Thermal and Electrical Behaviour of Epoxy-based
Microcomposites Filled with Al
2
O
3
and SiO
2
Particles
Roman Kochetov
Thomas Andritsch
Peter H.F. Morshuis
Johan J. Smit
Delft University of Technology High Voltage Components & Power Systems
Delft, the Netherlands [email protected]
Abstract—Epoxy resin is a polar thermosetting polymer that is widely employed in different branches of industry and everyday life, due to their stable physical and chemical properties. Of all the polymer materials currently being used in the electrical insulation industry, epoxy resin is the most widely used kind, together with polyethylene and chosen as the base polymer material in the present study. As a common practice, in order to obtain materials of the desired thermal, mechanical and electrical properties, polymers are processed with different types of inorganic fillers. In this paper, the authors made an attempt to thoroughly analyze both the thermal and the electrical behaviour of the created epoxy microcomposites. Epoxy-based composites containing microparticles of aluminum oxide and silicon dioxide were prepared by high shear mechanical mixing and ultrasonic processing to obtain a fine dispersion of the fillers in the matrix. The incorporation of all filler types led to noticeable improvement in thermal conductivity compared to the pure epoxy resin. The thermal conductivity and the relative permittivity of the composites were greatly influenced by the filler loading of the inorganic particles. Rules of mixture are used to predict the thermal conductivity and the relative permittivity of two-phase composites have been applied to compare experimental results with theoretical models.
Keywords - microcomposite, epoxy resin, Al2O3, SiO2, thermal conductivity, relative permittivity
I. INTRODUCTION
Epoxy resin (ER) is a common electrical insulating polymer, which is used in high voltage cast resin transformers; cable joints, terminations and other accessories; rotating machines; bushings; GIS spacers [1], etc. Polymers used for electrical insulation are usually reinforced with inorganic fillers to improve the thermal, electrical and mechanical properties. In previous studies we investigated the thermal and electrical properties of nanocomposites containing one or two types of nanoparticles with a concentration of up to 10 wt.% [2, 3]. This paper is focused on the study of the same properties of microcomposites but the concentration of the filler is much higher – up to 60 percent by weight.
II. EXPERIMENTAL
A. Sample fabrication
The epoxy resin system used in this study is a commercially available Araldite CY231 epoxy resin with
anhydride hardener Aradur HY925. The micro aluminum oxide (Al2O3) and silicon dioxide (SiO2) particles have an
average particle size of 4 and 20 μm, respectively.
Epoxy composites containing Al2O3 or SiO2 microparticles
were fabricated in six steps:
1) Mixing the epoxy resin, hardener and filler by conventional mechanical high shear stirring;
2) Degassing;
3) Mixing in an ultrasonic bath at 42 kHz;
4) Casting into the molds;
5) Curing for 3 hours at 140 oC;
6) Postcuring for 3 days at 140 oC.
Several types of alumina and silica microcomposites were fabricated with a fillgrade up to 60 percent by weight. The samples for thermal conductivity were prepared as plates with dimensions of 110×70×3 mm. For dielectric spectroscopy analysis the samples were prepared as discs with a diameter of 40 mm and a thickness between 0.4 - 0.7 mm.
Table I lists the set of materials used in this study.
B. Thermal conductivity and dielectric spectroscopy measurements
The thermal conductivity measurements were realized with a THASYS system, produced by Hukseflux Thermal Sensors. This system performs a direct measurement which allows the
TABLE I. SPECIMENS STUDIED
Specimen Nanofiller
Neat ER Neat Epoxy Resin system ER-Al2O3-5 Epoxy system + 5wt.% Al2O3
This work was performed for the nanoPOWER project, which is supported by a Dutch government IOP-EMVT grant.
determination of the absolute value of the thermal conductivity. With a combination of a thin heater, two samples of similar thickness and two heat sinks it is possible to generate a homogeneous thermal field with a well defined heat flux through the samples.
A straightforward calculation of the thermal conductivity was made using the following equation:
eff
H T
λ=Φ ⋅ Δ , (1)
where λis thermal conductivity, Φ is the heat flux derived from the heated power, Heff is the effective sample thickness
and ΔT is the differential temperature across the samples. The thermal conductivity data represent the average value for the thermal conductivity of both samples. The measurements are performed in a climate chamber at 18 °C to avoid any influence due to changes of the ambient temperature during measurement. The accuracy of the measurements is 6%. Each data point corresponds to an average value of 4 measurements.
The real part of the permittivity ε′(relative permittivity) was determined by dielectric spectroscopy (DS) using a Novocontrol ALPHA-A analyzer in combination with a ZGS active sample cell. All samples were measured in the frequency range of 10-2 - 107 Hz and at temperatures between -20 ºC and 1-20 ºC.
III. RESULTS AND DISCUSSIONS
A. Thermal Conductivity
The thermal conductivity of the ER-Al2O3 and ER-SiO2
composites as a function of the filler concentration is shown in Fig. 1. With an increase of the filler content, the thermal conductivity increases gradually. Neat epoxy shows a weak thermal conductivity because of its relatively low atomic density [4]. The thermal conductivity of the polymer matrix can be increased while leaving the dielectric properties intact by addition of an inorganic filler, which has a higher thermal conductivity while still being an electrical insulator. Values of the thermal conductivities of neat epoxy and composites filled with Al2O3 and SiO2 are presented in Table II. The addition of
Al2O3 resulted in a steady increase of the thermal conductivity
by about 300% at the weight fraction of 60%. Adding 60 wt.% SiO2 filler improves the thermal conductivity by about 340%
however.
The possible explanation for the higher thermal conductivity of ER-SiO2 compounds compared to ER-Al2O3
ones could be the larger particle size of silica. The size of the particles plays an important role in the heat transfer between polymer matrix and the incorporated filler. The addition of fillers with a higher thermal conductivity than ER improves the heat transfer. But the resulting values are much lower than the values of bulk crystalline silica or alumina would suggest. Phonons, which are responsible for heat conduction in dielectric materials, are scattering at the interface between dissimilar materials and the heat dissipates on the surface of
microparticles. The epoxy is a thermal barrier for heat propagation, while the filler material transmits the heat much faster. Since the particles of Al2O3 are smaller than SiO2, more
phonons are scattered on the boundary, which in turn results in a lower heat transmission of the compound.
Two theoretical models were used to predict thermal conductivity of the binary system – parallel model (2) and geometric model (3):
composite, filler and matrix, respectively, and φ is the volume fraction of the filler content.
The thermal conductivity values of the bulk Al2O3 and
SiO2 vary in the range 15-40 W/m·K and 1.3-10 W/m·K,
respectively. These values depend on the purity of the material, crystal structure, way of production and so on. But as mentioned earlier, the thermal conductivity of powders is significantly lower than their crystalline counterparts, because the interface area is much larger, which leads to scattering of phonons. Therefore we used lower values of the thermal conductivity for our calculations, namely 10 W/m·K for Al2O3
and 1.2 W/m·K for SiO2.
The measured values of the thermal conductivity are plotted in Fig. 2 and compared to values that were calculated using the parallel and geometric mean models.
For Al2O3 composites the measured values and values
estimated by using the geometric mean model correspond almost completely with each other, throughout the whole range. The parallel model agrees better with the SiO2
composites for our measured values of the thermal conductivity for silica powder, which is 1.2 W/m·K. However, the geometric mean model is fitting the experimental data of ER-SiO2 samples best with the assumption that the thermal
conductivity of silica is close to 7 W/m·K. According to literature, the value of a bulk of crystalline silica lies between 1.3 and 10 W/m·K, thus it is possible that the silica used has a thermal conductivity value of 7 W/m·K.
Figure 1. Thermal conductivity of investigated materials as a function of filler concentration.
Figure 2. The experimental and predicted thermal conductivity values for Al2O3-epoxy (a) and SiO2-epoxy (b) composites at 18 °C.
B. Dielectric Properties
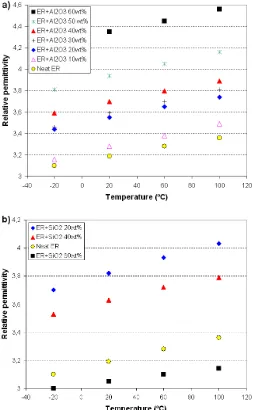
Fig. 3 shows the relative permittivity at 1.15 MHz as a function of temperature. A specimen of unfilled epoxy is used for comparison with the microcomposites. The relative permittivity ε ′ increases with the increase of both the filler content and temperature. The raised temperature increases the kinetic energy, which leads to faster movement of the particles on the molecular level.
Fig. 4 shows the typical behavior of the relative permittivity of composites with different particles inside as a function of frequency. The permittivity of all samples decreases with increasing frequency in the whole range due to a reduction in the polarization caused by a dipolar group of the organic matrix.
The interesting observation evident from Fig. 4b is that the permittivities of silica-epoxy composites decrease with an increasing concentration of SiO2, regardless of the fact that
permittivity of the filler is higher than matrix. The relative
.
permittivity of the silica is 4.3-4.7 [5], which is close to the value of the epoxy, i.e. 3.2-3.3 at 1 MHz. A possible explanation of this behaviour could be the immobilization of polymer chains. For a higher amount of filler material the mobility of the chains would be limited, lowering the effective relative permittivity.
In case of alumina the permittivity is much higher than that of ER (ε ′ of Al2O3 is about 9). The contribution of the
intrinsic permittivity of the filler plays a more important role in this case than the change of the molecular structure of the epoxy.
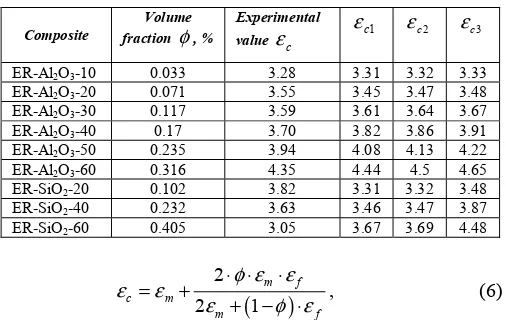
Rules of mixture are often used to predict the dielectric properties of ceramic composites. The relative permittivity of a composite at a certain frequency can be calculated using the following equations [6, 7, 8]:
logεc= ⋅φ logεf +(1−φ) log⋅ εm, (4)
TABLE III. THE EXPERIMENTAL AND CALCULATED VALUES OF RELATIVE PERMITTIVITY
filler and the matrix, respectively, φ is the volume fraction of the filler.
The experimental values and those calculated using (4)-(6) are presented in Table III.
As it can be see from the Table III, the calculated values are close to each other and predict the effective εc of the ER-Al2O3 binary system quite good. As discussed earlier, the εc
of ER-SiO2 composites becomes lower with addition of the
filler, therefore it cannot be predicted with the rules of mixture. The lower relative permittivity is an advantage which is currently exploited in different applications of epoxy with silica filler. A lower ε′ means lower capacity which leads to lower reactive currents.
IV. CONCLUSIONS
In this paper we investigated the influence of different types of particles and fillgrades on the thermal conductivity and dielectric properties of epoxy-based microcomposites.
Major findings from this study are as follows:
• Epoxy-based microcomposites containing Al2O3 and
SiO2 particles were successfully fabricated.
• The highest value of the thermal conductivity was achieved for ER-SiO2-60 with 0.745 W/m·K, which
means an improvement of 340% compared to the neat polymer. The addition of 60 wt.% Al2O3 improved the
thermal conductivity by 4 times. The reason for the higher thermal conductivity of composites filled with silica is the larger particle size of silica.
• The most interesting behavior of the relative
permittivity was observed for ER-SiO2 compounds.
The relative permittivity of ER-SiO2-60 became lower
than the one of neat epoxy. A possible explanation for this is an immobilization of polymer chains due to the filler.
mean model corresponds well, while the parallel model agrees better with SiO2 composites.
REFERENCES
[1] T. Shimizu, S. Kinoshita, S. Makishima, J. Sato, O. Sakaguchi, “Material and Simulation Technology for Solid Insulated Switchgear”, IEEE 7th International Conference on Properties and Application of Dielectric Materials (ICPADM), S22-5, pp. 1194-1197, 2003.
[2] R. Kochetov, T. Andritsch, U. Lafont, P.H.F. Morshuis, J.J. Smit, “The Thermal Conductivity in Aluminum Nitride and Epoxy-Aluminum Oxide Nanocomposite Systems”, Nordic Insulation Symposium (Nord-IS09), Gothenburg, Sweden, June 15-17, pp. 27-30, 2009.
[3] R. Kochetov, T. Andritsch, U. Lafont, P.H.F. Morshuis, J.J. Smit, “Thermal Conductivity of Nano-filled Epoxy Systems”, IEEE
Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Virginia Beach, USA, October 18-21, 2009.
[4] S.L. Shindé, J.S. Goela, High thermal conductivity materials, Springer, 2006.
[5] J. Kriegesmann, Technische Keramische Werkstoffe, Deutsche Keramische Gesellschaft, 1992.
[6] J.K. Nelson, J.C. Fothergill, “Internal charge behaviour of nanocomposites”, Nanotechnology 15, pp. 585-595, 2004.
[7] J.I. Hong, P. Winberg, L.S. Schadler, R.W. Siegel, “Dielectric properties of zinc oxide / low density polyethylene nanocomposites”, Materials Letters59, pp. 474-476, 2005.