Summary We assessed the effects of Cu on root growth and morphology of stone pine (Pinus pinea L.) and maritime pine (Pinus pinaster Ait.) seedlings grown in culture solutions sup-plied with 0.012 (control), 0.1, 1 or 5 µM CuSO4. The presence of 5 µM Cu in the nutrient solution completely inhibited root growth of both species within 3 days. In both species, taproot elongation was reduced in the presence of 1 µM Cu, although partial growth recovery occurred after 7 days of treatment. The presence of 0.1 µM Cu in the culture solution slightly enhanced root elongation in P. pinaster, but did not significantly influ-ence root elongation in P. pinea. In both species, root weight per unit length increased in response to Cu exposure, and in P. pinaster, root diameter was significantly increased. The Cu treatments also affected lateral root number and length. In the presence of 1 µM Cu, both species formed only short lateral primordia. The 1 µM Cu treatment increased the lateral root index (number of roots per cm of root length) of P. pinaster, but decreased that of P. pinea, compared with control values. Neither the 0.1 nor 1 µM Cu treatment had a significant effect on the mitotic index of either species. We conclude that cell elongation is more sensitive to Cu than cell division. Cell membrane damage, as indicated by Trypan blue staining, oc-curred after 10 days of exposure to 1 µM Cu.
Keywords: cell division, cell elongation, heavy metal toxicity, maritime pine, membranes, mitotic index, stone pine.
Introduction
Large amounts of heavy metals have been removed from natural ore deposits and redistributed in the environment as a result of human activity (Lepp 1981). For example, copper and other heavy metals have been introduced into soil by applica-tion of fungicides, fertilizers and, more recently, sewage sludge (Levi-Minzi and Riffaldi 1978). Italian soils have higher copper concentrations than those of other European countries and the world average (51 mg kg−1, Angelone and Bindi 1992), probably as a result of the widespread and pro-longed application of copper-based fungicides in Italian or-chards and vineyards (Lepp 1981).
Copper is an essential element for all forms of life, acting in the prosthetic group of many oxidizing enzymes. However, as
a result of the formation of organocopper complexes, excess copper may be highly toxic (Marschner 1986). Compared to other heavy metals, copper is not readily bioaccumulated, and so it is seldom dangerous for human and animal health, but it is highly toxic to plants (Fernandes and Henriques 1991). Because both root elongation and branching are sensitive to the presence of copper, excess copper reduces the ability of the plant to explore the soil for water and nutrients (Wong and Bradshaw 1982). In particular, copper reduces transroot poten-tial (Kennedy and Gonsalves 1987) and damages the plasma membrane, causing ion efflux and thereby inhibiting cell elon-gation (Woolhouse 1983, DeVos et al. 1989).
Copper is mostly present in soil in an organically bound form and is therefore strongly retained in the uppermost layers (Hunter et al. 1987). Because the mobility and bioavailability of copper increases with increasing soil acidity (Basta et al. 1993), soil acidification due to atmospheric inputs could en-hance the bioavailability of copper over time, as has been described for Al (Ulrich 1994). This may be particularly im-portant for seedlings because they are more sensitive to envi-ronmental stress than mature trees and initially develop in the most contaminated superficial soil layer (Patterson and Olson 1983). Among conifers, Pinus species often colonize superfi-cially dry soils (Sutton 1980). Because Pinus seedlings have large woody taproots that form few laterals and seem unable to develop adventitious roots (Coutts and Philipson 1987), the maintenance of a high root elongation rate is essential for seedling establishment in this genus.
To assess copper sensitivity of Pinus pinea L. and Pinus pinaster Ait. roots, daily root elongation rate, lateral root development and root morphology were investigated in seed-lings grown in nutrient solutions containing a range of copper concentrations. We also examined the effects of exposure to copper on root mitotic index, root weight per unit length, root thickness, and the integrity of the plasma membrane.
Materials and methods
Culture of plants
Seeds of P. pinea and P. pinaster were harvested from summer to autumn 1991 at the San Rossore Natural Park near Pisa,
Influence of copper on root growth and morphology of Pinus pinea L.
and Pinus pinaster Ait. seedlings
IDUNA ARDUINI,
1DOUGLAS L. GODBOLD
2and ANTONINO ONNIS
11 Dipartimento di Agronomia e Gestione dell’Agro-Ecosistema, Università degli Studi Pisa, Via S. Michele degli Scalzi 2, 56124 Pisa, Italy
2 Forstbotanisches Institut, Universität Göttingen, Büsgenweg 2, 37077 Göttingen, Germany
Received May 9, 1994
Italy, and stored at 20 °C.
To hasten germination, P. pinea seeds were first cracked and peeled. They were germinated in petri dishes on filter paper moistened with glass-distilled water. When the radicles were about 5 cm long, the seedlings were transferred to 2-l culture vessels (four seedlings per vessel) containing nutrient solution (based on Heller, in Gautheret 1959). The composition of the nutrient solution was (µM): 500 KCl, 435 NaNO3, 200 MgSO4, 45 NaH2PO4, 100 CaCl2, 0.37 FeCl3, 0.35 ZnSO4, 1.6 H3BO3, 0.6 MnSO4, 0.021 AlCl3, 0.023 NiSO4, 0.006 K, pH 5.5, to which was added 0.012 (control), 0.1, 1 or 5 µM CuSO4. Nutrient solutions were constantly aerated with sterile air and renewed every 2 days.
Growth conditions for the 4-week study period were 23/21 °C day/night temperatures, 35 ± 5% relative humidity, 100 µmol m−2 s−1 photon flux density (Osram L18W/25 lamps), and a 16-h photoperiod. Each treatment comprised 16 seedlings divided among four replicate culture vessels. Estimation of root elongation rate
Root elongation was estimated at intervals by measuring the distance between a reference point, marked 1 cm behind the tip at the start of the study, and the root tip.
Lateral root measurements
Numbers and lengths of lateral roots were estimated after 4 weeks of exposure to copper. Lateral root index was esti-mated by dividing the number of lateral roots developed during the 4-week treatment by the length of the main root.
Mitotic index
After 4 weeks of treatment, five root tips per treatment were fixed in Carnoy solution (3/1 v/v ethanol/acetic acid), hydro-lyzed in 1 M HCl and then stained by the Feulgen technique. The most intensely colored part of the root tip was smeared on slides, and the number of mitotic cells was estimated. Root weight per unit length
At the end of the 4-week treatment, seedlings were oven dried to constant weight at 70 °C. Root density was estimated by dividing the average dry weight by the average root length measured at the end of the 4-week treatment.
Root sections
At the end of the 4-week treatment, thin sections of roots were cut on a dry glass knife with an ultramicrotome (Ultracut E, Reichert-Jung, Vienna, Austria) at a distance of about 7 mm from the root tip. The sections were stained with methyl blue, and the root diameter and the thickness of cortex and central cylinder determined.
Integrity of plasma membranes
After 1, 3, 7 and 10 days of culture, five 1-cm long root tips per treatment were excised and placed in 0.5% (w/v) Trypan blue in glass-distilled water for 5 min (DeVos et al. 1989). Penetra-tion of the dye soluPenetra-tion was observed on cross and longitudinal sections with the aid of a binocular microscope.
Results
Root elongation rate
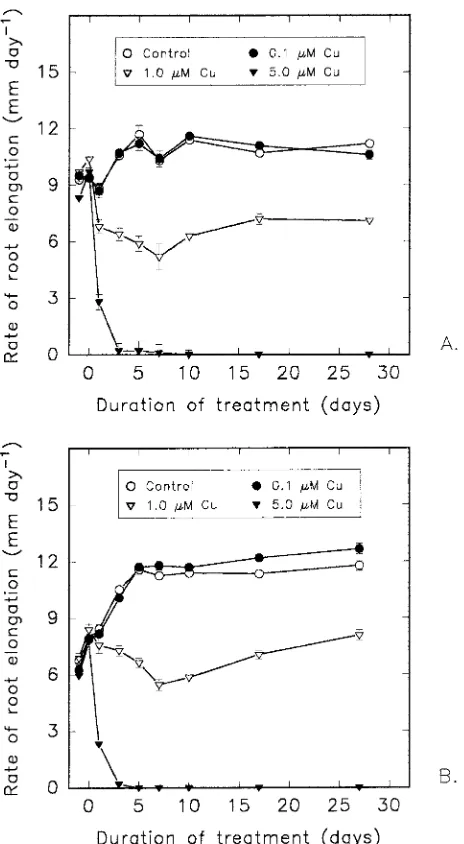
In both species, the presence of 5 µM Cu in the nutrient solution caused a large decrease in the rate of root elongation on Day 1 and complete inhibition of root growth after 3 days (Figures 1A and 1B). In the presence of 1 µM Cu, a progressive decrease in root elongation was observed from Day 1 until Day 7, after which there was a slight recovery in root growth. Exposure to 0.1 µM Cu had no effect on root elongation rates of P. pinea seedlings (Figure 1A), whereas it caused a slight stimulation of elongation growth in roots of P. pinaster seed-lings compared to the controls (Figure 1B).
Figure 1. Root elongation rates of 2-week-old seedlings of (A) Pinus
Lateral roots and root morphology
The 4-week treatment with 1 µM Cu decreased main root length by 38.7% in P. pinea and 35.1% in P. pinaster (Table 1). The same treatment caused a significant decrease in lateral root length in both species. After 4 weeks in the presence of 1 µM Cu, the lateral root index had decreased by 75% in P. pinea, whereas it had increased by 44% in P. pinaster, compared to the control value (Table 1). The 5 µM Cu treatment completely inhibited root growth and no laterals developed. The roots turned a brownish color and lost turgor (Table 1).
Mitosis
Neither the 0.1 nor the 1 µM Cu treatment had a significant effect on the mitotic index of either species (Table 2).
Root weight per unit length and root thickness
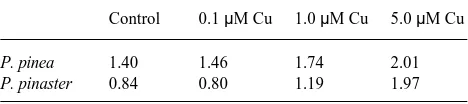
In the presence of 1 µM Cu, seedlings of both species showed a marked thickening of the apical part of the root. The dry weight per unit root length was 124.3% of the control value in P. pinea and 141.7% in P. pinaster (Table 3). The high values obtained in the 5 µM Cu treatment were not related to any visible thickening of the root.
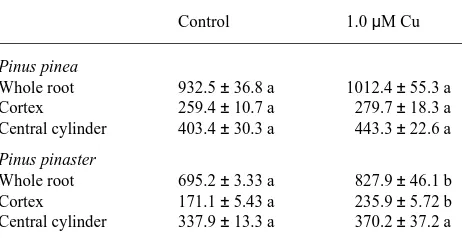
There was an increase in root diameter measured on thin sections in both species exposed to 1 µM Cu; however, the increase was only statistically significant for P. pinaster (Ta-ble 4). In this species, the thickening of the cortex layer was more marked than that of the central cylinder, although the width of both root sections increased in the Cu-treated seed-lings compared to the control seedseed-lings.
Plasma membrane integrity
No differences in plasma membrane integrity between Cu-treated and control roots were observed after 1 or 3 days of treatment. However, after a 10-day exposure to 1 µM Cu, penetration of Trypan blue was observed in the root tips of both species in contrast to the controls. Longitudinal sections showed that the most intense coloration occurred between 2 and 4 mm from the root tip where both the cortex and the central cylinder cells were stained.
Discussion
Root elongation of both P. pinea and P. pinaster seedlings was completely inhibited within 3 days of exposure to 5 µM Cu (Figures 1A and 1B). The presence of 1 µM Cu in the nutrient solution for 7 days caused the rate of root elongation to de-crease by 49.5% in P. pinea and 51.4% in P. pinaster. In both species, a slight recovery in root elongation growth was sub-sequently observed, followed by a marked thickening of the youngest root that did not turn brown. A similar recovery in the rate of root elongation after inhibition during the first days of exposure to Al has been reported for Picea abies (L.) Karst. seedlings (Godbold 1991). These observations suggest that some tolerance mechanisms, e.g., binding to metalloproteins (Rauser 1984) or the precipitation of Cu complexes in globular bodies (Sela et al. 1988), were activated in response to low concentrations of Cu. The root dry weight per unit length increased in all Cu treatments (Table 3), and a significant thickening of the cortex layer was observed in roots of P. pi-naster seedlings (Table 4). Shorter but thicker main and lateral
Table 1. Main root length, average lateral root length and lateral root index (number of roots cm−1 root length) of Pinus pinea and Pinus pinaster seedlings exposed for 4 weeks to nutrient solutions containing 0.012 (control), 0.1, 1 or 5 µM CuSO4. Treatments with no common indices in rows are significantly different (P < 0.05, Bonferroni test). Data are means ± SE, n = 5.
Control 0.1 µM Cu 1.0 µM Cu 5.0 µM Cu
Pinus pinea
Main root length (mm) 306.3 ± 15.2 a 301.8 ± 16.4 a 187.8 ± 17.2 b 4.0 ± 0.50 c
Lateral root length (mm) 5.4 ± 0.30 a 5.6 ± 1.17 a < 2 b -- b
lateral root index 3.2 ± 0.23 a 3.2 ± 0.21 a 0.8 ± 0.17 b -- c
Pinus pinaster
Main root length (mm) 298.6 ± 16.7 a 319.7 ± 12.2 a 193.9 ± 7.85 b 2.9 ± 0.35 c
Lateral root length (mm) 8.7 ± 0.83 a 8.5 ± 1.60 a 2.4 ± 0.09 b -- b
Lateral root index 5.0 ± 0.72 a 4.7 ± 1.04 a 7.2 ± 0.81 a -- b
Table 2. Mitotic index (% of mitotic cells) in the taproot meristem of
Pinus pinea and Pinus pinaster seedlings exposed for 4 weeks to
nutrient solutions containing 0.012 (control), 0.1 or 1 µM CuSO4. Treatments with no common indices in rows are significantly different (P < 0.05, Bonferroni test). Data are means ± SE, n = 5.
Control 0.1 µM Cu 1.0 µM Cu
P. pinea 7.4 ± 0.46 a 6.5 ± 0.36 a 6.2 ± 0.38 a
P. pinaster 3.8 ± 0.41 a 4.4 ± 0.35 a 4.5 ± 0.58 a
Table 3. Root weight per unit length (mg cm−1) of Pinus pinea and
Pinus pinaster seedlings exposed for 4 weeks to nutrient solutions
containing 0.012 (control), 0.1, 1 or 5 µM CuSO4. Data are average dry weight divided by average length of 15 roots.
Control 0.1 µM Cu 1.0 µM Cu 5.0 µM Cu
P. pinea 1.40 1.46 1.74 2.01
roots are frequently observed in crop (Przymusinski and Gwozdz 1994) and forest species (Heale and Ormrod 1982) grown in soils or culture solutions containing heavy metals. Root thickening may be a consequence of a decrease in cell elongation (Breckle 1991).
Under our experimental conditions, Cu was much more toxic than Cd to the roots of P. pinea and P. pinaster (cf. Arduini et al. 1994). In both species, exposure to 1 µM Cu greatly decreased root elongation, whereas the same concen-tration of Cd did not significantly influence root elongation in P. pinaster and stimulated root elongation in P. pinea (Arduini et al. 1994). The high toxicity of Cu has been reported for several species including Lolium perenne L. (Wong and Brad-shaw 1982) and Brassica chinensis L. (Wong et al. 1986). Barbolani et al. (1986) reported a higher and more rapid uptake of Cu than Cd in Iris pseudacorus L. Copper may be more phytotoxic than Cd because it is an essential element and may be absorbed and become involved in metabolic pathways more readily than Cd (Hardiman et al. 1984). In some species, high Cu sensitivity of root growth is related to disturbances of mitosis (Eleftheriou and Karataglis 1989) and especially to damage to the cell membrane, which is often the first target of Cu toxicity (Meharg 1993). Some authors have explained both effects as consequences of Cu toxicity on protein synthesis or activity (Przymusinski and Gwozdz 1994). DeVos et al. (1991) suggested that Cu-induced damage to integral proteins, through the formation of disulfide links, resulted in increased cell membrane permeability and ion efflux. We observed pene-tration of Trypan blue between 2 and 4 mm behind the root tips of seedlings that had been subjected to a 10-day exposure to 1 µM Cu, indicating that cell membranes had been damaged (DeVos et al. 1989). The absence of Trypan blue penetration at the root tips, which was also observed in Silene cucubalus Wibel (DeVos et al. 1991), suggests that the well-developed root cap protects the meristematic cells. This protection of the meristematic cells may also explain why Cu did not signifi-cantly affect mitotic processes. Copper also binds strongly to the root surface, decreasing transroot potential, which is essen-tial for water and ion uptake (Kennedy and Gonsalves 1987).
In addition to damaging the plasma membrane and reducing the transroot potential, Cu could affect cell elongation in the roots by stimulating lignification (Marschner 1986). Increased lignin synthesis could explain the brown color that developed on Cu-treated roots of both P. pinea and P. pinaster (cf. Heale and Ormrod 1982). We conclude that decreased cell elongation caused by increased plasmalemma permeability and cell wall lignification were the main mechanisms of Cu toxicity in these Pinus seedlings.
The presence of 1 µM Cu in the nutrient solution affected the morphology of the root system by decreasing elongation of laterals more than that of the main root (63.0 versus 38.7% in P. pinea, 72.5 versus 35.1% in P. pinaster, Table 1). Copper toxicity causes inhibition of lateral root development in many tree species including Acer pseudoplatanus L. (Turner and Dickinson 1993) and some conifers (Wotton et al. 1986). Although the length of the lateral roots decreased, there was an increase in the lateral root index in P. pinaster seedlings treated with 1 µM Cu (Table 1). Numerous stunted laterals or primor-dia were also observed in Cu-treated Acer rubrum L. and Pinus resinosa Ait. (Heale and Ormrod 1982), suggesting that root elongation was more sensitive to Cu toxicity than root initia-tion (Fernandes and Henriques 1991).
Acknowledgments
We thank Ms. C. Kettner for skillful technical assistance and Prof. A. Stefani for constructive comments during the analysis of cytological data. This work was supported, in part, by a European Community grant (STEP-CT90-0059).
References
Angelone, M. and C. Bindi. 1992. Trace elements concentrations in soils and plants of western Europe. In Biogeochemistry of Trace Metals. Ed. D.C. Adriano. Lewis Publications, New York, pp 19--60.
Arduini, I., D.L. Godbold and A. Onnis. 1994. Cadmium and copper change root growth and morphology of Pinus pinea and Pinus
pinaster seedlings. Physiol. Plant. 92:675--680.
Barbolani, E., M. Clauser, F. Pantani and R. Gellini. 1986. Residual heavy metal (Cu and Cd) removal by Iris pseudacorus. Water Air Soil Pollut. 28:277--282.
Basta, N.T, D.J. Pantone and M.A. Tabatabai. 1993. Path analysis of heavy metal adsorption by soil. Agron. J. 85:1054--1057. Breckle, S.W. 1991. Growth under stress. Heavy metals. In Plant
Roots: The Hidden Half. Eds. Y. Waisel, A. Eshel and U. Kafkafi. Marcelle Dekker Inc., New York, Basel, Hong Kong, pp 351--373. Coutts, M.P. and J.J. Philipson. 1987. Structure and physiology of
Sitka spruce roots. Proc. Roy. Soc. Edinburgh 93B:131--144. DeVos, C.H.R., H. Schat, R. Vooijs and W.H.O. Ernst. 1989.
Copper-induced damage to the permeability barrier in roots of Silene
cucu-balus. J. Plant Physiol. 135:164--169.
DeVos, C.H.R., H. Schat, M.A.M. DeWaal, R. Vooijs and W.H.O. Ernst. 1991. Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol. Plant. 82:523--528.
Eleftheriou, E.P. and S. Karataglis. 1989. Ultrastructural and morpho-logical characteristics of cultivated wheat growing on copper-pol-luted fields. Bot. Acta 102:134--140.
Table 4. Thickness (µm) of the whole root and the cortex and central cylinder measured 7 mm above the root tip in Pinus pinea and Pinus
pinaster seedlings exposed for 4 weeks to nutrient solutions
Fernandes, J.C. and F.S. Henriques. 1991. Biochemical, physiological, and structural effects of excess copper in plants. Bot. Rev. 57:246--273.
Gautheret, R.J. 1959. La culture des tissus vegetaux. Masson & Cle, Paris, 863 p.
Godbold, D.L. 1991. Die Wirkung von Aluminium und Schwermetal-len auf Picea abies Sämlinge. Schriften aus der Forstlichen Fakultät der Universität Göttingen und der Niedersächsischen Forstilchen Versuchsanstalt, Vol. 104. J.D. Sauerländers. Verlag, Frankfurt, 156 p.
Hardiman, R.T., B. Jacoby and A. Banin. 1984. Factors affecting the distribution of cadmium, copper and lead and their effect upon yield and zinc content in bush beans (Phaseolus vulgaris L.). Plant Soil 81:17--27.
Heale, E.L. and D.P. Ormrod. 1982. Effects of nickel and copper on
Acer rubrum, Cornus stolonifera, Lonicera tatarica and Pinus resinosa. Can. J. Bot. 60:2674--2681.
Hunter, B.A., M.S. Johnson and D.J. Thompson. 1987. Ecotoxicology of copper and cadmium in a contaminated grassland ecosystem. I. Soil and vegetation contamination. J. Appl. Ecol. 24:573--586. Kennedy, C.D. and F.A.N. Gonsalves. 1987. The action of divalent
zinc, cadmium, mercury, copper and lead on the trans-root potential and H+ efflux of excised roots. J. Exp. Bot. 38:800--817.
Lepp, N.W. 1981. Copper. In Effect of Heavy Metal Pollution on Plants, Vol. 1. Ed. N.W. Lepp. Applied Science Publications, Lon-don, pp 111--143.
Levi-Minzi, R. and R. Riffaldi. 1978. Ricerche preliminari sul con-tenuto in metalli pesanti dei fanghi di depurazione. Agricol. Ital. 107:169--178.
Marschner, H. 1986. Mineral nutrition of higher plants. Academic Press, London, pp 287--300.
Meharg, A.A. 1993. The role of plasmalemma in metal tolerance in angiosperms. Physiol. Plant. 88:191--198.
Patterson III, W.A. and J.J. Olson. 1983. Effects of heavy metals on radicle growth of selected woody species germinated on filter paper, mineral and organic soil substrates. Can. J. For. Res. 13:233--238.
Przymusinski, R. and E.A. Gwozdz. 1994. Increased accumulation of the 16 × 103 M polypeptide in lupin roots exposed to lead, copper and nitrite ions. Environ. Exp. Bot. 34:63--68.
Rauser, W.E. 1984. Partial purification and characterization of copper-binding protein from roots of Agrostis gigantea Roth. J. Plant Physiol. 115:143--152.
Sela, M., E. Tel-Or, E. Fritz and A. Hüttermann. 1988. Localization and toxic effects of cadmium, copper, and uranium in Azolla. Plant Physiol. 88:30--36.
Sutton, R.F. 1980. Root system morphogenesis. N.Z. J. For. Sci. 10:264--292.
Turner, A.P. and N.M. Dickinson. 1993. Survival of Acer
pseudopla-tanus L. (sycamore) seedlings on metalliferous soils. New Phytol.
123:509--521.
Ulrich, B. 1994. Nutrient and acid base budget of central European forest ecosystems. In Effects of Acid Rain on Forest Processes. Eds. D.L. Godbold and A. Hüttermann. John Wiley, New York, pp 1--50. Wong, M.H. and A.D. Bradshaw. 1982. A comparison of the toxicity of heavy metals, using root elongation of rye grass, Lolium perenne. New Phytol. 91:255--261.
Wong, M.K., G.K. Chuah, K.P. Ang and L.L. Koh. 1986. Interactive effects of lead, cadmium and copper combinations in the uptake of metals and growth of Brassica chinensis. Environ. Exp. Bot. 26:331--339.
Woolhouse, H.W. 1983. Toxicity and tolerance in the responses of plants to metals. In Physiological Plant Ecology III. Responses to the Chemical and Biological Environment. Encyclopedia of Plant Physiology, New Series, Vol. 12C. Eds. O.L. Lange, P.S. Nobel, C.B. Osmond and H. Ziegler. Springer Verlag, Berlin, Heidelberg, pp 245--300.