Properties of acid phosphatase–tannic acid complexes formed in the
presence of Fe and Mn
M.A. Rao*, L. Gianfreda
Dipartimento di Scienze Chimico-Agrarie, Universita` di Napoli “Federico II”, via Universita` 100, 80055 Portici, Naples, Italy
Accepted 15 May 2000
Abstract
The preparation and characterisation of synthetic enzyme complexes simulating those usually encountered in soil were investigated. Complexes were prepared at 308C by the interaction of acid phosphatase and tannic acid in the presence and absence of Fe and Mn ions (as chlorides) and oxides, with and without montmorillonite. In comparison with phosphatase–tannic acid complexes, the enzymatic complexes formed in the presence of Fe oxide showed higher activity levels (.70%). Non significant increases were measured in the presence of Fe and Mn ions and Mn oxide. In the presence of montmorillonite, a further increase in the activity of the resulting complexes was measured. For example, the activity of complexes formed with Fe31or Mn21increased by 25 and 28%, respectively. In all cases, the kinetics of the immobilised enzyme conformed to the Michaelis–Menten equation and the Vmaxvalues were significantly P0:001lower and the Km
values significantly higher than those of the free enzyme. For example, a two-fold reduction in the Vmaxand four-fold increase in the Km
parameters were measured for phosphatase complexed with tannic acid. In the presence of montmorillonite, no significant changes of Vmax
values were measured whereas a detectable reduction of Kmvalues was observed. Immobilised phosphatase showed a greater sensitivity to
increases in temperature, but higher stability to proteolytic attack. Complexes with Fe ions and MnO2were the most stable.q2000 Elsevier
Science Ltd. All rights reserved.
Keywords: Soil-enzymes; Organo-mineral complexes; Inorganic catalysts; Fe and Mn oxides and ions
1. Introduction
In soil, the Fe and Mn compounds are involved in numer-ous processes, including adsorption and/or transformation (Boyd and Mortland, 1990; Huang, 1990). An extensive literature is available concerning the role that Fe and Mn compounds may play in soil as catalysts of processes invol-ving simple or complex aromatic compounds (e.g. mono and polyphenols, humic-like precursors) (Burns, 1986; Huang, 1990). Biotic catalysts, such as oxidoreductive enzymes, may also promote similar processes (Bollag, 1992). Depending on the nature, number and type of aromatic compounds and/or catalysts involved, the ultimate result of such processes is the formation of polymers and/or copolymers of various sizes and complexity, that contribute to the pool of humic materials (Shindo and Huang, 1992; Naidja et al., 1998).
Recently, Naidja et al. (1998) have demonstrated that the catechol –melanin complexes were formed whether the polymerisation of a phenol such as catechol was catalysed
by birnessite (ad-manganese oxide) or an oxidative enzyme such as tyrosinase. However, the authors observed that the reaction products with a lower degree of aromatic ring condensation and lower molecular mass were produced by the abiotic catalysis as compared with those generated in the presence of tyrosinase.
Other molecules, such as proteins, may be involved in the polymerisation of phenolic compounds and polymeric organic aggregates entrapping protein molecules may form (Ladd and Butler, 1975). Adsorption of proteins on the exter-nal surfaces of already formed organic aggregates may also occur (Ladd and Butler, 1975). If the involved protein is an enzyme, the enzymatic-copolymer complexes showing a cata-lytic activity may result (Sarkar and Burns, 1983, 1984).
In the previous studies, we and others have demonstrated that active enzymatic complexes were generated by the interaction of urease, invertase b-glucosidase and acid phosphatase with tannic acid, a humic-like precursor (Sarkar and Burns, 1984; Gianfreda et al., 1993, 1995a; Rao et al., 1996). The presence of montmorillo-nite during the process usually produced organo-mineral complexes with higher enzymatic activity as compared with those complexes obtained without clay.
0038-0717/00/$ - see front matterq2000 Elsevier Science Ltd. All rights reserved.
PII: S 0 0 3 8 - 0 7 1 7 ( 0 0 ) 0 0 1 6 7 - X
www.elsevier.com/locate/soilbio
Further investigations showed that the addition of Fe and/ or Mn, (as ions or oxides) influenced significantly the formation and the resulting enzymic properties of urease– tannic acid complexes (Gianfreda et al., 1995a,b). The purpose of the work reported here was to compare the ability of Fe and Mn (as ions and oxides), to promote the formation of complexes of tannic acid and acid phosphatase and to affect their catalytic properties. Acid phosphatase was chosen as it is one of the most studied enzymes in soil because of its essential role in the phosphorus cycle (Speir and Ross, 1978). The influence of the clay montmorillonite on the enzymic properties of the various complexes was also investigated.
2. Materials and methods
2.1. Chemicals
Acid phosphatase (P) (EC. 3.1.3.2, from potato, MW,100 kDa, Type I, 60 U mg21) was purchased from Boehringer Mannheim, Germany. Proteinase K (EC 3.4.21.14, about 20 Anson U g21) was purchased from Sigma Chemical Co., St Louis, Mo, USA. Tannic acid (T) (MW 1701.23) was a reagent from Fluka AG. All the other chemicals were reagent grade and were supplied by Analar, BDH, Ltd Poole, UK.
2.2. Inorganic components
An Al-substituted hematite (a-Fe2O3) was synthesised
and characterised as described by Colombo et al. (1994). The analysis by X-ray dffraction of a commercial MnO2
indicated the presence of peaks at 31.4, 24.1, 21.3 and 16.3 nm which are characteristic of pyrolusite (g-MnO2)
(Brindley and Brown, 1980).
A Wyoming montmorillonite (M) (Source Clay Minerals Repository, University of Missouri-Columbia, USA) was ultrasonically dispersed and the fraction,0.2mm was sepa-rated by sedimentation (Gianfreda et al., 1993). The clay was Na-saturated with 0.1 M NaCl solution, then washed with deionized distilled water and dialysed until Cl2free.
2.3. Phosphatase complexes
Phosphatase–tannic acid complexes (P–T) were prepared at 308C in the presence or absence of different inorganic components. Usually, 1 ml of 0.1 M Na-acetate buffer solu-tion at pH 5.0 containing 0.02 mg of acid phosphatase, 0.2 mg of tannic acid [T/P (w:w) of 10] either 1 mM FeCl3 and MnCl2 (cation Fe31 or Mn21/tannic acid acid
molar ratio0.4) or 10 mg of Fe2O3and MnO2were
incu-bated at 308C for 1 h. Phosphatase–tannic acid–Fe and –Mn chloride or oxide complexes were also prepared in the presence of montmorillonite at the ratio tannic acid/ montmorillonite (T/M) (w:w) of 0.1. After incubation the residual activity of the suspensions were assayed
before centrifugation at 10,000g for 30 min. The pellets, consisting of the insoluble complexes, were collected, washed twice with 1 ml acetate buffer at pH 5.0 and resuspended in an equal volume of buffer. The activities of the insoluble complexes, supernatant fractions and washings were measured.
The insoluble complexes were usually used immediately after preparation but, if necessary, were stored at 108C and pH 5.0 and their residual activity periodically measured. When the enzymatic activity had declined by more than 20% (with respect to that of the initial preparation), new complexes were prepared.
2.4. Phosphatase assay
The activity of free and immobilised phosphatase was assayed with 1 ml of 6 mM p-nitrophenylphosphate (pNPP) in 0.1 M Na-acetate buffer at pH 5.0 and 308C. After 20 min incubation 1 M NaOH was added and the concentration of p-nitrophenol was determined by measur-ing the adsorbance at 405 nm with a spectrophotometer. To avoid interference by turbidity, the samples were centri-fuged at 3000g for 15 min prior to measurement.
One enzymatic unit was defined as themmol of p-nitro-phenol produced by 1 ml of (free or immobilised) enzyme in 1 min at 308C and at pH 5.0. The specific activity was expressed as the units measured per mg21of protein.
The dependence of activity on temperature was obtained using the range 10–608C and the activation energy (Ea) was
calculated by plotting the log of activities of free and immo-bilised phosphatase vs. 1/T (in K) according to the Arrhe-nius equation. The value of activation energy was obtained by a computed linear regression analysis of the experimental data. The enthalpies of activationDHawere evaluated as the
slopes of the curves obtained by plotting log activity/T (K) vs. 1/T (Segel, 1975).
2.5. Kinetic tests
The kinetics of free and immobilised phosphatase were determined by activity assays at 308C and pH 5.0 with pNPP substrate concentrations ranging from 0 to 6 mM. The kinetic parameters (Vmaxand Km) were evaluated, according
to Michaelis–Menten method (Segel, 1975), by a computed non-linear regression analysis.
2.6. Stability studies
The stability of free and immobilised phosphatase in the presence of proteinase was studied by determining the resi-dual enzymatic activity after 24 h-exposure to a bacterial protease (500:1, ratio of proteinase K to phosphatase activ-ity) at 378C and pH 5.0. Controls were performed in the absence of proteinase.
enzyme suspension were withdrawn and the residual activ-ity assayed under standard conditions.
All experiments were carried out in triplicate.
3. Results and discussion
3.1. Inhibition studies
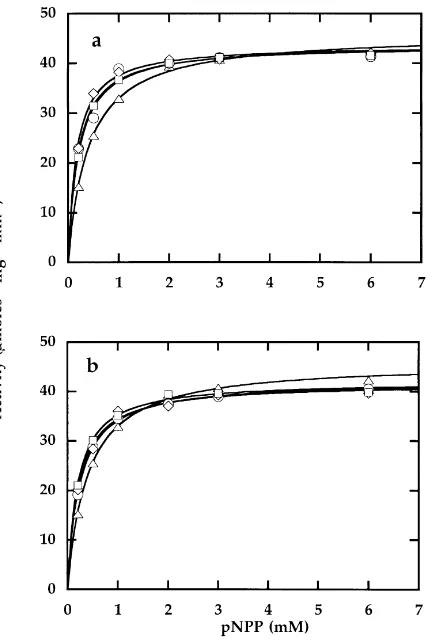
The activity and kinetics of free phosphatase was not significantly affected by chlorides or oxides of Fe and Mn (FeCl3, MnCl2, Fe2O3, MnO2). Fig. 1 shows the activity of
acid phosphatase at different substrate concentrations and in the presence of increasing amounts (0.3–1 mg ml21) of Fe2O3(Fig.1a) and MnO2(Fig. 1b). All data were fitted by
a single curve according to Michaelis–Menten equation, thus allowing the kinetic parameters Vmax
0:8mmol min
21
ml21and Km0:3 mMto be determined. Similar behaviour was observed with FeCl3and MnCl2(data
not shown). Analogous results have been obtained in studies performed with the same compounds and urease (Gianfreda et al., 1995a).
By contrast, tannic acid (at concentration.23mM) was demonstrated to behave as a strong inhibitor of acid
phosphatase activity (Rao et al., 1998). A kinetic study performed by changing both substrate (from 0.2 to 6.0 mM) and tannic acid (from 23 to 47mM) concentrations indicated that the inhibition by tannic acid followed Michaelis–Menten kinetics. In the presence of tannic acid a substrate inhibition effect appeared at a pNPP
concentrations$1 mM. The dependence of the kinetic parameters Vmaxand Km, (calculated according to the
modi-fied Michaelis–Menten equation taking into account the substrate inhibition effect (Laidler, 1958)) on tannic acid concentration suggested a mixed uncompetitive inhibition mechanism (Rao et al., 1998).
3.2. Formation and activity of complexes
To optimise the conditions under which maximum activ-ity of complexes are achieved, phosphatase–tannic acid complexes were prepared by changing: (i) Fe31 and/or Mn21/tannic acid molar ratios; (ii) amounts of oxides; and (iii) tannic acid/montmorillonite ratios. A tannic acid/ enzyme ratio (w:w) of 10 was adopted in all experiments as, at this ratio, the concentration of tannic acid in the reac-tion mixture (,23mM) shows negligible inhibition effects (Rao et al., 1998).
Experiments were performed using Fe or Mn ions/tannic acid molar ratios ranging from 2.5 to 8 (P–T–Fe31and P– T–Mn21 complexes), 5 and 10 mg of iron or manganese oxides per tube (P–T–Fe2O3and P–T–MnO2 complexes)
and at tannic acid/montmorillonite ratios ranging from 0.02 to 0.25 w:w (complexes with montmorillonite). The greatest recovery of residual enzymatic activity for the suspensions (up to 80%), the insoluble complexes (up to 58%) and the supernatants (up to 10%) was obtained when a Fe or Mn ions/tannic acid molar ratio of 2.5 and an oxide amount of 10 mg and a tannic acid/montmorillonite ratio of 0.1 were used. All results discussed in the following refer to these experimental conditions.
Table 1 reports the residual activities, expressed as percentages of the initial enzymatic units added, of
Fig. 1. Michaelis–Menten plot of specific acid phosphatase activity vs. pNPP concentrations in the absence w0 mg ml21and in the presence of increasing concentrations W0:3 mg ml
21
; — 0:5 mg ml
21
; A 1 mg ml21of Fe2O3(a) and MnO2(b).
Table 1
Residual activities (%) of suspensions (Sp), insoluble complexes (Cp) and supernatants (Sn) of acid phosphatase–tannic acid mixtures (P–T) obtained in the presence or in the absence of Fe and Mn ions and oxides, with or without montmorillonite. Data are mean^SD n3
Complexes Residual activity (%)a
Without montmorillonite With montmorillonite
Sp Cp Sn Sp Cp Sn
P–T 51^3.1 33^2.1 7^1.6
P–T–Fe31 41^2.8 33^2.5 6^1.1 75^2.9 41^2.1 0 P–T–Mn21 59^3.2 36^2.3 10^2.0 80^3.1 46^2.6 0 P–T–Fe2O3 72^3.8 58^3.4 0 57^2.7 55^3.2 0 P–T–MnO2 32^2.3 33^2.8 0 50^3.0 29^2.8 0
a
suspensions (Sp), insoluble complexes (Cp) and superna-tants (Sn) of tannic acid–phosphatase mixture obtained in the presence of metal chlorides or oxides, with and without montmorillonite. In all cases, phosphatase activities of suspensions were higher than the sum of the activities measured in the insoluble complex and in the respective supernatant.
The presence of Fe or Mn ions did not significantly change the activities of the suspensions, insoluble complexes and supernatants as compared with tannic acid–phosphatase preparations. Higher activity levels of both the suspensions and the insoluble complexes were measured with Fe2O3 (increases of 72 and 58%,
respectively), whereas MnO2 had no significant effect.
In both cases, there was no activity in the respective supernatants.
In the presence of montmorillonite, no activity was detected in the supernatants but increases in the activity of the suspensions of 75% (Fe) and 80% (Mn) and insoluble complexes of 41% (Fe) and 46% (Mn) were recorded. The Fe and Mn oxides had no effect.
The first observation may be explained by the experimen-tal procedure used to separate insoluble complexes and supernatants from the original suspensions. Insoluble complexes were recovered by repeated high-speed centrifu-gation. This treatment probably led to aggregation of the colloidal particles of the insoluble complexes, thus prevent-ing their total resuspension in buffer solution. The entrap-ment of some enzyme molecules within the organic aggregate may have given rise to substrate diffusional limitations, which would result in a lower apparent residual activity of the complexes.
As regards the other observations, various aspects should be considered. There is much experimental evidence to suggest that enzyme–phenolic complexes with different levels of activity may form if phenolic compounds and macromolecules, such as proteins, interact in the presence of ions and oxides (e.g. hydrolytic species of Al and Fe, or Ca21, Cu21, Mn21, Al31 and Fe31 ions (Maignan, 1983; Gianfreda et al., 1993; Gianfreda et al., 1995a; Violante and Gianfreda, 2000).
Tannic acid–invertase (Gianfreda et al., 1993), tannic acid–urease (Gianfreda et al., 1995a) and humic acid– invertase (Maignan, 1983) complexes all had significantly higher activities when prepared in the presence than in the absence of Fe, Al and/or Cu species. The authors attributed this effect to the capacity of inorganic species to promote the flocculation of enzymatic complexes with higher activity levels. The data in Table 1 indicate that no similar effects occurred with acid phosphatase and other explanations must be considered.
Metal oxides may catalyse the abiotic polymerisation of phenolic compounds giving rise to the formation of organic polymers. Furthermore, Mn oxide usually displays a stron-ger polymerising effect as compared with that of the well crystallised or short-range ordered oxides of Fe (Gianfreda
et al., 1995a). The adsorption of organics, such as phenolics and proteins, on the surface of oxides might be also considered.
It is possible that both Fe and Mn oxides accelerated the polymerisation of tannic acid and that a higher number of phosphatase molecules was involved in this process and, therefore, removed from the solution phase; this would account for the absence of activity in the supernatant fractions (Table 1). However, Fe2O3is less effective in
poly-merising phenolic compounds (Huang, 1990). Probably, small size phosphatase–tannic acid–Fe2O3 complexes
were formed and phosphatase molecules were adsorbed on the external surfaces of the tannic acid polymers rather than being entrapped within them. Exposed adsorbed enzymes would give higher activities. However, the formation of tannic acid–phosphatase complexes and their subsequent adsorption to the surface of the oxide cannot be excluded.
By contrast, polymerisation was probably more efficient in the presence of manganese oxide (Huang, 1990), gener-ating insoluble complexes or polymers entrapping many enzyme molecules. A hindrance to substrate access or irre-versible conformational changes of enzyme molecules prob-ably occurred and lowered the activity of these complexes (Table 1).
The binding of active enzyme molecules to insoluble complexes was enhanced by the presence of montmorillo-nite during the interaction of tannic acid and enzyme with Fe and Mn ions. The activities of the insoluble complexes and the supernatants further increased (Fe) or decreased (Mn), compared with those in the absence of montmorillo-nite (Table 1). These results confirm those with invertase incubated with tannic acid, OH–Al polymers and montmor-illonite (Gianfreda et al., 1993). An increase of activity in suspensions and insoluble complexes with a concomitant decrease of activity in the supernatants was observed in that study.
It is possible that Fe and/or Mn ions acted as bridges between tannic acid molecules and montmorillonite surfaces (Violante and Gianfreda, 2000) and favoured the attachment of soluble enzyme–tannic acid complexes to the external surfaces of the clay. The exposure of these to the substrate could have resulted in higher activity levels.
3.3. Catalytic and stability properties of complexes
Investigations were carried out to evaluate the catalytic behaviour and stability of the insoluble phosphatase complexes. As described in Section 2, the complexes were tested immediately after their preparation.
Kinetic parameters — Kinetic behaviour, described by a
Michaelis–Menten kinetics, was observed for all phospha-tase complexes. The kinetic parameters Vmaxand Km,
equation (e.g. Eadie–Hofstee, Hanes–Wolf and Eadie– Scatchard equations) to the data. In all cases values for
Vmaxand Km were in agreement with those obtained by the
Michaelis–Menten equation, thus indicating a proper weighting of experimental data.
Vmax values for all complexes were reduced when
compared to that of the free enzyme (Table 2). By contrast,
Kmvalues of immobilised phosphatase were greater than of
the free enzyme. Km values doubled for P–T and P–T–
MnO2, whereas they increased 2.7- and 3.3-fold for P–T–
Mn21and P–T–Fe31complexes, respectively (Table 2). In contrast, the Kmof P–T–Fe2O3was significantly reduced by
30%.
In agreement with others (Mc Laren and Packer, 1970; Goldstein, 1976), the catalytic efficiency of immobilised phosphatase, measured by Vmax/Km ratio, was lower than
that of the free enzyme. For enzyme–tannic acid complexes formed in the presence of iron oxide (with and without montmorillonite) the Vmax/Km values were similar to that
of free phosphatase.
Lower Vmaxand higher Kmvalues are usually expected for
enzymes immobilised on or within insoluble supports (Nannipieri and Gianfreda, 1998) although variable results are reported in the literature when immobilised phosphatase
is involved. Whereas Vmaxvalues are usually reduced
(Gian-freda and Bollag, 1994; Huang et al., 1995), varying effects on Kmvalues have been found. Compared to the free form
alkaline phosphatase adsorbed on Ca21-montmorillonite had a higher Vmax, and a lower Kmwhen adsorbed on illite
(Makboul and Ottow, 1979). Acid phosphatase adsorbed to montmorillonite and montmorillonite covered by OH–Al species showed lower Km values than free phosphatase
(Rao et al., unpublished results). In contrast, the binding of phosphatase to montmorillonite by covalent bridges (achieved by treatment with glutaraldehyde) decreased
Vmax and increased Km, indicating that the mechanism of
immobilisation affected substrate affinity (Gianfreda and Bollag, 1994). Lower Vmaxand higher Kmvalues were also
measured by Huang et al. (1995) when acid phosphatase was adsorbed on montmorillonite.
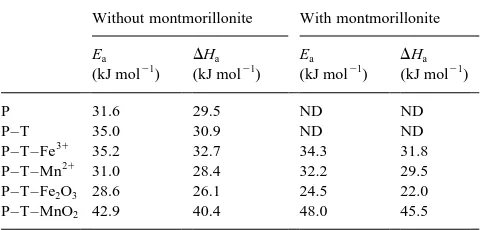
The activation energy, Ea, of free and immobilised
phos-phatase ranged from 28.6 kJ mol21 for the enzyme complexed with tannic acid and iron oxide to a maximum value of 42.9 for the complex obtained with tannic acid and manganese oxide (Table 3). There was no significant change when the enzyme was complexed in the presence of montmorillonite, except for P–T–MnO2 whose Ea value
increased to 48.0 kJ mol21. According to the theoretical equation EaDHa1RT;DHavalues decreased by an aver-age 2.5 kJ mol21with respect to Ea.
An activation energy of about 30 kJ mol21is reasonable for reactions catalysed by free enzymes (Laidler, 1958), although lower values (13–15 kJ mol21) have been calcu-lated for urease from jack bean (Gianfreda et al., 1995b) and for free (19.95 kJ mol21) and immobilised (20.91 kJ mol21) invertase (Gianfreda et al., 1991). When immobilised on solid supports, a general increase of the thermodynamic parameters Ea and DHa usually occur if modified
reac-tion mechanisms are involved in the catalytic process (Lai and Tabatabai, 1992). When compared to the free enzyme, the lower Ea values of P–T–Fe2O3 and the
higher Ea values of P–T–MnO2 support the hypothesis
that the enzyme is located in different positions in the two complexes. Enzyme molecules located on the exter-nal surfaces of P–T–Fe2O3behaved more closely to that
of the free enzyme.
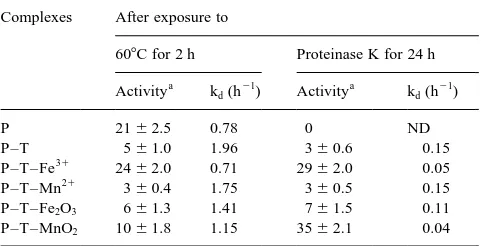
Stability properties — In order to check to what extent
the resistance of the enzyme to various denaturing condi-tions is influenced by the immobilization process and the nature of the support, the activity of free and immobilised phosphatase was measured after 2h at 608C and after 24 h-exposure to proteinase K. Immobilised enzyme showed an increased sensitivity to elevated temperature (except P–T– Fe2O3) and a greater resistance to proteolysis as compared to
the free form (Table 4).
Deactivation constants (kd) at 608C for immobilised
phos-phatase were in general higher than that of the free enzyme and reduced activities were measured. A non significant increase in stability was observed for P–T–Fe31. The lowest stability was shown by phosphatase complexed
Table 3
Activation energy (Ea) and activation enthalpy (DHa) for free acid phos-phatase (P) and acid phosphos-phatase immobilised on tannic acid (T) in the presence of Fe or Mn ions or oxides
Without montmorillonite With montmorillonite
P–T–MnO2 42.9 40.4 48.0 45.5
Table 2
Kinetic parameters of free acid phosphatase (P) and acid phosphatase immobilised on tannic acid (T) in the presence of Fe or Mn ions or oxides
with tannic acid, either alone or in the presence of manga-nese ions or iron oxide
Soluble phosphatase did not survive protease treatment. In comparison, all phosphatase complexes showed some degree of resistance to protease. The complexes least sensi-tive to protease were also those that showed the highest residual activity to exposure to 608C. For example, P–T– Fe31and P–T–MnO2complexes, which showed the highest
values of activity after 2 h exposure to 608C, showed also the lowest sensitivity to the action of the proteolytic agent. An activity of 29–35% of the initial value was measured after 24 h contact with proteinase K.
In conclusion, the interaction of acid phosphatase with tannic acid was affected to different extents by the presence of Fe and Mn ions or oxides. Iron oxide showed a positive effect on the activity and the catalytic behaviour of phos-phatase–tannate–Fe oxide complexes. Stability was also enhanced in the simultaneous presence of Fe or Mn ions and montmorillonite. No significant increase of stability to temperature was measured for the immobilised enzyme, whereas a general higher resistance to proteolysis was observed.
References
Bollag, J.-M., 1992. Enzymes catalyzing oxidative coupling reactions of pollutants. In: Siegles, H., Siegel, A. (Eds.). Metal Ions in Biological Systems, Marcel Dekker, New York, pp. 205–217.
Boyd, S.A., Mortland, M.M., 1990. Enzyme interactions with clays and clay-organic matter complexes. In: Bollag, J.-M., Stotzky, G. (Eds.). Soil Biochemistry, Vol. 6. Marcel Dekker, New York, pp. 1–28. Brindley, G.W., Brown, G., 1980. Crystal structures of clay minerals and
their X-ray identification. Mineralogical Society, London.
Burns, R.G., 1986. Interactions of enzymes with soil mineral and organic colloids. In: Huang, P.M., Schnitzer, M. (Eds.). Interactions of Soil Minerals with Natural Organics and Microbes, SSSA, Madison, pp. 429–451.
Colombo, C., Barron, V., Torrent, J., 1994. Phosphate adsorption and deso-rption in relation to morphology and crystal properties of synthetic hematites. Geochimica Cosmochimica Acta 58, 1261–1269. Gianfreda, L., Bollag, J.-M., 1994. Effect of soils on the behaviour of
immobilized enzymes. Soil Science Society of American Journal 58, 1672–1681.
Gianfreda, L., Rao, M.A., Violante, A., 1991. Invertase (b-fructosidase): effects of montmorillonite. Al-hydroxide and Al(OH)x-montmorillonite
complex on activity and kinetic properties. Soil Biology & Biochem-istry 23, 581–587.
Gianfreda, L., Rao, M.A., Violante, A., 1993. Interactions of invertase with tannic acid, OH-Al-species and/or montmorillonite. Soil Biology & Biochemistry 25, 671–677.
Gianfreda, L., Rao, M.A., Violante, A., 1995a. Formation and activity of urease–tannate complexes affected by aluminum, iron, and manganese. Soil Science Society of America Journal 59, 805–810.
Gianfreda, L., De Cristofaro, A., Rao, M.A., Violante, A., 1995b. Kinetic behaviour of synthetic organo- and organo-mineral complexes. Soil Science Society of America Journal 59, 811–815.
Goldstein, L., 1976. Kinetic behaviour of immobilized enzyme systems. In: Mosbach, K. (Ed.). Methods in Enzymology, Vol. 44. Academic Press, London, pp. 397–443.
Huang, P.M., 1990. Role of soil minerals in transformations of natural organics and xenobiotics in soil. In: Bollag, J.-M., Stotzky, G. (Eds.). Soil Biochemistry, Vol. 6. Marcel Dekker, New York, pp. 29–115. Huang, Q., Shindo, H., Goh, T.B., 1995. Adsorption, activities and kinetics
of acid phosphatase as influenced by montmorillonite with different interlayer material. Soil Science 159, 271–278.
Ladd, J.N., Butler, J.H., 1975. Humus-enzyme systems and synthetic, organic polymer–enzyme analogs. In: Paul, E.A., McLaren, A.D. (Eds.). Soil Biochemistry, Vol. 4. Marcel Dekker, New York, pp. 143–194. Lai, M.C., Tabatabai, M.A., 1992. Kinetic parameters of immobilized
urease. Soil Biology & Biochemistry 24, 225–228.
Laidler, K.J., 1958. The Chemical Kinetics of Enzyme Action. Clarendon, Oxford.
Makboul, H.E., Ottow, J.C.G., 1979. Alkaline phosphatase activity and the Michaelis constant in the presence of different clay minerals. Soil Science 128, 129–135.
Maignan, C., 1983. Activite´ des complexes acides umiques-invertase: influ-ence des cations flocculants. Soil Biology & Biochemistry 15, 651–655. Mc Laren, A.D., Packer, L., 1970. Some aspects of enzyme reaction in
heterogeneous systems. Advances in Enzymology 33, 245–308. Naidja, A., Huang, P.M., Bollag, J.-M., 1998. Comparison of reaction
products from the transformation of catechol catalyzed by birnessite or tyrosinase. Soil Science Society of America Journal 62, 188–195. Nannipieri, P., Gianfreda, L., 1998. Kinetics of enzyme reactions in soil
environments. In: Huang, P.M., Senesi, N., Buffle, J. (Eds.). Structure and Surface Reactions of Soil Particles, Wiley, New York, pp. 449–479. Rao, M.A., Gianfreda, L., Palmiero, F., Violante, A., 1996. Interaction of acid phosphatase with clays, organic molecules and organo-mineral complexes. Soil Science 161, 751–760.
Rao, M.A., Gianfreda, L., Violante, A., 1998. Interactions between tannic acid and acid phosphatase. Soil Biology & Biochemistry 30, 111–112. Sarkar, J.M., Burns, R.G., 1983. Immobilization ofb-d-glucosidase andb -d-glucosidase-polyphenolic complexes. Biotechnology Letters 5, 619– 624.
Sarkar, J.M., Burns, R.G., 1984. Synthesis and properties ofb-d -glucosi-dase-phenolic copolymers as analogues of soil humic–enzyme complexes. Soil Biology & Biochemistry 16, 619–625.
Segel, I.K., 1975. Enzyme Kinetics. Wiley, New York.
Shindo, H., Huang, P.M., 1992. Comparison of the influence of Mn(IV) oxide and tyrosinase on the formation of humic substances in the envir-onment. Science of the Total Environment 117/118, 103–110. Speir, T.W., Ross, D.F., 1978. Soil phosphatases and sulphatases. In: Burns,
R.G. (Ed.). Soil Enzymes, Academic Press, New York, pp. 198–250. Violante, A., Gianfreda, L., 2000. Role of biomolecules in the formation
and reactivity towards organics and plant nutrients of variable-charge minerals and organo-mineral complexes in soil. In: Bollag, J.-M., Stotzky, G. (Eds.). Soil Biochemistry, Vol. 10. Marcel Dekker, New York, pp. 207–270.
Table 4
Thermal and proteolytic stability of free acid phosphatase (P) and acid phosphatase immobilised on tannic acid (T) in the presence of Fe or Mn ions or oxides. Data are mean^SD n3