Accumulation of antibody fusion proteins in the cytoplasm and
ER of plant cells
Holger Spiegel
a, Stefan Schillberg
a,b,*, Markus Sack
a, Achim Holzem
a,
Jo¨rg Na¨hring
a, Michael Monecke
a, Yu-Cai Liao
a, Rainer Fischer
a,b aInstitut fu¨r Biologie I(Botanik/Molekulargenetik),RWTH Aachen,Worringerweg1,D-52074Aachen,Germany bFraunhofer Department for Molecular Biotechnology,IUCT,Grafschaft,Auf dem Aberg1,D-57392Schmallenberg,GermanyReceived 17 February 1999; received in revised form 19 July 1999; accepted 20 July 1999
Abstract
To test whether the accumulation of cytoplasmically targeted recombinant antibodies could be improved by fusion to a cytoplasmic protein, we generated a series of single chain antibody-fusion proteins and assayed the levels of functional protein. Glutathione S-transferase (GST) fromSchistosoma japonicum, coat protein (CP) from TMV, thioredoxin from tobacco (TRXt) or thioredoxin from Escherichia coli (TRXe) was fused to the N-terminus of scFv24, a TMV specific single chain antibody. Accumulation of functional fusion proteins in the endoplasmic reticulum (ER) and plant cell cytoplasm was analysed by transient expression in tobacco leaves. ELISA analysis demonstrated that the fusion partners did not prevent the binding of scFv24 to TMV virions. However, accumulation of functional scFv24 was dependent on the fusion partner coupled to it. CP-scFv and GST-scFv fusion protein accumulation amounted to 1mg and 3mg/g of leaf material, respectively, whereas the thioredoxin fusion proteins were produced at low levels. Western blot and surface plasmon resonance analysis confirmed the integrity of the ER retained CP and GST fusion proteins. In the cytoplasm, only the CP fusion protein was detectable (1 – 5 ng/gram of leaf material) and levels of scFv24 alone or fused to the other three fusion partners were below the ELISA detection limit. Addition of a KDEL sequence to the C-terminus of the cytoplasmic CP fusion resulted in a 3-fold increase in protein accumulation indicating that an N-terminal CP and the C-terminal KDEL sequence are suitable elements to stabilize scFv antibodies in the cytoplasm. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Recombinant antibodies; Transgenic plants; Protein targeting; Protein stability
www.elsevier.com/locate/plantsci
1. Introduction
Recombinant antibody and antibody fragments (rAb) have been expressed in different plant cell compartments (for review see [1]). The highest accumulation of functional rAb protein has been obtained in the apoplast and ER, reaching up to 6.8% of total soluble protein [2,3]. This has offered
the opportunity for using plants as production vehicles for large quantities of diagnostic or thera-peutically useful antibody molecules. However, in several cases cytoplasmic expression of antibodies is desirable — to inhibit physiological functions [4 – 6] or inactivate pathogens [7 – 9]. Single chain antibodies (scFv) are the preferred rAb for cyto-plasmic expression since they consist of a single polypeptide that does not require in vivo assembly or complex folding [10]. Nevertheless, cytoplasmic protein levels of scFv fragments in plant cells, derived from monoclonal antibodies, are low and do not exceed 0.1% of total soluble protein [7]. It seems likely that the lack of protein disulphide isomerase and specific chaperones in the cyto-plasm results in scFv misfolding and contribute to Abbre6iations: GST, glutathione S-transferase from Schistosoma
japonicum; CP, coat protein from TMV; TRXt, thioredoxin h2 from tobacco; TRXe, thioredoxin fromEscherichia coli; scFv, single chain antibody; TMV, tobacco mosaic virus; N. tabacum, Nicotiana tabacum; N.benthamiana, Nicotiana benthamiana; ELISA, Enzyme-linked immunosorbent assay.
* Corresponding author. Tel.: +49-241-806629; fax: + 49-241-871062.
enhanced proteolysis [11]. However, a recent re-port demonstrated that scFv fragments derived from phage display reached up to 0.3 and 1% of total soluble protein in the cytoplasm of Petunia
leaves and petals, respectively, indicating that phage display selection can be used for enrichment of more stable scaffolds which tolerate the absence of disulfide bonds [12].
Accumulation of cytoplasmic recombinant proteins in prokaryotes has been enhanced by the addition of a stabilizing fusion protein, and anti-body stability in plants has been improved by the fusion of peptide sequences to the antibody C-ter-minal. Schouten et al. [13,14] demonstrated that addition of a C-terminal KDEL sequence signifi-cantly increased scFv protein levels in the plant cytoplasm, indicating that fusion to short polypep-tides may protect the scFv fragment from prote-olytic degradation. In Escherichia coli, linkage to N-terminal fusion partners significantly increased cytoplasmic accumulation levels of functionally active protein. Small cytoplasmic proteins, such as thioredoxin (TRX), glutathione S-transferase (GST) or the maltose binding protein [15 – 17] were successfully used and improved the solubility and stability of heterologous proteins.
The success of protein stabilization with fusion partner proteins in the E.coli system prompted us to evaluate the suitability of fusion partners to stabilize scFv fragments in the plant cytoplasm. Here, we used the TMV-specific scFv24, which has a high affinity towards epitopes present on intact virions and confers virus resistance to transgenic plants [9]. scFv24 has been successfully expressed to high level in the apoplast of tobacco plants but expression only reaches low levels in the cytoplasm [9]. Our rationale was to improve cytoplasmic protein levels by linking scFv24 to small cytoplas-mic proteins. scFv24 was fused to GST from
Schistosoma japonicum, TMV coat protein (CP), thioredoxin from tobacco (TRXt) or thioredoxin from E. coli (TRXe) and these scFv-fusion proteins were expressed in the ER or the cyto-plasm of tobacco leaves. The results demonstrated that all four scFv24 fusion proteins were func-tional but their protein levels were directly related to the fusion partner. The highest levels of func-tional fusion protein were observed for the ER targeted GST-scFv24 fusion protein, whereas in the cytoplasm only the CP fusion gave a signifi-cantly increased level of functional fusion protein.
Cytosolic levels of CP-scFv24 could be further increased by the addition of a C-terminal KDEL sequence.
2. Materials and methods
2.1. Plasmids, bacteria, plants
The following plasmids, bacterial strains and plants were used throughout this study. Plasmids: pUC18 [18], pSS [19]; bacterial strains: E. coli
SCS110 (Stratagene, Heidelberg, Germany),
Agrobacterium tumefaciens GV3101: (pMP90RK, GmR KmR RifR), [20]; plants:Nicotiana tabacum
cv. L. Petite Havana SR1, Nicotiana benthamiana.
2.2. Vector design and construction
The gene fusion partners glutathione S-trans-ferase (GST) from S.japonicum, coat protein (CP) from TMV, thioredoxin from tobacco (TRXt) and thioredoxin fromE.coli(TRXe) were amplified by PCR or RT-PCR. cDNAs were amplified from the pGEX-5x-3 vector (Pharmacia, Freiburg, Ger-many) containing GST, a cDNA clone from TMV (kindly provided by Dr. Dennis Lewandowski, CREC, FA, USA), total RNA isolated from to-bacco [9] and the pTrxFus vector (Invitrogen, Leek, The Netherlands) containing bacterial thioredoxin as a template. The forward primers introduced a NcoI restriction site (5% end) and the backward primers a C-terminal (Gly4Ser)2 linker
5%-ACT GCG CCA TGG GGA GCG ATA AAA TTA TT-3%, TRXe-back 5%-CCG TCA GAC GTG AGA ACC TCC ACC TCC ACT TCC GCC GCC TCC GGC CAG GTT AGC GTC GAG GAA CTC TTT CAA-3%. The 5%-NcoI and 3%
-AatII restricted PCR fragments were subcloned into a pUC18 derivative containing the TMV spe-cific scFv24 [9] flanked by the 5% untranslated region (omega-sequence, V) and 3% untranslated region (Pw sequence) from TMV [21,22]. A C-ter-minal His6- (H) or a KDEL-sequence (K) were added to scFv24 of all four constructs by PCR using the backward primers: His6-back 5%-CTA CCC CTC GAG TTT AGT GAT GGT GAT GGT GAT GAG CGG CCG CGT CGA CTG CAG AGA CAG TGA CCA GAG TC-3% and KDEL-back 5%-CCC TCA CTC GAG TTT AGA GCT CAT CTT TCT CAG ATC CAC GAG CGG CCG CAG AAC CTC CAC CTC CGT CGA CTG CAG AGA CAG TGA CCA G-3%. The subsequent ligation of the EcoRI-AscI
frag-ments into the plant expression vector pSS, con-taining an double enhanced 35S promoter [19], resulted in the final expression constructs GST-scFv24H, GST-scFv24K, CP-scFv24H, CP-scFv24K, TRXt-scFv24H, TRXt-scFv24K, TRXe-scFv24H and TRXe-scFv24K, which were used for analyzing scFv-fusion protein accumula-tion in the cytoplasm (Fig. 1A).
For ER targeting and retention, the plant codon optimized leader sequence derived from the light chain of the murine monoclonal antibody mAb24 [19] was integrated between the 5% V untranslated region and scFv24 of the four different cytoplas-mic constructs containing the KDEL sequence, giving the four plant expression vectors L-GST-scFv24K, L-CP-L-GST-scFv24K, L-TRXt-scFv24K and L-TRXe-scFv24K (Fig. 1C).
Two expression vectors lacking a leader se-quence and an N-terminal fusion partner but con-taining scFv24 with a C-terminal His6 or KDEL sequence were used as controls for cytoplasmic accumulation (scFv24H, scFv24K, Fig. 1B).
Fig. 1. Constructs for expression of scFv24 fusion proteins in the cytoplasm and ER of plant cells. scFv24 cDNA, comprising variable light chain (VL) and heavy chain (VH) domains connected by a 14 amino acid 212 linker (linker 2), were fused to GST,
CP, TRXt or TRXe using the (Gly4Ser)2linker (linker 1) and subcloned into the plant expression vector pSS [19]. (A) Cytoplasmic
targeting vectors containing a C-terminal His6 or KDEL sequence. (B) Cytoplasmic targeting control vectors lacking a fusion partner. (C) ER retention vectors. 35SS=enhanced CaMV-35S promoter; V=5% untranslated region of TMV; LP=codon
2.3. Transformation of Agrobacterium tumefaciens and tobacco plants
Plant expression constructs were transferred intoA. tumefaciensGV3101 by N2transformation [23]. Transient transformation of Nicotiana tabacum cv. Petite Havana SR1 and N. benthami
-ana was performed as described [24].
2.4. Generation of anti-mAb24 polyclonal antibodies
Anti-mAb24 polyclonal antibodies were raised in rabbits (Charles River Wiga, Hannover, USA) and affinity purified, as described [9].
2.5. Protein extraction and analysis
To extract total soluble proteins, tobacco leaves were frozen and ground in liquid nitrogen and scFv-fusion protein level was analysed by ELISA and Western blot ([25], ELISA III). A Fab frag-ment of the mAb24 was used as the ELISA stan-dard. For surface plasmon resonance analysis, tobacco leaves were extracted using HBS-buffer containing dextran matrix (150 mM NaCl, 3.4 mM EDTA, 0.05% (v/v) Surfactant P20 (BioSen-sor, Upppsala, Sweden), 10 mM HEPES, pH 7.4, 1 mg/ml dextran matrix (BioSensor)) and cen-trifuged at high speed (40 000×g, 15 min, 4°C) to remove insoluble precipitate. Protein concentra-tions were determined with the BioRad protein assay using bovine serum albumin (BSA) as the standard.
2.6. Affinity purification
For affinity purification of scFv-fusion proteins from plant extracts (prepared as described above), TMV virions were coupled to an activated CNBr sepharose matrix. 300 mg of CNBr activated sep-harose 4B matrix (Pharmacia, Freiburg, Germany) was resuspended in 1 ml PBS pH 7.4 (1.37 M NaCl, 27 mM KCl, 81 mM Na2HPO4, 15 mM KH2PO4) and incubated for 1 h at RT on a rotator. The matrix was pelleted (5000×g, 5 min, RT), resuspended in 1 ml PBS pH 7.4 containing 10 mg TMV virions and incubated for 2 h at RT on a rotator. The TMV coupled matrix was cen-trifuged (5000×g, 5 min, RT), resuspended in 1 ml PBS pH 7.4 containing 1% (w/v) BSA and 1%
(w/v) powdered milk and rotated over night at 8°C to block nonspecific binding sites. The TMV cou-pled matrix was washed three times with PBS pH 7.4 and resuspended in 1 ml PBS pH 7.4. 30 ml TMV-matrix was added to 1.5 ml plant extract (prepared as described above) and incubated for 1 h at RT on a rotator. Then the TMV-matrix was washed three times with PBS and the TMV-matrix bound proteins were solubilised in sample buffer and analysed by SDS-PAGE [26].
2.7. Surface plasmon resonance
Biomolecular interaction analyses were carried out in HBS-buffer (150 mM NaCl, 3.4 mM EDTA, 0.05% (v/v) Surfactant P20, 10 mM HEPES, pH 7.4) using the BIAcore® 2000 (BioSensor, Uppsala, Sweden). TMV was immobi-lized on a CM5-rg sensorchip (BioSensor) using the Amine Coupling Kit (BioSensor, Uppsala, Sweden) [27]. The surface of the sensorchip was activated with 70 ml EDC/NHS buffer (100 mM
N- ethyl -N%- (dimethylaminopropyl) - carbodimide-hydrochloride, 400 mM N-hydroxy-succinimide) using a flow rate of 10ml/min. For immobilization of the virus, 200 mg of TMV in 100 ml 10 mM formic acid pH 3.0 were applied (flow rate: 5
ml/min). Subsequently, the sensorchip was deacti-vated with 70 ml 1M ethanolamine hydrochloride pH 8.5 (flow rate: 10ml/min) and conditioned with 10 ml 100 mM HCl (flow rate: 5 ml/min). Between sample injections the surface was regenerated with 10 ml 30 mM HCl (flow rate: 30 ml/min).
3. Results
3.1. Analysis of fusion protein accumulation in the ER
Four different cytoplasmic expressed proteins were selected as fusion partners to analyse their effects on the function and stability of the TMV-specific scFv24. Glutathione S-transferase (GST) from S. japonicum [16], the tobacco mosaic virus coat protein (CP) [28], thioredoxin h2 from to-bacco (TRXt) [29] and thioredoxin from E. coli
sig-Fig. 2. Protein levels of ER retained scFv-fusion proteins.N.
tabacumcv. Petite Havana SR1 leaves were transiently trans-formed with recombinant agrobacteria containing the con-structs L-TRXt-scFv24K, L-TRXe-scFv24K, L-CP-scFv24K, L-GST-scFv24K and incubated for 3 days. Total soluble protein was isolated and levels of functional scFv24-fusion proteins, were quantified in a TMV-specific ELISA and indi-cated as ng/g of leaf material. Each column represents the mean value of four leaves. Standard deviations are indicated.
mg/g of leaf material) and the thioredoxin fusion proteins L-TRXt-scFv24K and L-TRXe-scFv24K showed the lowest accumulation (average 190 ng or 40 ng/g of leaf material, respectively).
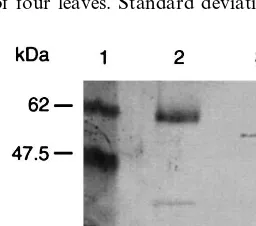
To verify the integrity of scFv24 fusion proteins, western blot analysis was carried out using affinity purified L-GST-scFv24K and L-CP-scFv24K, which showed the highest protein levels in tran-sient expression assays. In both cases, intact fusion proteins were detected with the expected size of 56.5 kDa for L-GST-scFv24K and 49.5 kDa for L-CP-scFv24K (Fig. 3). However, a faint prote-olytic 27 kDa product of the GST-scFv24 fusion protein was detected using an anti-GST antisera. It is likely that cleavage occurred in the linker region 1 because the detected faint band corre-sponds to the predicted size of GST (27 kDa).
In addition to ELISA the capacity of the GST-fusion protein scFv24 domain to bind antigen was confirmed by surface plasmon resonance based biomolecular interaction analysis [31]. The dextran matrix of a CM5-rg sensorchip was coated with intact TMV virions, using standard amine cou-pling chemistry. After surface stabilization, a protein extract from a non transformed tobacco leaf was injected followed by anti-GST antisera, to monitor nonspecific binding. As shown in Fig. 4, no binding was observed when these controls were applied. A protein extract from a tobacco leaf
Fig. 3. Western blot analysis of ER retained fusion proteins. Affinity purified L-GST-scFv24K and L-CP-scFv24K were separated by 12% SDS-PAGE and proteins were transferred to a nitrocellulose membrane. Blots were probed with GST rabbit antisera and CP-specific mAb29 primary anti-body followed by alkaline phosphatase conjugated goat-anti rabbit and goat-anti mouse secondary antibody and NBT/ BCIP staining. Lane 1: Prestained protein marker; lane 2: affinity purified L-GST-scFv24K; lane 3: affinity purified L-CP-scFv24K.
Fig. 4. Surface plasmon resonance analysis of the L-GST-scFv24K fusion protein. TMV virions were immobilized on the surface of a CM5rg sensorchip and extracts from a non-transformed and a transformed tobacco leaf were analysed by surface plasmon resonance using a BIAcore 2000®. The sensogram shows injection of: (1)
non-trans-formed tobacco leaf extract; (2) 25 mg/ml anti-GST antisera; (3) total soluble protein extract from a L-GST-scFv24K producing tobacco leaf; (4) 25 mg/ml anti-GST antisera. All injections were made in HBS-buffer with a flow rate of 5 ml/min.
nificantly higher in the ER than cytoplasmic accu-mulation [1,13], which facilitates analysis of func-tionality and integrity of the recombinant fusion proteins.
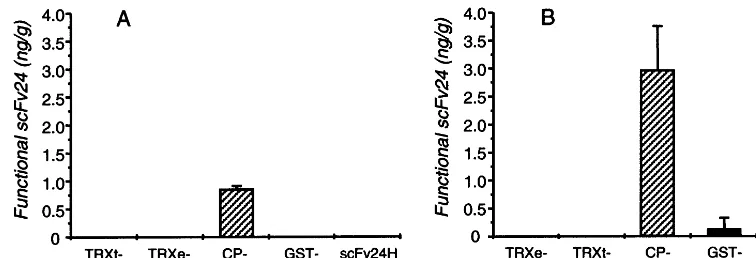
Fig. 5. Levels of cytoplasmic expressed fusion proteins. N. benthamiana leaves were transiently transformed with recombinant agrobacteria and incubated for 3 days. Total soluble protein was isolated and levels of functional scFv24, expressed as part of the fusion proteins, were quantitated in a TMV-specific ELISA and indicated as ng/g of leaf material. Each column represents the mean value of four leaves. Standard deviations are indicated with bars. (A) Protein levels of constructs GST-scFv24H, CP-scFv24H, TRXt-scFv24H, TRXe-scFv24H and scFv24H containing a C-terminal His6 sequence. (B) Protein levels of constructs GST-scFv24K, CP-scFv24K, TRXt-scFv24K, TRXe-scFv24K and scFv24K containing a C-terminal KDEL sequence.
producing L-GST-scFv24K was injected and bind-ing was observed (1470 response units), as the TMV-specific scFv24 domain of the fusion protein was captured by the TMV virions. The presence of the fusion partner GST was subsequently confi-rmed by additional binding of an anti-GST antis-era (800 response units, Fig. 4).
3.2. Analysis of fusion protein accumulation in the cytoplasm
For cytoplasmic accumulation of fusion proteinsN. benthamiana leaves were used, because protein level was higher than usingN.tabacum cv. Petite Havana SR1 leaves (data not shown). Anal-ysis using the four constructs containing the C-ter-minal His6 sequence demonstrated that only CP-scFv24H was detectable in a TMV-specific ELISA, with an average protein level of 0.9 ng functional active scFv24/g of leaf material. Protein levels of GST-scFv24H, TRXt-scFv24H and TRXe-scFv24H were below the ELISA detection limit (0.5 ng) as was the control construct scFv24H lacking an N-terminal fusion partner (Fig. 5A).
We evaluated the influence of a C-terminal KDEL sequence on the accumulation of scFv24 fusion proteins. Addition of the C-terminal KDEL sequence increased the level of fusion proteins (Fig. 5B). The average protein level of the KDEL tagged scFv24K was 3-fold higher than CP-scFv24H (2.9 ng per gram leaf material). However, levels of GST-scFv24K, TRXt-scFv24K, TRXe-scFv24K and the control construct TRXe-scFv24K were
below the ELISA detection threshold. A control ELISA performed without the antigen TMV gave no signal, indicating that values of CP-scFv24H and CP-scFv24K did not correspond to binding of CP-fusions to anti TMV polyclonal sera (data not shown).
4. Discussion
In this paper, the hypothesis was tested that small cytoplasmic proteins could stabilize ex-pressed single chain antibodies in the plant cytosol and ER. Glutathione S-transferase (GST) from S.
ER environment is oxidizing, but the thioredoxin oxido-reductase activity in the ER may be suffi-cient to affect the formation of disulphide bridges. Disulphide bridges are a prerequisite for the func-tion of scFv fragments [33] and this may explain why levels of functional TRX-scFv24 fusions de-tected by ELISA were low.
High protein levels in the ER were obtained using the fusion partners CP and GST, reaching a maximum of 3mg GST-scFv24 fusion protein/g of leaf material. A possible explanation is that since GST is also natural component of the ER [34], it is intrinsically stable in the compartment and so stabilizes scFv24.
In contrast to ER expression, functional scFv24 was only detectable in the cytoplasm when fused to a TMV coat protein partner. A further 3-fold increase of the CP fusion protein level was at-tained by the addition of a C-terminal KDEL sequence. The stabilizing effect of the short KDEL sequence was also demonstrated for other cytoplasmically expressed scFv fragments and may be based on C-terminal protection against proteolytic degradation [13,14]. In case of the thioredoxin and GST fusion proteins as well as the scFv24 construct without any fusion partner, values did not exceeded the ELISA detection limit. Why the CP fusion partner significantly improved scFv24 stability is unclear. CP is very stable in the cytoplasm and is produced in high amounts during the TMV infection cycle [35]. It seems likely that CP facilitates folding and pro-tects scFv24 from proteolytic degradation. A sec-ond possibility is that CP could form proto-viral disks, the preliminary stage of intact virion forma-tion [36], thereby generating the neotope recog-nized by scFv24 [19]. Binding to the neotope epitope would promote an antigen-dependent folding leading to higher levels of functional scFv24 [37]. However, such proto-viral disk for-mation is unlikely since the levels of CP detected in tobacco cytoplasm were far below the threshold required to induce proto-viral disk formation. In vitro experiments indicated that 106 higher levels are needed for the formation of disks [36]. Bind-ing and stabilization through bindBind-ing the CP can also be excluded, because ELISA studies, as well as western blot analysis, have demonstrated that scFv24 does not bind CP, but only recognises intact virus particles (data not shown).
The transient state of accumulation may not be
fully comparable to results after stable integration into tobacco chromosomes and extended screen-ing of different individual lines. However, a num-ber of different antibody fragments targeted to different plant cell compartments were used in transient and stable transformation experiments in our lab. The data from these experiments indicate that similar recombinant protein levels were found in transient and stably transformed plants (un-published results). Therefore, we anticipate that the accumulation of scFv24 fusion protein de-tected in transient assays will be found in a simi-lar range in stably transformed plant lines. Hondred et al. [38] have shown that N-terminal fusion partners could augment expression of re-combinant proteins in stably transformed plants. Although the fusion partner (ubiquitin) was cleaved off during processing, it led to an in-creased accumulation of recombinant protein, which was independent of steady-state levels of the transcripts. Therefore, this is an attractive alternative which could compliment our ap-proach.
Our data have shown that cytoplasmic accumu-lation of functional scFv fragments was improved by linkage to a tobacco mosaic viral coat protein fusion partner. We demonstrated that cytoplasmic stabilization of scFv fragments with a N-terminal fusion protein such as TMV coat protein has the potential to provide a rapid and easy alternative to time consuming methods for scFv stability im-provement, such as molecular evolution [37,39] or loop grafting [40]. This alternative approach is particularly important when generating an scFv from an existing hybridoma cell line producing a monoclonal antibody of interest, for these tend to be unstable upon cytosolic expression when com-pared to phage derived scFvs. As there are still a great number of high affinity monoclonal anti-bodies available that bind to cytosolic targets, combined N- and C-terminal scFv stabilization has a great potential to interfere in plant patho-genesis and modify metabolic pathways.
Acknowledgements
References
[1] U. Conrad, U. Fiedler, Compartment-specific accumula-tion of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomod-ulation of physiological functions and pathogen activity, Plant Mol. Biol. 38 (1998) 101 – 109.
[2] A. Hiatt, R. Cafferkey, K. Bowdish, Production of anti-bodies in transgenic plants, Nature 342 (1989) 76 – 78. [3] U. Fiedler, J. Phillips, O. Artsaenko, U. Conrad,
Opti-mization of scFv antibody production in transgenic plants, Immunotechnology 3 (1997) 205 – 216.
[4] M. Owen, A. Gandecha, B. Cockburn, G. Whitelam, Synthesis of a functional anti-phytochrome single-chain Fv protein in transgenic tobacco, Biotechnology 10 (1992) 790 – 794.
[5] O. Artsaenko, M. Peisker, U. zur Nieden, U. Fiedler, E.W. Weiler, K. Muntz, U. Conrad, Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco, Plant J. 8 (1995) 745 – 750.
[6] J. Phillips, O. Artsaenko, U. Fiedler, C. Horstmann, H.P. Mock, K. Muntz, U. Conrad, Seed-specific im-munomodulation of abscisic acid activity induces a de-velopmental switch, EMBO J. 16 (1997) 4489 – 4496. [7] P. Tavladoraki, E. Benvenuto, S. Trinca, D. De
Mar-tinis, A. Cattaneo, P. Galeffi, Transgenic plants express-ing a functional sexpress-ingle-chain Fv antibody are specifically protected from virus attack, Nature 366 (1993) 469 – 472. [8] L.F. Fecker, R. Koenig, C. Obermeier, Nicotiana ben
-thamianaplants expressing beet necrotic yellow vein virus (BNYVV) coat protein-specific scFv are partially pro-tected against the establishment of the virus in the early stages of infection and its pathogenic effects in the late stages of infection, Arch. Virol. 142 (1997) 1857 – 1863. [9] S. Zimmermann, S. Schillberg, Y.C. Liao, R. Fischer,
Intracellular expression of TMV-specific single-chain Fv fragments leads to improved virus resistance inNicotiana tabacum, Mol. Breeding 4 (1998) 369 – 379.
[10] R.E. Bird, K.D. Hardman, J.W. Jacobson, S. Johnson, B.M. Kaufman, S.M. Lee, T. Lee, S.H. Pope, G.S. Riodan, M. Whitlow, Single-chain antigen-binding proteins, Science 242 (1988) 226 – 423.
[11] S. Biocca, F. Ruberti, M. Tafani, P. Pierandrei-Amaldi, A. Cattaneo, Redox state of single chain Fv fragments targeted to the endoplasmic reticulum, cytosol and mito-chondria, Biotechnology 13 (1995) 1110 – 1115.
[12] G. De Jaeger, E. Buys, D. Eeckhout, C. De Wilde, A. Jacobs, J. Kapila, G. Angenon, M. Van Montagu, T. Gerats, A. Depicker, High level accumulation of single-chain variable fragments in the cytosol of transgenic
Petunia hybrida, Eur. J. Biochem. 259 (1998) 426 – 434. [13] A. Schouten, J. Roosien, F.A. van Engelen, G.A. de
Jong, A.W. Borst-Vrenssen, J.F. Zilverentant, D. Bosch, W.J. Stiekema, F.J. Gommers, A. Schots, J. Bakker, The C-terminal KDEL sequence increases the expression level of a single-chain antibody designed to be targeted to both the cytosol and the secretory pathway in transgenic tobacco, Plant Mol. Biol. 30 (1996) 781 – 793.
[14] A. Schouten, J. Roosien, J.M. de Boer, A. Wilmink, M.N. Rosso, D. Bosch, W.J. Stiekema, F.J. Gommers, J. Bakker, A. Schots, Improving scFv antibody expression levels in the plant cytosol, FEBS Lett. 415 (1997) 235 – 241.
[15] E.R. LaVallie, E.A. DiBlasio, S. Kovacic, K.L. Grant, P.F. Schendel, J.M. McCoy, A thioredoxin gene fusion expression system that circumvents inclusion body for-mation in theE.colicytoplasm, Biotechnology 11 (1993) 187 – 193.
[16] D.B. Smith, K.S. Johnson, Single-step purification of polypeptides expressed inEscherichia colias fusions with glutathione S-transferase, Gene 67 (1988) 31 – 40. [17] C.V. Maina, P.D. Riggs, A.G.D. Grandea, B.E. Slatko,
L.S. Moran, J.A. Tagliamonte, L.A. McReynolds, C.D. Guan, An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from mal-tose-binding protein, Gene 74 (1988) 365 – 373.
[18] J. Messing, New M13 vectors for cloning, Methods Enzymol. 101 (1983) 20 – 78.
[19] A. Voss, M. Niersbach, R. Hain, H.J. Hirsch, Y.C. Liao, F. Kreuzaler, R. Fischer, Reduced virus infectivity inN.
tabacum secreting a TMV-specific full-size antibody, Mol. Breeding 1 (1995) 39 – 50.
[20] C. Koncz, J. Schell, The promoter of TL-DNA gene 5 controls the tissue specific expression of chimeric genes carried by a novel type ofAgrobacteriumbinary vector, Mol. Gen. Genet. 204 (1986) 383 – 396.
[21] J. Schmitz, D. Pru¨fer, W. Rohde, E. Tacke, Non-canon-ical translation mechanisms in plants: efficient in vitro and in planta initiation at AUU codons of the tobacco mosaic virus enhancer sequence, Nucleic Acids Res. 24 (1996) 257 – 263.
[22] D.R. Gallie, M. Kobayashi, The role of the 3% -untrans-lated region of non-polyadeny-untrans-lated plant viral mRNAs in regulating translational efficiency, Gene 142 (1994) 159 – 165.
[23] R. Ho¨fgen, L. Willmitzer, Storage of competent cells for
Agrobacterium transformation, Nucleic Acids Res. 16 (1988) 9877.
[24] J. Kapila, R. De Rycke, M. Van Montagu, G. Angenom, An Agrobacterium-mediated transient gene expression system for intact leaves, Plant Sci. 122 (1996) 101 – 108. [25] R. Fischer, J. Drossard, Y.C. Liao, S. Schillberg, in: C.
Cunningham, A.J.R. Porter (Eds.), Methods in Biotech-nology, Production and Isolation of Clinically Useful Compounds, Recombinant Proteins in Plants, vol. 3, Humana Press, Totowa, NJ, 1998.
[26] U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
[27] B. Johnsson, S. Lo¨fas, G. Lindquist, Immobilization of proteins to a carboxymethyldextran-modified gold sur-face for bispecific interaction analysis in sursur-face plasmon resonance sensors, Anal. Biochem. 198 (1991) 268 – 277. [28] P. Goelet, G.P. Lomonossoff, P.J. Butler, M.E. Akam, M.J. Gait, J. Karn, Nucleotide sequence of tobacco mosaic virus RNA, Proc. Natl. Acad. Sci. USA 79 (1982) 5818 – 5822.
[29] C. Brugidou, I. Marty, Y. Chartier, Y. Meyer, The
thioredoxin genes which are differently expressed, Mol. Gen. Genet. 238 (1993) 285 – 293.
[30] A. Holmgren, Thioredoxin, Ann. Rev. Biochem. 54 (1985) 237 – 271.
[31] M.H. Van Regenmortel, Use of biosensors to character-ize recombinant proteins, Dev. Biol. Stand. 83 (1994) 143 – 151.
[32] H. Eklund, F.K. Gleason, A. Holmgren, Structural and functional relations among thioredoxins in different spe-cies, Proteins: Structure, Functions and Genetics 11 (1991) 13 – 28.
[33] R. Glockshuber, T. Schmidt, A. Pluckthun, The disulfide bonds in antibody variable domains: effects on stability, folding in vitro, and functional expression inEscherichia coli, Biochemistry 31 (1992) 1270 – 1279.
[34] I. Asakura, M. Sugimoto, K. Ito, GlutathioneS -trans-ferases in primary rat hepatocytes, Gastroenterol. Jpn. 28 (1993) 34 – 45.
[35] L.C. van Loon, O. van Kooten, E.G.A. Linders, C. Meurs, M.M.G. Wijdeveld, in: R.S.S. Fraser (Ed.), Recognition and Response in Plant – Virus Interactions,
vol. H41, Springer – Verlag, Berlin, 1990, pp. 311 – 328. [36] K. Raghavendra, J.A. Kelly, L. Khairallah, T.M.
Schus-ter, Structure and function of disk aggregates of the coat protein of tobacco mosaic virus, Biochemistry 27 (1988) 7583 – 7588.
[37] K. Tsumoto, Y. Nishimiya, N. Kasai, H. Ueda, T. Nagamune, K. Ogasahara, K. Yutani, K. Tokuhisa, M. Matsushima, I. Kumagai, Novel selection method for engineered antibodies using the mechanism of Fv frag-ment stabilization in the presence of antigen, Protein Eng. 10 (1997) 1311 – 1318.
[38] D. Hondred, J.M. Walker, D.E. Mathews, R.D. Vierstra, Use of ubiquitin fusions to augment protein expression in transgenic plants, Plant Physiol. 119 (1999) 713 – 772. [39] K. Proba, A. Worn, A. Honegger, A. Plu¨ckthun, Anti-body scFv fragments without disulfide bonds made by molecular evolution, J. Mol. Biol. 275 (1998) 245 – 253. [40] S. Jung, A. Pluckthun, Improving in vivo folding and
stability of a single-chain Fv antibody fragment by loop grafting, Protein Eng. 10 (1997) 959 – 966.