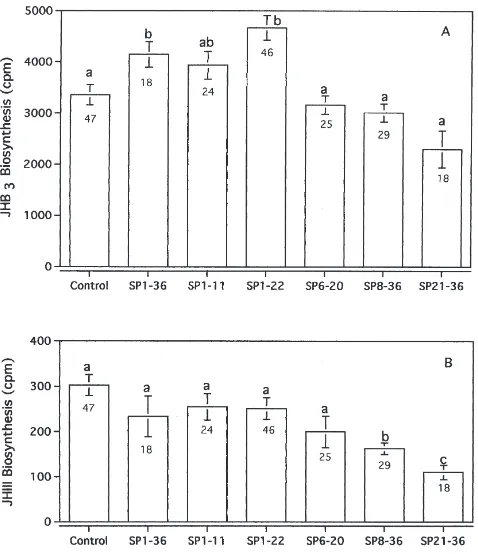
biosynthesis of CA excised from D. melanogaster (Moshitzky et al., 1996) and similarly stimulates the CA of the noctuid moth H. armigera (Fan et al., 1999a). In contrast, truncated N-terminal partial sequences of SP, lacking the first five amino acid residues, do not stimu-late in the D. melanogaster CA system in vitro (Fig. 2).
Fig. 2. The effects of SP, and partial SP sequence peptides on CA activity of D. melanogaster. A: JHB3biosynthesis; B: JH-III
biosynth-esis. Values with different letters indicate significant differences (P<0.05). Numbers below the error bars indicate the number of repli-cates.
SP partial sequences, containing only the full C-terminal, are similarly inactive. This suggests that the first five amino acid residues of SP, at the least, are essential for allatal activation. The longer N-terminal peptide with 22 amino acid residues is more active than the shorter N-terminal peptide with the first 11 N-N-terminal amino acid residues.
JHB3 normally comprises about 95% of the total JH
(Richard et al., 1989). We observed differential effects
on JHB3and JH-III, the two D. melanogaster JH
homo-logs produced in vitro: the full-length SP and SP1–22
sig-nificantly stimulated JHB3 synthesis but had no effect
on JH-III synthesis. The truncated N-terminal sequence SP6–20 had no effect on either JHB3 or JH-III
biosynth-esis. The shorter N-terminal SP1–11 slightly stimulated
JHB3 synthesis but had no effect on JH-III synthesis.
The C-terminal sequences SP8–36and SP21–36had no
sig-nificant effect on JHB3 synthesis, but did significantly
inhibit the synthesis of JH-III.
Similarly, peptides lacking the N-terminal of SP had no effect on JH-II biosynthesis by excised H. armigera
CA (Table 1). The N-terminal peptide SP1–22stimulated
at 10 pmol per incubation medium, whereas a 10-fold higher titer (100 pmol per incubation medium) of the
shorter N-terminal sequence SP1–11 was necessary for
eliciting significant stimulation. The full length Dup99B, with high homology to SP in the C-terminal region, but no real homology in the N-terminal region, was also inefficient in stimulating JH-II biosynthesis, confirming the hypothesis that the N-terminal of SP may be neces-sary for allatal stimulation in the H. armigera system as well.
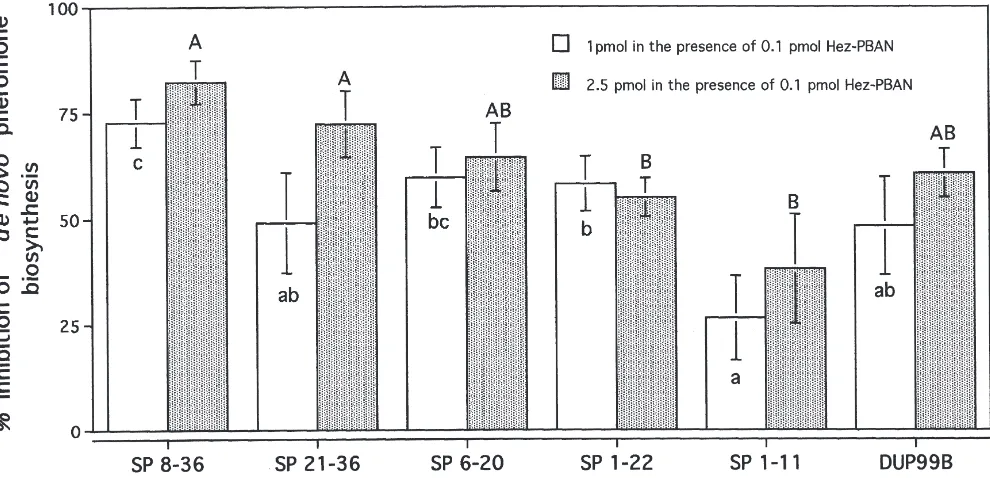
3.2. Inhibition of H. armigera female sex pheromone biosynthesis by SP and partial sequences in vitro
We have previously shown that full length SP depresses pheromone biosynthesis in H. armigera in vivo and in vitro (Fan et al., 1999a). We herein examined the relative contribution of SP partial sequences to the inhibition of PBAN-stimulated pheromone production in vitro.
The C-terminal partial sequence SP8–36, at low titers,
strongly depressed about 80–90% of PBAN-stimulated pheromone biosynthesis of H. armigera. Other partial
peptides depressed PBAN-stimulated pheromone
biosynthesis to different extents (Fig. 3). To attain simi-lar levels of inhibition (72.8±5.7%; n=15) caused by 1 pmole of SP8–36, 10-fold higher amounts (10 pmoles) of
the short N-terminal peptide SP1–11 were found to be
necessary, reaching levels of 78.8±5.9% (n=8) inhibition (data not shown).
3.3. Pheromone biosynthesis and calling in decapitated female moths
Dup99B, SP8–36 and SP21–36 strongly inhibited sex
decapi-Table 1
The effect of Dup99B, SP and partial sequences on in vitro JH-II biosynthesis by excised female adult H. armigera CA
JH-II biosynthesis (cpm/CA)
Expt. no. Treatment 2 h incubation 3 h incubation
I Control 26,526±2245 (n=10a) a
SP (10 pmol) 41,398±2470 (n=9) bb
Dup99B (10 pmol) 27,340±2413 (n=8) a SP21–36(10 pmol) 23,539±5151 (n=4) a
SP8–36(10 pmol) 28,860±1840 (n=9) a
II Control 28,381±3113 (n=8) A
SP6–20(10 pmol) 28,068±1780 (n=8) A
III Control 32,515±2396 (n=9) B 34,639±2388 (n=9) c SP1–11(10 pmol) 30,136±3896 (n=9) B 43,315±3564 (n=9) d
SP1–11(100 pmol) 42,259±1491 (n=9) C 62,707±6869 (n=7) e
IV Control 26,012±5009 (n=7) D 37,783±7172 (n=8) f
SP1–22(10 pmol) 35,016±3580 (n=8) E 66,106±4202 (n=9) g
SP1–22(100 pmol) 41,359±2100 (n=9) F 80,398±10,754 (n=9) h
aNumbers in parentheses indicate the number of replicates.
b Values with different letters indicate significant differences for each experiment separately (P ,0.05).
Fig. 3. The inhibition of H. armigera pheromone production in vitro as a result of SP, Dup99B and partial SP sequence peptides. Values with different letters indicate statistically significant differences (P<0.05).
tated photophase and in intact virgin female moths dur-ing the scotophase. Pheromone production was inhibited by about 60% of control (PBAN-injected only) in both cases (Table 2).
In contrast to its effect on pheromone biosynthesis, even an excessive dose of 10 pmole Dup99B did not significantly affect calling behavior; the percentage of females exhibiting calling behavior was 58% after Dup99B injection, compared to 63% where a saline con-trol injection replaced the Dup99B (Table 2).
4. Discussion
The immediate post-mating response of gravid females of D. melanogaster and H. armigera to mating is to terminate reproductive behavior. This is done in D.
melanogaster by actively rejecting male courtship. In H. armigera females, cessation of pheromone production
Table 2
Effect of SP partial-sequence peptides (10 pmol/female) on pheromone production in vivo
Treatment % Inhibition of mean pheromone % Inhibition of mean pheromone % Females calling during the production by decapitated femalesa production by females during the scotophase
scotophaseb
Control 0 0 63±24.0 (n=15)
SP8–36 55.5±11.1 (n=10) 73.3±7.7 (n=12) Not tested
SP21–36 56.2±7.9 (n=16) Not tested Not tested
Dup99B 56.7±10.6 (n=9) 63.1±8.8 (n=17) 58±18.5 (n=20)
aStimulated to synthesize pheromone by an injection of 5 pmol PBAN/female: mean production of main pheromone component
(Z11-hexadecenal) by decapitated females amounted to 51.2±7 ng/female (n=31).
b Mean production of Z11-hexadecenal during the scotophase amounted to 35.7±6.7 ng/female (n=22).
Dup99B, both bioactive seminal fluid peptides (Chen et al., 1988; Schmidt et al., 1993; Saudan, Hauck and Kubli, unpublished).
The results obtained with partial sequences with intact N-terminus of SP, and in contrast, with truncated N-ter-minal partial sequences, are consistent with the hypoth-esis that the N-terminus is responsible for CA activation in Drosophila and Helicoverpa, leading to oogenesis and perhaps oviposition in Drosophila (Soller et al., 1999). In Helicoverpa, the C-terminus is responsible for control
of pheromone production (regulating reproductive
behavior) and in Drosophila for reduction of receptivity and increase of oviposition. This conclusion is strongly supported by the lack of allatal stimulation obtained with Dup99B, which is very dissimilar to SP in its N-terminal amino acid residues (see Fig. 1) on Drosophila CA (Moshitzky, Kubli and Applebaum, unpublished and on
Helicoverpa CA (Table 1)).
The physiological significance of inhibition of D.
mel-anogaster JH-III biosynthesis by the short and long
C-terminals (SP21–36and SP8–36) is unclear at this time, as
no distinctive function has hitherto been attributed to this minor component, nor for that matter to JHB3, the major
homolog produced by D. melanogaster CA. In principle, partially degraded peptides could serve to suppress the initial physiological action of the full-length peptide, as part of a regulatory system for terminating hormonal effects subsequent to initial activation. Endogenous pro-teolytic processing of SP in D. melanogaster is not examined in the present study.
Pheromone production in noctuid moths is dependent on the action of pheromone biosynthesis activating neur-opeptide (PBAN), which regulates the synthesis and release of sex pheromone from abdominal integumental glands (Raina, 1993; Raina and Rafaeli, 1995). PBAN has been shown to act directly on the pheromone produc-ing glands in many moth species (Soroker and Rafaeli, 1989; Rafaeli et al., 1990; Arima et al., 1991; Jurenka et al., 1991; Jacquin et al., 1994; Matsumoto et al., 1995; Ramaswamy et al., 1995). The action of D. melanogaster SP and partial derivatives in vitro suggests that the pher-omone gland is a direct target for putative moth SP-like
peptides. It is inappropriate to draw physiological con-clusions from cross-reactivity of a D. melanogaster par-tial peptide in the heterologous H. armigera system. Nevertheless, the 10-fold higher concentration required
for the shorter N-terminal sequence SP1–11 to depress
PBAN-stimulated activity suggests that at least pharma-cologically, its effect is less pronounced.
The differential effects of D. melanogaster SP, Dup99B and partial peptides on in vitro pheromone pro-duction by pheromonal glands of H. armigera suggest the existence of multiple binding sites of the presumptive PBAN-receptor in the pheromone glands towards several regions of SP. The possibility of more than one popu-lation of receptors or receptor-sites has been previously suggested on the basis of structure-function and dose-response studies (Raina and Kempe, 1992; Rafaeli, 1994; Rafaeli and Gileadi, 1996).
The maximal inhibition of H. armigera pheromone biosynthesis in vivo in decapitated photophase females by Dup99B and SP C-terminal partial sequences is less than that obtained in vitro. This could simply be due to the fact that the peptides are diluted when injected into the hemolymph, and may also be more accessible to pro-teolytic degradation. These peptides are from a heterol-ogous system; factors endogenous to the male moth, unidentified as yet, may act in the regulation of phero-mone production in H. armigera.
Calling behavior, a major characteristic of female receptivity in H. armigera, expedites the dispersal of volatile sex pheromones after secretion by the gland tissue. It is not terminated after SP injection in vivo, although pheromone production is significantly reduced (Fan et al., 1999b). Dup99B, with high sequence hom-ology to SP in the C-terminal region, also has no effect on calling behavior.
attained during scotophase (Rafaeli et al., 1997), but do not exhibit calling behavior. Similarly, octopamine can inhibit pheromone biosynthesis (Rafaeli et al., 1997) but calling behavior is unaffected.
We are presently studying retrogonadal secretory pep-tides of H. armigera which affect post-mating female receptivity. By distinguishing between these two func-tions with the heterologous peptides SP and Dup99B, we hope to define moth-derived retrogonadal peptides which depress calling behavior and pheromone production.
Acknowledgements
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (DFG) to SWA, from the Swiss National Science Foundation (grant no. 31-52440.97) and Hescheler-Stiftung to EK, and the Israel Academy of Sciences and Humanities to AR. This con-tribution is acknowledged as No. 418 (1999 series) of AR from the Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel.
References
Altaratz, M., Applebaum, S.W., Richard, D.S., Gilbert, L.I., Segal, D., 1991. Regulation of juvenile hormone synthesis in wild-type and apterous mutant Drosophila. Molec. Cell. Endocrinol. 81, 205– 216.
Arima, R., Takahara, K., Kadoshima, T., Numazaki, F., Ando, T., Uchiyama, M., Nagasawa, H., Kitamura, A., Suzuki, A., 1991. Hor-monal regulation of pheromone biosynthesis in the silkworm moth, Bombyx mori (Lepidoptera: Bombycidae). Appl. Entomol. Zool. 26, 137–148.
Chen, P.S., 1984. The functional morphology and biochemistry of insect male accessory glands and their secretions. Annu. Rev. Ento-mol. 29, 233–255.
Chen, P.S., 1991. Biochemistry and molecular regulation of the male accessory gland secretions in Drosophila (Diptera). Annl. Soc. Entomol. Fr. (N.S.) 27, 231–244.
Chen, P.S., Stumm-Zollinger, E., Aigaki, T., Balmer, J., Bienz, M., Bohlen, P., 1988. A male accessory gland peptide that regulates reproductive behaviour of female D. melanogaster. Cell 54, 291– 298.
Cusson, M., Yu, C.G., Carruthers, K., Wyatt, G.R., Tobe, S.S., McNeil, J.N., 1994. Regulation of vitellogenin production in armyworm moths, Pseudaletia unipuncta. J. Insect Physiol. 36, 139–146. Dunkelblum, E., Gothilf, S., Kehat, M., 1980. Identification of the sex
pheromone of the cotton bollworm, Heliothis armigera, in Israel. Phytoparasitica 8, 209–211.
Fan, Y., Rafaeli, A., Gileadi, C., Kubli, E., Applebaum, S.W., 1999a. Drosophila melanogaster sex peptide stimulates JH-synthesis and depresses sex pheromone production in Helicoverpa armigera. J. Insect Physiol. 45, 127–133.
Fan, Y., Rafaeli, A., Gileadi, C., Applebaum, S.W., 1999b. Juvenile hormone induction of pheromone gland PBAN-responsiveness in Helicoverpa armigera females. Insect Biochem. Molec. Biol. 29, 635–641.
Hammock, B.D., Abdel-Aal, Y.A.I., Mullin, C.A., Hanzlik, T.N., Row, R.M., 1984. Substituted thiofluoropropnones as potent selective
inhibitors of juvenile hormone esterase. Pestic. Biochem. Physiol. 22, 209–223.
Herndon, L.A., Wolfner, M.F., 1995. A Drosophila seminal protein, Acp26Aa, stimulates egg laying in females for one day after mat-ing. Proc. Natl. Acad. Sci. USA 92, 10114–10118.
Jacquin, E., Jurenka, R.A., Ljungberg, H., Nagnan, P., Lofstedt, C., Descoins, C., Roelofs, W.L., 1994. Control of sex pheromone biosynthesis in the moth Mamestra brassicae by the pheromone biosynthesis activating neuropeptide. Insect Biochem. Molec. Biol. 24, 203–211.
Jurenka, R.A., Jacquin, E., Roelofs, W.L., 1991. Stimulation of phero-mone biosynthesis in the moth Helicoverpa zea: action of a brain hormone on pheromone glands involves Ca2+and cAMP as second
messengers. Proc. Natl. Acad. Sci. USA. 88, 8621–8625. Kingan, T.G., Bodnar, W.M., Raina, A.K., Shabanowitz, J., Hunt, D.F.,
1995. The loss of female sex pheromone after mating in the corn earworm moth Helicoverpa zea: identification of a male phero-monostatic peptide. Proc. Natl. Acad. Sci. USA 92, 5082–5086. Matsumoto, S., Ozawa, R., Nagamine, T., Kim, G.-H., Uchiumi, K.,
Shono, T., Mitsui, T., 1995. Intracellular transduction in the regu-lation of pheromone biosynthesis of the silkworm, Bombyx mori: suggested involvement of calmodulin and phosphoprotein phospha-tase. Biosci. Biotech. Biochem. 59, 560–562.
Moshitzky, P., Fleischmann, I., Saudan, P., Klauser, S., Kubli, E., Applebaum, S.W., 1996. Sex-peptide activates juvenile hormone biosynthesis in Drosophila melanogaster corpus allatum. Arch. Insect Biochem. Physiol. 32, 363–374.
Park, M., Wolfner, M.F., 1995. Male and female cooperate in the pro-hormone-like processing of a Drosophila melanogaster seminal fluid protein. Develop. Biol. 171, 694–702.
Pratt, G.E., Tobe, S.S., 1974. Juvenile hormones radiosynthesized by corpora allata of adult female locusts in vitro. Life Sci. 14, 575– 586.
Rafaeli, A., 1994. Pheromonotropic stimulation of moth pheromone gland cultures in vitro. Arch. Insect Biochem. Physiol. 25, 287– 299.
Rafaeli, A., Gileadi, C., 1995. Modulation of the PBAN-induced pher-omonotropic activity in Helicoverpa armigera. Insect Biochem. Molec. Biol. 25, 827–834.
Rafaeli, A., Gileadi, C., 1996. Down regulation of pheromone biosynthesis: cellular mechanisms of pheromonostatic responses. Insect Biochem. Molec. Biol. 26, 797–807.
Rafaeli, A., Gileadi, C., Fan, Y., Cao, M., 1997. Physiological mech-anisms of pheromonostatic responses: effects of adrenergic agonists and antagonists on moth pheromone biosynthesis. J. Insect Physiol. 43, 261–269.
Rafaeli, A., Soroker, V., 1989. Influence of diel-rhythm and brain hor-mone on pherohor-mone production in two Lepidopteran species. J. Chem. Ecol. 15, 447–455.
Rafaeli, A., Soroker, V., Kamensky, B., Raina, A.K., 1990. Action of PBAN on in vitro pheromone glands of Heliothis armigera females. J. Insect Physiol. 36, 641–646.
Raina, A.K., 1993. Neuroendocrine control of sex pheromone biosynthesis in Lepidoptera. Ann. Rev. Entomol. 38, 320–349. Raina, A.K., Kempe, T.G., 1992. Structure activity studies of PBAN of
Helicoverpa zea (Lepidoptera: Noctuidae). Insect Biochem. Molec. Biol. 22, 221–225.
Raina, A.K., Rafaeli, A., 1995. PBAN: What all do we know? In: Matsumoto, S., Suzuki, A. (Eds.), Molecular Mechanisms of Insect Metamorphosis and Diapause. Industrial Publishing and Con-sulting, Inc, Tokyo, Japan, pp. 151–160.
Ramaswamy, S.B., Cohen, N.E., 1991. Comparative activity of juven-ile hormones I, II, and III in promoting egg maturation in the moth Heliothis virescens (Noctuidae). Zool. Sci. 8, 747–750.
hemo-lymph and regulation of sex pheromone production. J. Insect Phy-siol. 41, 501–508.
Ramaswamy, S.B., Mbata, G.N., Cohen, N.E., 1990. Necessity of juv-enile hormone for choriogenesis in the moth Heliothis virescens (Noctuidae). Int. J. Invert. Reprod. Develop. 17, 57–63.
Ramaswamy, S.B., Shu, S., Park, Y.I., Zeng, F., 1997. Dynamics of juvenile hormone-mediated gonadotropism in the Lepidoptera. Arch. Insect Biochem. Physiol. 35, 539–558.
Richard, D.S., Applebaum, S.W., Sliter, T.J., Baker, F.C., Schooley, D.A., Reuter, C.C., Henrich, V.C., Gilbert, L.I., 1989. Juvenile hor-mone bisepoxide biosynthesis in vitro by the ring gland of Droso-phila melanogaster: a putative juvenile hormone in the higher Dip-tera. Proc. Natl. Acad. Sci. USA 86, 1421–1425.
Satyanarayana, K., Bhaskaran, G., Dahm, K.H., Meola, R., 1992. Regulation of vitellogenin synthesis by juvenile hormone in the corn earworm, Helicoverpa zea. Invert. Reprod. Develop. 21, 169–178.
Satyanarayana, K., Yu, J.H., Bhaskaran, G., Dahm, K.H., Meola, R., 1991. Hormonal control of egg maturation in the corn earworm, Heliothis zea. Entomol. Exp. Appl. 59, 135–143.
Schmidt, T., Choffat, Y., Klauser, S., Kubli, E., 1993. The Drosophila melanogaster sex-peptide: a molecular analysis of structure-func-tion relastructure-func-tionships. J. Insect Physiol. 39, 361–368.
Soller, M., Bownes, M., Kubli, E., 1999. Control of oocyte maturation in sexually mature Drosophila females. Develop. Biol. 208, 337– 351.
Soroker, V., Rafaeli, A., 1989. In vitro hormonal stimulation of [14
C]-acetate incorporation by Heliothis armigera pheromone glands. Insect Biochem. 19, 1–5.