T
he scale of both the economic and environmental costs of insect control in agriculture, and of the losses incurred in spite of such measures, is high (Fig. 1). Therefore, it is not surprising that insect-resistant transgenic plants were among the first products of plant biotechnology to reach the marketplace. Recent commercial releases of genetically engineered crops have included transgenic corn, cotton and potato, which express Bacil-lus thuringiensis (Bt) toxins (Box 1). In North America, these crops are already being grown on a considerable proportion of the arable land. An even more diverse array of transgenic crops are scheduled for release in several countries. Here we review the use of Bt, the underlying scientific developments and the creation and introduction of transgenic Btplants. The safety issues involved, and the concerns raised by the introduction of transgenic Btplants into agricultural systems, are also discussed.Bacillus thuringiensis: a natural insecticide
Bacillus thuringiensisis a gram-positive, spore-forming bacterium that exists in many locations, such as the soil, plant surfaces and in grain storage dust. B. thuringiensiscan be distinguished from re-lated Bacillusspecies by the presence of parasporal crystals that are formed during sporulation. The parasporal crystals consist of one or more δ-endotoxins or crystal (Cry) proteins of ~130 kDa (although truncated forms also occur). It is these that makes Btan
effective insect pathogen. Following ingestion, the alkaline en-vironment of the insect midgut causes the crystals to dissolve and release their constituent protoxins. The protoxins are subsequently trimmed by gut proteases to an N-terminal, 65–70 kDa truncated form – the activated toxin (Fig. 2). The toxin binds to specific receptors on the cell membranes of the midgut epithelial cells, inserts itself into the membrane, and forms pores that kill the epi-thelial cells (and eventually the insect) by colloid osmotic lysis1.
B. thuringiensisδ-endotoxins are part of a large and still growing family of homologous proteins – more than 130 genes have been identified to date (see Bttoxin nomenclature at: http://epunix.biols. susx.ac.uk/Home/Neil_Crickmore/Bt/index.html). These genes generate a rich source of diversity on which to draw for differing insect specificities. This specificity is an important aspect of the Bt Cry proteins: each protein is active in only one or a few insect species. Specificity is to a large extent determined by the toxin–receptor interaction2, although solubility of the crystal and
protease activation also play a role. The members of the Cry gene family are grouped in subfamilies according to their specificity for members of the insect families Lepidoptera (caterpillars), Diptera (flies and mosquitoes) and Coleoptera (beetles)3. Some Btstrains
have also been reported to be active against other insect families and also mites, nematodes, flatworms, and protozoa, but few details as to their practical use are available4. It is also significant
Bacillus thuringiensis toxin-mediated
insect resistance in plants
Ruud A. de Maagd, Dirk Bosch and Willem Stiekema
We are currently in an interesting phase of plant biotechnology releases, both for the scientists responsible for these innovations who are beginning to see their ideas realized, and for the biotechnology companies that are starting to see a return on their investment. One of the most notable examples, is the introduction of transgenic crops that are engineered to express a Bacillus thuringiensis toxin that confers resistance to insect predation. However, the picture is not altogether positive – there is concern that the introduction of this technology was premature or should not have happened at all, and that the valuable insecticidal properties of Bacillus thuringiensis will be lost.
Fig. 1.(a) Worldwide expenditure on insecticides (in millions of US $) for different crop categories. (b) Worldwide crop losses caused by insect pests, in spite of the use of chemical insecticides (in millions of US $). The percentage of the total crop volumes lost are: maize, 12%; fruit, 6%; vegetables, 9%; rice, 27%. The data for cotton are not available. Data for 1994, adapted from Krattiger et al.35
Fruit and Vegetables 2465
Vegetables 25 000 Fruit 20 000 Cotton
1870
Other
1965 Rice
1190 Rice
45 000 Maize
620
Maize 8000
that several important insect pests appear to be insensitive to known Cry proteins (for example, the Corn root worm, aphids, and white flies).
Bacillus thuringiensis formulations (spore and crystal mix-tures) were used as insecticidal sprays in the 1930s, but large scale production only started with the introduction of Thuricidein the late 1950s, and this was followed by similar products from several companies5. In spite of their environmentally friendly reputation, Bt sprays have never occupied a large share of the insecticide market, and are largely used by organic farmers and gardeners and in forestry. Three factors are responsible for this:
• Lack of stability.
• Failure to penetrate tissues, and therefore to reach insects in all parts of the plant.
• Too narrow a specificity.
Crystal proteins degrade rapidly in UV light, loosing their activity. It is therefore necessary to make multiple applications throughout the growing season, which raises the costs of pesticide treatment. Although some improvements have been made in this area, it re-mains the biggest single drawback to the use of Btsprays. Further-more, Btsprays are non-systemic insecticides and are therefore ineffective against insects that do not come into direct contact with the crystals, such as sap sucking and piercing insects, against root dwelling pests, or larvae that after hatching rapidly burrow or bore into plant tissues. In addition, crops are often subject to pre-dation by a variety of pests that cannot currently be controlled by a single Btproduct. The first two problems have been effectively addressed by creating transgenic plants that express the crystal
proteins. In these plants, the toxin is continuously produced and protected against degradation, and provided it is expressed in the appropriate tissues it will also be ingested by boring larvae. The problem of narrow specificity may be overcome by simultaneously expressing genes for proteins with different specificities (resistance gene ‘stacking’ or ‘pyramiding’).
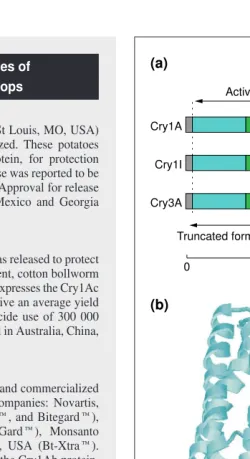
Fig. 2. (a) Schematic representation of the primary structure of some typical Bacillus thuringiensis delta-endotoxins (Cry pro-teins). The images shown are the full-length gene products, the protoxins found in Bt crystals (size in number of amino acid residues, is shown at the bottom). Insect gut proteases trim the N-terminal and C-N-terminal ends (gray), resulting in the activated toxin (indicated by arrowheads). Transgenic plants were made to produce either C-terminally truncated or full-length protoxins. (b) Three-dimensional structure of an activated toxin, Cry1Aa. The toxin has three stuctural domains (see also the primary structure). Domain I (blue) is involved in membrane insertion and pore formation. Domain II (green) and domain III (red) are in-volved in receptor recognition and binding. Structure determined by Grochulski et al.36
Activated toxin
Truncated forms in transgenic plants
0 Cry1A
(a)
(b)
Cry1I
Cry3A
600 1200
Box 1. Commercial releases of Bacillus thuringiensis crops
Potato
In 1995, Bt-potato (NewLeaf™, Monsanto, St Louis, MO, USA) became the first Bt-crop to be commercialized. These potatoes were engineered to express the Cry3A protein, for protection against Colorado potato beetles. Insecticide use was reported to be reduced by up to 40% for Bt-plants in 1997a. Approval for release
has since been granted in Canada, Japan, Mexico and Georgia (former Soviet Republic).
Cotton
In 1996, Bt-cotton (Bollgard™, Monsanto) was released to protect against tobacco budworm, and to a lesser extent, cotton bollworm and pink bollworm. The transgenic Bt-cotton expresses the Cry1Ac protein. In 1997, Bt-cotton was reportedato give an average yield
increase of 14%, with a reduction in insecticide use of 300 000 gallons. Approval for release has been granted in Australia, China, Mexico, South Africa and the USA.
Maize
Several companies have developed Bt-maize and commercialized its use since 1996 by licensing it to seed companies: Novartis, Basel, Switzerland (YieldGard™, Knockout™, and Bitegard™), Mycogen, San Diego, CA, USA (NatureGard™), Monsanto (YieldGard™), and DEKALB Genetics, IL, USA (Bt-Xtra™). With the exception of Bt-Xtra™, all produce the Cry1Ab protein. In field experiments, Bt-maize gives 99% control of first gener-ation European corn borer larvae. Approval for release has been granted in Argentina, Canada, Japan, the USA and within the European Community.
Transgenic Bacillus thuringiensis-plants
The economic need for an effective insecticide, the availability of Btgenes encoding relevant insecticidal activity and the proven safety of Btsprays, have all made transgenic Btplants obvious candidates for early commercial exploitation of plant biotech-nology. In early experiments, both full-length and truncated cry genes (the latter almost exclusively encoding the activated toxin; Fig. 2) were introduced into tobacco and tomato by Agrobac-terium tumefaciens-mediated transformation6–8. Constructs
con-taining truncated genes, but not those concon-taining full-length genes, yielded plants showing some measure of protection against insect predation. However, it soon became clear that the expression level of unmodified crygenes was too low (typically only a few ng mg21
total protein) to confer sufficient protection against pests under field conditions.
Bacillus thuringiensis cry genes are typical bacterial genes in that they have a high A/T-content compared with plant genes (typically values are 60–70% for Btgenes and 40–50% for plant genes). As a consequence cry gene codon usage is inefficient in plants, and the A/T-rich regions may contain transcription termination (polyadenylation) sites (AATAAA and variations thereof), cryptic mRNA splice sites, and mRNA instability motifs (ATTTA). Indeed, truncated transcripts were often observed and most attempts to raise expression levels were performed with the intention of removing sequence motifs that could cause these truncations. The effects of different degrees of gene modification were investigated in the cry1Aband cry1Acgenes9. For cry1Ab,
partial modification (removal of seven out of 18 polyadenylation sites and seven out of 13 ATTTA sequences) increased the pro-portion of protected transformed plants and raised Bt protein concentrations to detectable levels (up to tenfold higher than for the unmodified genes). Removal of the remaining poly-adenylation sites and ATTTA sequences, and changes to a total of 356 of the 615 codons, raised the levels
still higher (up to 0.2–0.3% of total soluble protein) – 100-fold higher than the level for unmodified genes. These ef-fects were observed in transgenic tobacco, tomato and cotton9,10. By comparing
con-structs containing different modifications it became apparent that it is not the pres-ence of rare codons, but other mecha-nisms that are primarily responsible for the low expression of wild-type genes. Further analysis of Cry1Ab transcript formation revealed that although tran-scription initiation occurs at a normal fre-quency, unmodified cry gene sequences interfere with transcript accumulation11,
probably as a result of mRNA splicing caused by the presence of three cryptic introns (i.e. functioning as plant introns) in the unmodified gene. Codon optimiz-ation further increased expression levels. A similar approach was used to modify the cry3A gene for use in potato, which re-sulted in resistance to Colorado potato beetle predation12. Even more radical
changes were made to the cry1Abgene to make it fit maize-specific codon usage and to transform the first cereal, creating trans-genic maize lines with resistance against European corn borer13. Once adapted to
the plant transcriptional and translational
machinery, both full-length and truncated versions of various cry genes can be successfully expressed in plants14 (see Fig. 3).
Modification of Btgenes for expression in transgenic plants is now common practice. A variety of plant promoters have been used in combination with cry genes: the CaMV 35S promoter is the most commonly used, but wound-inducible promoters6,
chemically inducible promoters15 and tissue specific promoters
have all been used (the latter with Bt protein levels of up to 0.4% of total protein in maize13). A combination of the use of the
Rubisco small subunit promoter, a translational fusion to its chloroplast transit peptide and a modified cry1Accoding region resulted in Bt protein levels of up to 0.8% of total protein in tobacco16. Even higher levels were achieved by transforming
tobacco chloroplasts with an unmodified, full length cry1Ac coding region. As the transcriptional and translational machinery of plastids is similar to that of bacteria, modification of the wild-type cry1Acsequence was not necessary. The effects of amplifi-cation of the integrated gene in the plastid genome resulted in Bt protein levels of 3–5% of total soluble protein. This level of expression even provided protection against relatively Cry1Ac-tolerant plant pests17.
Many crop plants have now been successfully transformed to provide resistance against one or several of their respective pests18
(see also: USDA/APHIS Biotechnology Permit Database: http:// www.aphis.usda.gov/bbep/bp/).
Concerns raised by the introduction of transgenic plants Plants expressing Bttoxins were among the first plant biotechnol-ogy products to be approved for commercial use. However, oppo-sition against the use of transgenic Bt-plants arose even before their release. The most important objections are:
• Toxins could negatively affect non-target animals such as predators or parasites that feed on pests.
• Continuous exposure of pest insects to the Bttoxin would cre-ate a high selection pressure for the development of resistance against the toxin.
Effects on non-target organisms
One of the ecological risks of releasing transgenic Bt-plants, would be the unforeseen effects of the toxin on organisms that are not pests of the crop itself – especially if those organisms are predators and parasites of pests and therefore of benefit to agri-culture. In the past, indiscriminate use of broad spectrum chemi-cal insecticides that killed natural enemies along with the primary pests, often led to outbreaks of secondary pests. Testing for the ef-fects on beneficial Arthropod species is nowadays part of procedure for gaining approval for new insecticides.
According to most published reports, Btsprays have no detri-mental effects on natural enemies (because of their high speci-ficity and low persistence), and are considered to be compatible with integrated pest management (IPM) practices. These results cannot be readily extrapolated to transgenic Bt-plants, because of differences in the duration and form of an insect’s exposure to the toxin (continuous versus intermittent exposure, soluble and par-tially activated toxin versus protoxin in crystals). In general, Bt -plants would be expected to be even more insect-specific because they only produce one toxin, whereas Btstrains in sprays usually produce several different toxins, sometimes including the rela-tively non-specific β-exotoxins.
Procedures for ecotoxicological risk assessment of Bt-plants that have been proposed include a list of organisms that should be tested for effects19. However, few studies on the effects of Bt-plants on beneficial insects have been published to date. In one study, two species of caterpillars were reared on transgenic or non-transgenic maize and fed to predatory lacewing larvae. The caterpillars from Cry1Ab-producing maize led to increased mortality in lacewing larvae20, but it was not clear whether this
was a direct effect of the toxin that accumulated in the prey lar-vae, or whether the increased mortality was an indirect effect caused by suboptimal prey quality. In another study, there was no detrimental effect of Cry1Ab in pollen of transgenic maize on the insect predators examined, indicating that the toxin has no direct effects21. There have also been very few field studies
on the effects of Bt-plants on non-target organisms. Those that are available appear to confirm the original assumption that Bt -plants either have no effect on beneficial insect populations22,
or that they even cause an increase in numbers of non-target insects23; this allows an increased reliance on biological control
of secondary pests by eliminating the need for nonselective sprays24.
Insect resistance to toxins
Concern about insects rapidly becoming resistant to Bttoxins as a consequence of the release of transgenic Bt-plants has received a great deal of attention. Insects are highly adaptable and have evolved resistance to many chemical insecticides. In this con-text, Bt toxins are not expected to be different from other in-secticides. Laboratory studies have shown that resistance that is already present in the gene pool of a population, can be selected for with purified toxins or Bt formulations in several insect species and for several different toxins25,26. The occurrence of
re-sistance in field populations in response to extensive applications of Btsprays are rare, but it has been reported27,28. Resistance in
response to Bt-plants has not been reported to date, but of course this may be attributed to the fact that Bt-plants have not been around for very long. However, there is little doubt that the gen-etic potential for resistance is present. Many scientists, as well as
members of environmental pressure groups, believe that continu-ous exposure to Bt-plants will lead to selection for resistance, and that the large scale introduction of Bt-crops endangers the dur-ability of Btas an insecticide, both in crops and in sprays. This would have an impact on the growers of transgenic Bt-crops as well as on organic, conventional and IPM farmers who use Bt sprays.
Research into the mechanism of insect resistance to Bttoxins indicates that a seemingly common factor is the loss or modifi-cation of the midgut binding sites for the toxin25, which leads to
resistance to only one or a few related toxins that recognize the same receptor. Increased protease activity may also render the toxin inactive. Other mechanisms also exist, but have yet to be elucidated; some of these may lead to cross-resistance to other cry proteins. It is possible that, because of the specific form in which the toxin is expressed in the plant, the type of resistance mecha-nisms selected for with transgenic plants may differ from, or only partially overlap, with those that occur in response to Btsprays or purified toxins.
Several strategies that should prevent or delay the rapid de-velopment of resistance to Bt-plants have been proposed and com-pared29–32. The efficacy of these strategies is difficult to prove
without large-scale cultivation, but simulation modeling has been used extensively to predict results. The most plausible strategies are:
• The use of multiple toxin genes with different modes of action so that cross-resistance is unlikely to occur (i.e. two crygenes for toxins with different receptors, or a crygene in combination with an altogether different toxin gene).
• The use of tissue-specific or inducible promoters to achieve spatial or temporal variation in the expression levels of the toxin. The use of tissue-specific promoters would decrease se-lection pressure by allowing pests to feed unharmed on eco-nomically less important parts of the plant. The use of inducible promoters would decrease selection pressure over time, as ex-pression would only be induced when a certain economical threshold of damage was crossed.
• Use of temporal or spatial refuges. Rotation of Bt-crops with non-transgenic plants would slow down development of resist-ance, particularly if resistance is not stable in the insect popu-lation. With spatial refuges, part of a field is set aside for non-transgenic plants. This allows Bt-resistant insects that have survived on the transgenic plants to mate with non-selected, sensitive insects from the non-transgenic plants, and prevents the rise of a population that is homozygous for a recessive or semi-dominant resistance allele.
In all three cases, a thorough understanding of the biology of the crop–pest complex, the possible mechanisms of resistance, and the frequency of resistance alleles in the insect population would be necessary to devise an optimum resistance management strategy.
Future developments and conclusions
The first generation of insect-resistant transgenic plants are now in large-scale use in agriculture. The use of Bt-crops will probably increase as more crop species that express different cry genes are developed. However, several important pest species remain tolerant of the available Bttoxins, and screening for active Bt pro-teins is in progress. In the future, other insecticidal propro-teins, such as the VIPs (vegetative insecticidal proteins of Bacillus), lectins, protease inhibitors, α-amylase inhibitors, chitinases and choles-terol oxidase may come to complement or even substitute for the Bttoxins18,34. In anticipation of the potential for insects to adapt to Bt-plants, resistance-management plans have been adopted, with farmers and seed companies working together to ensure the dur-ability of this environmentally friendly strategy for crop protec-tion. Viewed cynically, one might say that the commercialization of Bt-crops has created large-scale field trials to test this dur-ability. On a more positive note, the introduction of Bt-crops has led to a reduction in insect pest damage and the simultaneous elimination of chemical pesticides. Our efforts should now be di-rected towards maintaining this advantage and ensuring its long term viability.
References
1Knowles, B.H. and Dow, J.A.T. (1993) The crystal delta-endotoxins of Bacillus thuringiensis– models for their mechanism of action on the insect gut, BioEssays15, 469–476
2van Rie, J. et al. (1990) Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxin, Appl. Environ. Microbiol.56, 1378–1385
3Höfte, H. and Whiteley, H.R. (1989) Insecticidal crystal proteins of Bacillus thuringiensis, Microbiol. Rev.53, 242–255
4Feitelson, J.S., Payne, J. and Kim, L. (1992) Bacillus thuringiensis: insects and beyond, Biotechnology10, 271–275
5Beegle, C.C. and Yamamoto, T. (1992) History of Bacillus thuringiensis berliner research and development, Can. Ent.124, 587–616
6Vaeck, M. et al. (1987) Transgenic plants protected from insect attack, Nature 328, 33–37
7Fischoff, D.A. et al. (1987) Insect tolerant transgenic tomato plants, Biotechnology5, 807–813
8Barton, K.A., Whiteley, H.R. and Yang, N-S. (1987) Bacillus thuringiensis delta-endotoxin expressed in transgenic Nicotiana tabacumprovides resistance to lepidopteran insects, Plant Physiol.85, 1103–1109
9Perlak, F.J. et al. (1991) Modification of the coding sequence enhances plant expression of insect control protein genes, Proc. Natl. Acad. Sci. U. S. A.88, 3324–3328
10Perlak, F.J. et al. (1990) Insect resistant cotton plants, Biotechnology8, 939–943
11van Aarssen, R. et al. (1995) CryIA(b)transcript formation in tobacco is inefficient, Plant Mol. Biol.28, 513–524
12Perlak, F.J. et al. (1993) Genetically improved potatoes: protection from damage by Colorado potato beetles, Plant Mol. Biol.22, 313–321 13Koziel, M.G. et al. (1993) Field performance of elite transgenic maize plants
expressing an insecticidal protein derived from Bacillus thuringiensis, Biotechnology11, 194–200
14Koziel, M.G., Carozzi, N.B. and Desai, N. (1996) Optimizing expression of transgenes with an emphasis on post-transcriptional events, Plant Mol. Biol. 32, 393–405
15Williams, S. et al. (1992) Chemical regulation of Bacillus thuringiensis delta-endotoxin expression in transgenic plants, Biotechnology10, 540–543
16Wong, E.Y., Hironaka, C.M. and Fischhoff, D.A. (1992) Arabidopsis thaliana small subunit leader and transit peptide enhance the expression of Bacillus thuringiensisproteins in transgenic plants, Plant Mol. Biol.20, 81–93
17McBride, K.E. et al. (1995) Amplification of a chimeric Bacillus gene in chloroplasts leads to an extraordinary level of an insecticidal protein in tobacco, Biotechnology13, 362–365
18Schuler, T.H. et al. (1998) Insect-resistant transgenic plants, Trends Biotechnol.16, 168–175
19Jepson, P.C., Croft, B.C. and Pratt, G.E. (1994) Test systems to determine the ecological risks posed by toxin release from Bacillus thuringiensis genes in crop plants, Mol. Ecol.3, 81–89
20Hilbeck, A. et al. (1998) Effects of transgenic Bacillus thuringiensis corn-fed prey on mortality and development time of immature Chrysoperla carnea (Neuroptera: Chrysopidae), Environ. Entomol.27, 480–487
21Pilcher, C.D. et al. (1997) Preimaginal development, survival, and field abundance of insect predators on transgenic Bacillus thuringiensiscorn, Environ. Entomol.26, 446–454
22Orr, D.B. and Landis, D.A. (1997) Oviposition of European corn borer (Lepidoptera, Pyralidae) and impact of natural enemy populations in transgenic versus isogenic corn, J. Econ. Entomol.90, 905–909
23Fitt, G., Mares, C. and Llewellyn, D. (1994) Field evaluation and potential ecological impact of transgenic cottons (Gossypium hirsutum) in Australia, Biocontrol Sci. Technol.4, 535–548
24Roush, R. (1997) Managing resistance to transgenic crops, in Advances in Insect Control: The Role of Transgenic Plants (Carozzi, N. and Koziel, M., eds), pp. 271–294, Taylor & Francis
25Ferré, J. et al. (1995) Biochemistry and genetics of insect resistance to Bacillus thuringiensisinsecticidal crystal proteins, FEMS Microbiol. Lett.132, 1–7
26Tabashnik, B.E. (1994) Evolution of resistance to Bacillus thuringiensis, Annu. Rev. Entomol.39, 47–79
27Tabashnik, B.E. et al. (1990) Field development of resistance to Bacillus thuringiensisin diamondback moth (Lepidoptera: plutellidae), J. Econ. Entomol.83, 1671–1676
28Liu, Y. et al. (1996) Field-evolved resistance to Bacillus thuringiensistoxin CryIC in diamondback moth (Lepidoptera: Plutellidae), J. Econ. Entomol.89, 798–804
29Alstad, D.N. and Andow, D.A. (1995) Managing the evolution of insect resistance to transgenic plants, Science268, 1894–1896
30Gould, F. (1988) Genetic engineering, integrated pest managment and the evolution of pests, Trends Biotechnol. 3, 515–519
31McGaughey, W.H. and Whalon, M.E. (1992) Managing insect resistance to Bacillus thuringiensistoxins, Science258, 1451–1455
32McGaughey, W.H., Gould, F. and Gelernter, W. (1998) Bt resistance management, Nat. Biotechnol.16, 144–146
33Mellon, M. and Rissler, J. (1998) Now or Never: Serious New Plans to Save a Natural Pest Control, Union of Concerned Scientists, Cambridge, MA, USA 34Carozzi, N. and Koziel, M., eds (1997)Advances in Insect Control: The Role
of Transgenic Plants, Taylor & Francis
35Krattiger, A. (1997) Insect Resistance in Crops: a Case Study of Bacillus thuringiensis (Bt) and its Transfer to Developing Countries, International Service for the Acquisition of Agri-biotech Applications Briefs No. 2, ISAAA, Ithaca, NY, USA
36Grochulski, P. et al. (1995) Bacillus thuringiensisCryIA(a) insecticidal toxin–crystal structure and channel formation, J. Mol. Biol. 254, 447–464
Ruud A. de Maagd*, Dirk Bosch and Willem Stiekema are at the Dept of Molecular Biology, DLO-Centre for Plant Breeding and Reproduction Research, PO Box 16, 6700 AA Wageningen, The Netherlands.
*Author for correspondence