305
Studies of the reduction of fitness in plants expressing resistance characteristics have always been popular. New techniques for manipulating defense expression have recently resulted in a greater understanding of the mechanisms through which different types of resistance strategies produce costs, especially those costs associated with inducible defenses.
Addresses
Department of Biology, Swarthmore College, Swarthmore, Pennsylvania 19081, USA; e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:305–308 1369-5266/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved.
Introduction
A surprisingly large number of articles on the costs of resis-tance in plants begin with a statement marveling at the small number of studies showing costs. In reality, hundreds of papers have concluded that resistance can be cost-ly [1,2], and thus it is a relief that recent research seems to focus on more interesting issues such as the mechanisms by which costs are realized, why certain resistance traits cost as much as they do, the effect of environmental con-ditions on the magnitude of costs, and the implications of such costs. This review summarizes the important contri-butions made in understanding these issues during the past year, and concludes with some prospects for future research in the field.
It is important to stress that curiosity over why resistance polymorphisms persist in plant populations has been the primary motivation for much of the research on the costs and benefits of resistance [3,4]. A popular view is that plant populations and their pests are in equilibrium, with the frequency of resistance alleles highest in those populations in which the virulence of the pest is highest or in which the pest is common. The most recent paper on this front is that by Berenbaum and Zangerl [5], reviewed by [6], who showed that the frequency of a resistance polymorphism in a plant species is very closely related to the level of viru-lence of the plant’s primary pest (a specialist herbivore). This work provides evidence that there is a coevolutionary relationship between the plant and pest species, and it is possible that costs play at least some role in maintaining that relationship.
Mechanisms of costs
When resistant genotypes have relatively low fitness (e.g. poor seed production) in the absence of pests, it is often assumed that their poor performance must be explained by the energetic drain involved in making and maintaining a chemical or structural (e.g. thorns) defense, even if such a character has not been identified. Because the mechanism
of resistance can itself vary (Table 1), the assumption that costs accrue by allocation costs — the costs due to diver-sion of energy and resources away from reproductive function — is a dangerous one. Indeed, even for the pro-duction of a chemical defense, costs (if present) are likely to be caused by a combination of factors.
A notable aspect of many recent studies is the focus on the ecological costs of resistance. For example, Strauss et al. [7••] found that plants with extensive chemical defenses were visited less frequently by pollinators. This phenomenon could lead to reduced pollen or seed produc-tion as demonstrated by Agrawal et al. [8•]. A different type of ecological cost was demonstrated by Agrawal et al. [9••], who found that even when allocation costs are absent, resistance to one type of herbivore can translate into greater susceptibility to another more specialized pest.
It is important to note that linkage effects — the fitness difference that can be caused by alleles linked to the resis-tance locus — is not a mechanism that produces resisresis-tance costs per se, but rather a mechanism by which such costs are obscured. Despite great optimism by most researchers, successive backcrossing will not reliably eliminate linkage effects within an average human life span [10,11], although breeders might be successful in removing the majority of unlinked alleles that were initially associated with the resistant allele(s). An argument is often made, that if close-ly linked loci contribute to the poor fitness of resistant genotypes for hundreds of generations, then in functional terms their costs can be attributed to the resistance allele when making assessments. Over evolutionary time, how-ever, such linkages will ultimately be broken [12], and thus this source of fitness reduction should never be considered as part of the true cost of resistance. Moreover, because resistance alleles might be simultaneously selected along with growth-related alleles that are adapted to low resource availability in conditions, of limited resource availability [13,14], it might be expected that resistant biotypes would have low fitness relative to susceptible biotypes in pest-free conditions, even if the resistance mechanism itself accrued no cost. An important consideration related to this issue is that when several generations of breeding result in a reduction or elimination of costs, then the most likely explanation is the loss of linked alleles, rather than the often-invoked phenomenon of compensatory muta-tions — i.e. new mutamuta-tions that somehow decrease the fitness cost of the resistance phenotype. In time, a greater knowledge of DNA-sequence variation and the use of marker-assisted backcrossing will hopefully minimize the complications associated with effects caused by linked alleles. Until then, experiments involving transgenes will remain the only methods by which costs can be assessed in the absence of linkage effects [15•].
Costs of resistance
306 Biotic interactions
Another exciting area of research surrounds the issue of whether there is a tradeoff between resistance and toler-ance, such that highly-resistant plants might be less able to tolerate herbivory when it occurs [8•,16,17]. Stowe [16] found that Brassica rapa(Brassicaceae) lines that were arti-ficially selected to contain high amounts of glucosinolate were less fit in the absence of pests and were also less able to compensate for defoliation. This tradeoff is not neces-sarily attributable to the defense itself (i.e. linkage may exist). Similar results have been reported in wheat [17], in which highly aphid-resistant lines are hypothesized to be less able to tolerate the toxins released by feeding aphids. Although reduced tolerance might be expected, given that cost-burdened resistant plants will have less photosynthet-ic resources available to devote to tolerance responses (or to any other function, for that matter), it is interesting to consider whether herbivore damage itself might release toxic compounds into plant cells, and thus cause further fitness reductions in form of reduced regrowth potential. Such a loss of fitness would presumably not be caused by morphological defenses.
Effect of environment
There has been an increased interest in examining whether the costs of defense vary under different environ-mental conditions. It is typically predicted that the energetic drain of producing a defense will be greatest when resources are limiting. Four recent studies have, however, failed to confirm this prediction [18,19,20•,21]. An exception to this trend is reported by Pavia et al.[22••] who showed that nitrogen limitation in a brown algae (admittedly not a plant) eliminates the cost of a carbon-rich defensive compound. Future studies might also ask (as do Pavia et al.[22••]) whether increased costs in certain envi-ronments are merely due to an increase in the production
of a chemical defense — perhaps through a generalized stress response or through a redirection of surplus carbon — rather than to an increase in the relative value of diverted resources.
The effects of environment on costs are particularly impor-tant when the mechanism of resistance depends on environmental conditions, as does the hyperaccumulation of metals [23•]. Costs associated with this type of resis-tance have not been explored, but if they exist they would probably develop primarily through the toxic effect of the metal on plant tissues themselves (i.e. self-toxicity).
Inducible defenses
In contrast to constitutive defenses, inducible defenses are commonly thought not to accrue fitness costs in the absence of pests and pathogens. In reality, it is more likely that inducible systems will simply be less costly than con-stitutive systems, because inducible resistance depends on maintaining wound-detection pathways, defense precur-sors, and storage vesicles, all of which require allocation of both energy and resources away from growth and repro-duction [24,25]. The fitness costs of inducible resistance are thus difficult to measure because one must compare the fitness of uninduced plants that possess the above components (i.e. resistant genotypes) to the fitness of plants that lack all of the components (i.e. susceptible genotypes). In other words, to successfully incorporate costs and benefits of inducible defenses into an evolution-ary model costs must be determined using genotypes in entirely uninduced states. Such susceptible genotypes are rare, although there is a recent report of a plant that lacks both constitutive and inducible expression of a single class of defense compounds [9••]. New molecular research on alleles involved in the recognition pathways of induced Table 1
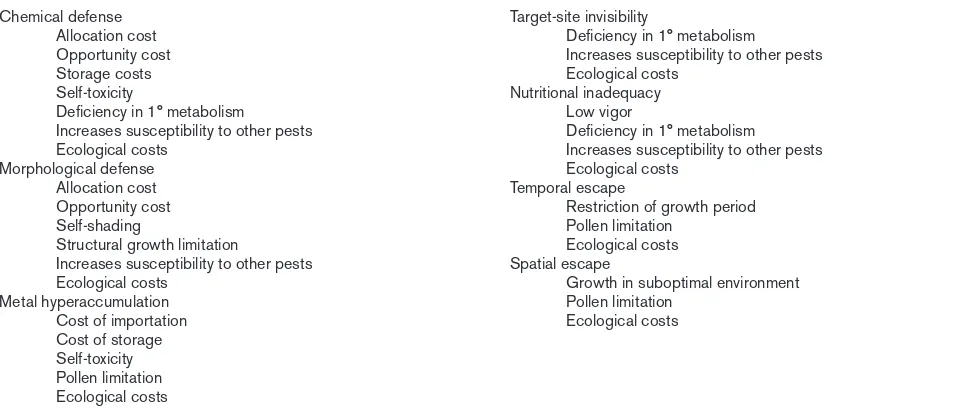
Mechanisms of defense and potential sources of costs.
Chemical defense Target-site invisibility
Allocation cost Deficiency in 1° metabolism
Opportunity cost Increases susceptibility to other pests
Storage costs Ecological costs
Self-toxicity Nutritional inadequacy
Deficiency in 1° metabolism Low vigor
Increases susceptibility to other pests Deficiency in 1° metabolism
Ecological costs Increases susceptibility to other pests
Morphological defense Ecological costs
Allocation cost Temporal escape
Opportunity cost Restriction of growth period
Self-shading Pollen limitation
Structural growth limitation Ecological costs
Increases susceptibility to other pests Spatial escape
Ecological costs Growth in suboptimal environment
Metal hyperaccumulation Pollen limitation
Cost of importation Ecological costs
Costs of resistancePurrington 307
defenses may provide indirect estimates of the costs of this component of the inducibility phenotype [3,4]. When inducibility mutants are eventually studied in this context, it will of course be necessary to also account for linked alle-les in both the resistant and susceptible genotypes because some mutations that are unrelated to defense may also be inducible, and thus affect fitness.
Because the overall costs of an inducible defense system cannot be easily measured, researchers have instead com-pared the fitness of induced resistant plants with the fitness of uninduced resistant plants. Application of methyl jasmonate (or jasmonic acid), in particular, has become increasingly common as a means of inducing a wide variety of defenses in taxonomically diverse plant lin-eages [26,27]. Such experiments generally conclude that defenses, when induced, reduce at least some component of fitness (but see [28•]). A related methodology, that is antisense inhibition of jasmonic acid synthesis, has showed similar costs [29•]. Despite the tendency of such studies to interpret the fitness differences as allocation costs of chemical defenses, it should be emphasized that methyl jasmonate has many effects on plants in addition to the desired effect of increasing many secondary compounds (see references in [28•]). In this light, a simple experiment that is begging to be performed is to examine the fitness of induced and uninduced individuals of a genotype that lacks the ability to produce its normal chemical defense (e.g. nicotine); a fitness drop in the ‘induced’ plant would not be expected if jasmonic acid had no effects beyond that of elevating resistance. The uncertainty over the effects of jasmonic acid suggests that more traditional approaches to induction, such as minimal levels of wound-ing, are still justified [9••]. Experiments aimed at estimating the costs of induction are also of great interest.
With all techniques of induction, however, it is important to consider whether a single induction event is sufficient to maintain the production of the defense for a duration that is experimentally relevant [28•]. Some methods of induction may cause only temporary bursts of defense expression, and thus might underestimate the cost of a fully induced response. A related concern is that some species may have difficulty in standing down a defense repsonse. Indeed, Agrawal et al. [30••] found that resis-tance can even be transferred to the next generation, and thus might translate into burdensome costs being inher-ited by progeny through nongenetic maternal and paternal effects.
Conclusions
Several dozen recently published articles on the costs of resistance characteristics in plants are reviewed above. Among recent research trends are the increasing frequen-cy of inducibility experiments and the use of transgenic technology, both of which allow estimation of costs without the complications of linked effects, but which have their own shortcomings.
Although the presence of costs has ceased to surprise most researchers in the field, examples of cost-free resistance con-tinue to excite [28•,31•]. Snow et al. [31•], for example, found no costs associated with an herbicide resistance gene developed by a biotechnology company. They speculated that resistance genes used by the private sector may be pre-selected to be cost-free, and thus profitable for farmers even under pest-free conditions (but see [15•]). Nevertheless, as Siemens and Mitchell-Olds [18] suggest, a lack of costs reported could be explainable by low statistical resolution.
A big question that remains unexplored is whether ‘non-host’ resistance (see review by MC Heath, this issue, pp 315–319], which describes complete resistance to a par-ticular pest or pathogen, is costly. Although, by definition such resistance is fixed (i.e. not polymorphic) and thus dif-ficult to study, it may still be true that such resistance is costly. As interest in cloning non-host resistance genes increases, however, research over the next few years may elucidate the evolutionary factors behind such loci relative to their more popular polymorphic varieties.
Of all of the fields in evolutionary biology, the study of costs of resistance traits in plants is probably the most blessed. Because of the potential profits for creating trans-genic crops with enhanced resistance to herbicide, pests and pathogens, the number of cloned resistance genes and even already-transformed plants will increase dramatically, thus facilitating the study of the fitness consequences of a large variety of defense-related pathways. One drawback of transgenic technology, the large variation among inde-pendently-transformed lines, provides a powerful opportunity to examine the effect of expression levels of resistance genes on the severity of costs of defense (see [15•] for example). Transgenic technology may also help to solve a vexing question in the field: whether a par-ticular gene product is part of primary or secondary metabolism or both. Specifically, if an antisense transgene can be constructed to eliminate accumulation of a putative secondary compound, then mortality of the transformed plant, or even a persistent failure to recover the transfor-mants, might indicate a primary role for the gene product.
Acknowledgements
I am grateful to Anna Hess, Joy Bergelson, and an anonymous reviewer for reading an earlier draft of this paper. Research in my laboratory is supported by the Howard Hughes Medical Institute.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Simms EL: Cost of plant resistance to herbivory.In Plant Resistance to Herbivores and Pathogens. Edited by Fritz RS, Simms EL. Chicago: University of Chicago Press; 1992:392-425.
2. Bergelson J, Purrington CB: Surveying costs of resistance in plants.Amer Naturalist 1996, 148:536-558.
308 Biotic interactions
Brassica and Arabidopsis. Proc Natl Acad Sci USA 1998,
95:15843-15848.
4. Stahl EA, Dwyer G, Mauricio R, Kreitman M, Bergelson J: Dynamics of disease resistance polymorphism at the Rpm1 locus of
Arabidopsis. Nature 1999, 400:667-671.
5. Berenbaum MR, Zangerl AR: Chemical phenotype matching between a plant and its insect herbivore.Proc Natl Acad Sci USA
1998, 95:13743-13748.
6. Kareiva P: Coevolutionary arms races: is victory possible?Proc Nat Acad Sci USA1999, 96:8-10.
7. Strauss SY, Siemens DH, Decher MB, Mitchell-Olds T: Ecological •• costs of plant resistance to herbivores in the currency of
pollination.Evolution1999, 53:1105-1113.
A very careful study that goes beyond the typical interpretation of costs via allocation costs. Among other findings, the authors show that some compo-nent of cost is due to the pollinator's preference of low-resistance plants. 8. Agrawal AA, Strauss SY, Stout MJ: Costs of induced responses and • tolerance to herbivory in male and female fitness components of
wild radish.Evolution1999, 53:1093-1104.
The authors found no costs associated with tolerance but did detect costs of induced defense, which were incurred primarily via reduced male function.
9. Agrawal AA, Gorski PM, Tallamy DW:Polymorphism in plant •• defense against herbivory: constitutive and induced resistance in
Cucumis sativus.J Chem Ecol1999, 25:2285-2304.
An excellent demonstration that shows that ecological costs can affect fit-ness even when allocation costs are absent.
10. Stam P, Zeven AC: The theoretical proportion of the donor genome in near-isogenic lines of self-fertilizers bred by backcrossing.Euphytica1981, 30:227-238.
11. Young ND, Tanksley SD: RFLP analysis of the size of chromosomal segments retained around the Tm-2locus of tomato during backcross breeding.Theor Appl Genet1989, 77:353-359. 12. Mauricio R:Costs of resistance to natural enemies in field
populations of the annual plant Arabidopsis thaliana.Amer Naturalist1998, 151:20-28.
13. Coley PD, Bryant JP, Chapin FS III: Resource availability and plant antiherbivore defense.Science1985, 230:895-899.
14. Loreau M, de Mazancourt C: Should plants in resource-poor environments invest more in antiherbivore defence?Oikos1999,
87:195-200.
15. Purrington CB, Bergelson J: Exploring the physiological basis of • costs of herbicide resistance in Arabidopsis thaliana.Amer
Naturalist 1999, 154:S82-S91.
This paper explores the physiological mechanism of an already identified cost of herbicide resistance in Arabidopsis. In addition, the authors demon-strate the controls that are necessary for the proper interpretation of transgenic experiments.
16. Stowe KA: Experimental evolution of resistance in Brassica rapa: correlated response of tolerance in lines selected for
glucosinilate content.Evolution1998, 52:703-712. 17. Haile FJ, Higley LG, Ni XZ, Quisenberry SS: Physiological and
growth tolerance in wheat to Russian wheat aphid (Homoptera: Aphididae) injury.Environ Entomol1999, 28:787-794.
18. Siemens DH, Mitchell-Olds T: Evolution of pest-induced defenses in Brassicaplants: tests of theory.Ecology1998, 79:632-646. 19. Jordan N, Kelrick M, Brooks J, Kinerk W: Biorational management
tactics to select against triazine-resistant Amaranthus hybridus: a field trial.J Appl Ecol1999, 36:123-132.
20. Elle E, van Dam NM, Hare JD: Cost of glandular trichomes, a • ‘resistance’ character in Datura wrightiiRegel (Solanaceae).
Evolution1999, 53:22-35.
An interesting investigation of resistance costs and the effect of environment on costs. This paper is especially notable for its good introductory remarks on the effect of experimental design on the ability to detect costs. 21. van Dam NM, Baldwin IT: Costs of jasmonate-induced responses
in plants competing for limited resources.Ecol Lett1998, 1:30-33. 22. Pavia H, Toth G, Aberg P: Trade-offs between phlorotannin •• production and annual growth in natural populations of the brown
seaweed Ascophyllum nodosum.J Ecol1999, 87:761-771. The authors detected costs associated with producing defensive polyphe-nolics in brown seaweed. Although the organism featured in this paper is not a plant, this paper is extraordinarily clear and well-written, and the data are very carefully analyzed.
23. Boyd RS, Moar WJ: The defensive function of Ni in plants: • response of the polyphagous herbivore Spodoptera exigua
(Lepidoptera: Noctuidae) to hyperaccumulator and accumulator species of Strepthanthus(Brassicaceae).Oecologia1999,
118:218-224.
The authors demonstrate that metal hyperaccumulation can indeed confer resistance to herbivory. This paper does not discuss costs, but the authors will hopefully publish on this aspect in the future.
24. DeWitt TJ, Sih A, Wilson DS: Costs and limits of phenotypic plasticity.Trends Ecol Evol1999, 13:77-81.
25. Cipollini D: Induced defenses and phenotypic plasticity.Trends Ecol Evol1998, 13:200.
26. Baldwin IT: Jasmonate-induced responses are costly but benefit plants under attack in native populations.Proc Nat Acad Sci USA
1998, 95:8113-8118.
27. Agrawal AA: Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness.Ecology1999,
80:1713-1723.
28. Thaler JS: Induced resistance in agricultural crops: effects of • jasmonic acid on herbivory and yield in tomato plants.Environ
Entomol 1999, 28:30-37.
The author found no costs of induced resistance, but pests (i.e. thrips) could not be completely controlled, so it is unclear whether true costs were mea-sured in the complete absence of benefits.
29. Royo J, León J, Vancanneyt G, Albar JP, Rosahl S, Ortego F,
• Castañera P, Sánchez-Serrano JJ: Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insects.Proc Natl Acad Sci USA1999, 96:1146-1151.
Suppression of endogenous lipoxygenase activity disables production of proteinase inhibitors, and response results in a plant with high tuber yield. Thus, this paper shows nicely how antisense transgene technology can be used to determine costs.
30. Agrawal AA, Laforsch C, Tollrian R: Transgenerational induction of •• defences in animals and plants.Nature1999, 401:60-63.
The authors found that the progeny of plants that had been exposed to cater-pillar damage were more resistant than progeny collected from undamaged parents. Those researchers who believe that maternal effects do not apply to the study of plant resistance should please read this article.
31. Snow AA, Andersen B, Jorgensen RB: Costs of transgenic • herbicide resistance introgressed from Brassica napusinto
weedy B. rapa.Molec Ecol 1999, 8:605-615.