Differences in shear bond strengh of metal bracket
on porcelain surface using three silane coupling
agents
Diah Adisty, Krisnawati, and Faruk Hoesin Department of Orthodontics
Faculty of Dentistry, Universitas Indonesia Jakarta - Indonesia
Correspondence: Diah Adisty, c/o: Departemen Ortodonsia, Fakultas Kedokteran Gigi Universitas Indonesia. Jl. Salemba Raya 4 Jakarta 10430, Indonesia. E-mail: [email protected]
ABSTRACT
Background: Porcelain crown restorations are often found in patients. Problems that may occur when bonding the bracket on the restoration is dislodged bracket and there is damage to the porcelain. The use of silane coupling agent has been reported to increase the bond strength and maintain the integrity of the porcelain. Purpose: This study aimed to see differences of shear bond strength using three materials of silane coupling agent with different organosilane composition. Method: This study uses 21 porcelain plates that are divided into three groups, they are silane coupling agent Monobond-Plus (Ivoclar Vivadent), Ultradent Silane (Ultradent product) and Porcelain Repair Primer (Ormco). The surfaces of 21 porcelain plates are given a 37% phosphoric acid etching, silane coupling agent material and Transbond XT adhesive (3M Unitek). Once the bracket is bonded on the surface of the porcelain plate then soaking/immersed in distilled water for 24 hours and the shear bond strength was tested with test equipment Universal Testing Machine Shimazu AG-5000. Result: The results showed statistically significant differences in shear bond strength from three silane coupling agent material. Material A has the highest shear bond strength (12.827 ± 1.228 MPa) and B has the lowest shear bond strength (6.295 ± 0.642 MPa). Those three ingredients meet the minimum criteria of shear bond strength,which is 6-8 MPa. ARI scores on material B showed a clean porcelain surface, while the materials A and C show the presence of bonding adhesive on the entire surface of the porcelain. Conclusion: Materials A and C are used if the porcelain will be replaced, while materials B is used if the porcelain will not be replaced.
Keywords: Shear bond strength; metal bracket; porcelain surfaces; silane coupling agent
ABSTRAK
permukaan porselen yang bersih, sedangkan bahan A dan C memperlihatkan adanya bahan adhesif yang merekat seluruhnya di permukaan porselen. Simpulan: Bahan A dan C baik digunakan jika porselen akan diganti, sedangkan bahan B baik digunakan jika porselen tidak akan diganti.
Kata kunci: Kuat rekat geser; braket metal; permukaan porselen; silane coupling agent
INTRODUCTION
Adult patients in general have a complex oral cavity conditions, such as periodontal tissue disorders, tooth loss, dental caries, and dental restorations (fillings) for example: porcelain restorations.1,2 Porcelain restoration is usually used to correct tooth with extensive cavities, tooth after root canal treatment, and to replace tooth loss. Porcelain restoration is also more expensive than other types of restorations.3,4 Therefore, dentist or dental specialist must be careful in handling porcelain restoration.3,5,7
Handling porcelain restorations associated with orthodontic treatment is generally associated with the process of bonding and de-bonding bracket. The bonding process is an essential step in order to produce good orthodontic treatment.5 Previous studies explained that, the bracket bonding on the surface of porcelain restorations resulting in damage to the restoration and cannot be avoided.3,8 To generate a good bonding, the surface of the porcelain restoration is usually done by coarsening process or grinding glaze and etching applications, where all the process of bonding mechanism is done mechanically.3,9-14 This process can cause cracks and fractures in the porcelain restoration. The mechanism of chemical bonding has been reported would help to maintain the integrity of the porcelain surface so that the restoration can still be used well after orthodontic treatment is completed.7,15,16 The mechanism of chemical bonding is done with the use of a silane coupling agent material.17 Material used in bonding with silane coupling agent is influenced by several factors, one of which is the concentration of organosilane. Therefore, it is important for the orthodontist to determine the composition of the silane coupling agent material in order to produce an enough bond strength (6-8 MPa) between the metal bracket and porcelain restoration.18 Silane coupling agent are available on the market with a wide range of brand name and its composition. Ivoclar Vivadent manufactures silane coupling agent materials under the trade name Monobond-Plus and has organosilane composition of < 2.5%. Ultradent Product manufactures silane coupling agent
materials under the trade name Ultradent Silane and it has composition of organosilane at a range of 5-15%. Ormco manufactures silane coupling agent materials under the trade name Porcelain Repair Primer and it has composition of organosilane at a range of 15–20%.
The strength of bond friction/shear (shear bond strength) is the ability to hold while taking friction/shear coming from the parallel direction to the surface of the object, such as the force while performing intrusion; extrusion; distalisation; and mesialisation of teeth.15,19,20,21 In orthodontic treatment, shear bond strength is more applicable than the tensile bond strength.
Based on the above explanation, the researchers wanted to know the difference between the use of three silane coupling agent materials, namely Monobond-Plus, Ultradent Silane Primer and Porcelain Repair, in the application of shear bond strength metal bracket to the surface of the porcelain.
MATERIAL AND METHODS
Transbond XT 3M, 37% phosphoric acid etching with Ultraetch brand-Ultradent Product, distilled water (aquadest), and red wax.
The 21 porcelain specimens were divided into 3 groups: the porcelain surface applied with silane coupling agent Monobond-Plus (A) material, the porcelain surface applied with Ultradent Silane silane coupling agent (B) material, the porcelain surface applied with silane coupling agent Porcelain Repair Primer (C) material. The 21 incisor bracket of right central maxillary (Densply, GAC) is divided evenly into 3 groups (each group totalled 7). The surface of 21 porcelain cylindrical specimens (diameter 8 mm and height 3 mm) coated with red wax and then implanted into the PVC cylinder (diameter 18.8 mm and height 10 mm) with decorative resin as the mediator. The surface 21 specimens were cleaned with pumice powder with a low-speed brush, rinse with water and dried off. Then, the 21 specimens were put into ultrasonic cleaner to be cleaned and then dried off. Then, on to the 21 specimens, applied the etching using 37% phosphoric acid for 60 seconds, rinse with water and then dried off, group A (7 specimens) has been given the silane coupling agent A material for 60 seconds and dried off with air spray, group B (7 specimens) has been given silanes coupling agent B material for 60 seconds and dried off with air spray, group C (7 specimens) has been given silane coupling agent C material for 60 seconds and dried off with air spray, then apply bonding material on the bracket mesh. Excess of bonding material are disposed and then irradiate with light-cured for 20 seconds each from the mesial, distal, occlusal and cervical, then the
specimens were stored in distilled water (aquadest) at a temperature of 37°C for 24 hours,23 shear bond strength measurements performed on the second day of the test using Universal testing Machine Shimazu AG-5000 with a speed of 0.5 mm/min and a maximum load of 50 kg. Force value obtained will be noted. Research data will be processed and tested statistically with SPSS 17 computer program. Univariate analysis was performed to obtain an average value, median, maximum, minimum, and standard deviation of each group. Bivariate analysis is to analyze differences in shear bond strength for the three types of materials silane coupling agent using one-way Anova and Post Hoc.
RESULTS
The study was conducted in the Laboratory of Dental Materials Faculty of Dentistry, University of Indonesia. Shear bond strength measurements were performed on each specimen with a load of research used by 50 kgf and a cross head speed in this study of 0.5 mm/min.
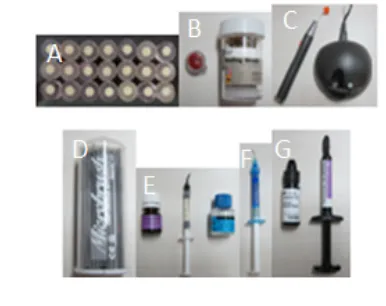
The results of the test for normality and variance of data showed that in group A, B and C have a normal distribution of data and there is no difference in the variance of the data. After the measurement is completed, the average was obtained from shear bond strength and metal bracket standard deviation on the surface of the porcelain using three materials of silane coupling agent A, B and C (Table 2). These results indicate that a large group of A has high shear bond strength while group B has low shear bond Figure 1. Tools and Material Research. A) 21 porcelain
speciments; B) pumice powder and low speed brush; C) light cured; D) microbrush; E) silane coupling agents; F) etsa (37% phosphoric acid); G) bonding material.
Table 1. Mean and standard deviation data of the metal bracket shear bond strength to porcelain surfaces using the three materials of silane couping agent (MPa)
Group N Mean ± s.d
A 7 12,827 ± 1,228
B 7 6,295 ± 0,642
C 7 11,098 ± 0,646
Table 2. One way Anova test of differences between metal bracket shear bond strength to the surface of the porcelain using three silane coupling agent material (MPa)
N Mean ± SD P
Silane Coupling Agent A 7 12,827 ± 1,228 < 0,001
strength. All three groups showed a shear bond strength that meets the minimum criteria of shear bond strength according to Whitlock and Reynolds which is 6–8 MPa.
To know the significant difference of metal bracket shear bond strength to the porcelain surface to the three groups, the data were tested using the bivariate analysis of oneway Anova (Table 3).
One way Anova result shows a p-value < 0.001 (p < 0.05). The hypothesis that stated that there are differences in the metal brackets shear bond strength to porcelain surfaces on the use of silane coupling agent A, B and C was accepted. Then from the data above, Post-Hoc statistical tests was performed. Post-hoc tests showed that there were significant differences between the application of a silane coupling agent A material with silane coupling agent B (p < 0.001), there were significant differences between the application of a silane coupling agent A material with silane coupling agent (Cp = 0.005) and there were a significant difference between the
application of a silane coupling agent B with a silane coupling agent C (p < 0.001) (Table 4).
Besides shear bond strength measurement, to complete this study, the residual adhesive on the bracket and porcelain surfaces was observed visually using a stereomicroscope. The result of these observations was assessed using the calculation of Adhesive Remnant Index (ARI).
These results indicate that in group A and C residual adhesive is almost entirely stuck to the porcelain surface, while in group B there is only a fraction/no residual adhesive on the surface of the porcelain.
DISCUSSION
The principle of the bracket bonding to the tooth surface is through the presence of microporosity in tooth enamel, which is obtained by the application of phosphoric acid etching. The problem is if the surface of the tooth is restored to get its integrity, then the formation of microporosity for bonding process becomes difficult. This is common on the ceramic restoration. In the anterior teeth that require high aesthetic, feldspathic ceramic or porcelain is usually used. Porcelain crown is a restoration which more expensive than other types of restorations. Therefore, in the process of bonding bracket on porcelain restorations, there are two things that should be concern to the orthodontist, which is to get enough bond strength and maintain the integrity of the porcelain after orthodontic treatment.
Silane coupling agent application in the bonding process allows achieving sufficient bonding strength and to maintain the integrity of the porcelain. To improve the bonding strength, the rules of a silane coupling agent to use the product in the market usually involve the application of acid etching before application of silanes. There are several factors that indirectly affect the strength of the bonding which is silane concentration, solvent, and temperature.
In group A, a silane coupling agent used is Monobond plus that content < 2.5% organosilane Table 3. Post-hoc Bonferroni test of significant differences in the
metal bracket shear bond strength to the surface of the porcelain using three silane coupling agent material (MPa)
Group Nilai p
A-B 0,000 p < 0,05
A-C 0,005 p < 0,05
B-C 0,000 p < 0,05
Value of p < 0.05 = statistically significant difference
8
Figure 2
Table 4
DISCUSSION
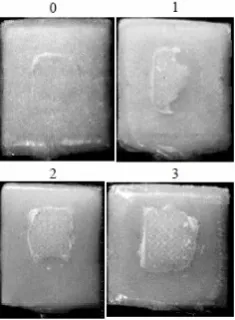
Figure 2. Adhesive Remnant Index 0 = no adhesive left on the tooth surface/other substrates, 1 = less than 50% of adhesive left on the tooth surface/other substrates, 2 = more than 50% of adhesive left on the surface dental/other substrates, 3 = entire adhesive left on the tooth surface/other substrates, characterized by the presence of a basic imprint bracket.
Table 4. Adhesive Remnant Index (ARI)
Kelompok Adhesive Remnant Index
0 1 2 3
A 0 0 6 1
B 5 2 0 0
composition (MPS), 50–100% ethanol and < 2.5% phosphoric acid ester methacrylated. Group B, a silane coupling agent used is Ultradent Silane that content 5–15% organosilane content (MPS), 92% isopropyl alcohol, < 1% acetic acid. Group C, a silane coupling agent used is Primary repair Porcelain that content 15–20% organosilane, 80–85% ethyl alcohol.
Group A which have the lowest organosilane (< 2.5%), has the highest shear bond strenght compared with groups B and C that content much more organosilane. This probably occurs because of the presence of phosphoric acid ester methacrylated of <2.5% in the preparation of a silane coupling agent. Methacrylated phosphoric acid ester is the active ingredient in self-etching solution primer. Phosphoric acid and methacrylate groups combined into a molecule that can simultaneously function as etching and primer. Therefore methacrylated phosphoric acid ester possible binding to the adhesive resin and porcelain materials, so that increase the covalent bond between the materials and also increase shear bond strength between the metal bracket with porcelain surface.
Group B which have 5-15% organosilane (MPS), has the lowest shear bond strength compared with groups A and C. Silane coupling agent B has an additional composition which is acetic acid. Acetic acid is used to accelerate the process of silanol condensation into oligomers, so that oligomers can be maximum formed automatically. These conditions may increase the covalent bond between the OH group of oligomers with inorganic substances (porcelain). This condition result the increase of shear bond strenght between metal brackets and porcelain surfaces. However, when compared with group A, which has higher shear bond strength but less organosilane content, the addition of phosphoric acid esters methacrylated may be more effective than the addition of acetic acid. Meanwhile, when compared with group C, which has higher shear bond strength organosilane and more content of organosilane, the addition of acetic acid may be less effective for generating maximum shear bond strength.
Group C which have the highest organosilane (15–20%) has higher shear bond strength than group B and lower than group A. In group C there is no additional material other than the organosilane and the main solvent. When compared with group A, which has higher shear bond strength and lower organosilane, then the addition of phosphoric acid
ester methacrylated effective to improve shear bond strength equivalent to group C that content 15–20% organosilane.
In this study, in addition to the measurement of shear bond strength, observations of adhesive remnant index that bond to the surface of the porcelain and bracket base were also carried out.
The lower the ARI score, the lower the power of bonding, but the bracket de-bonding and cleaning of the tooth surface/other substrate will be easier, and porcelain fracture may be lower.24 ARI scores in B shows a clean porcelain surface, while the A and C show the adhesive glue on the entire surface of porcelain.
Shear bond strength average of the three study groups meets the minimum criteria of shear bond strength according to Whitlock and Reynolds which is 6–8 MPa. Therefore, seen from the integrity of the porcelain surface, group B showed the best results because the shear bond strength hold enough to receive an orthodontic force and on the porcelain surface there is no/few remaining adhesive, so the possibility of porcelain fracture is small. Silane coupling agent A and C materials can be used only if the porcelain will be replaced, while the Silane coupling agent B can be used if the porcelain will not be replaced.
REFERENCES
2. Akhoundi MSA, Kamel MR, Hashemi SHM. Tensile bond strength of metal bracket bonding to glazed ceramic surfaces with different surface conditionings. Journal of Dentistry 2011; 8: 201–8.
3. Bishara SE, Ajlouni R, Oonsombat C. Bonding orthodontic brackets to porcelain using different adhesives/enamel conditiones: A comparative study. World J Orthod 2005; 6: 17–24.
4. Sakaguchi RL, Powers JM. Craig’s restorative dental materials. 13th ed. St. Louis: Elsevier; 2012. p. 254–5. 5. Larmour CJ, Bateman G, Stirrups DR. An investigation
into the bonding of orthodontic attachments to porcelain. Eur J Orthod 2006; 28: 74–7.
6. Alhaija ESJA, AlReesh IAA, AlWahadni AMS. Factors affecting the shear bond strength of metal and ceramic brackets bonded to different ceramic surfaces. Eur J Orthod 2010; 32: 274–80.
7. Yadav S, Upadhyay M, Borges GA. Influence of ceramic (feldspathic) surface treatment on the micro-shear bond strength of composite resin. Angle Orthod 2010; 80: 756–70.
8. Kojima I, Komori A, Nakahara R. An experimental study on the direct bonding method to reduce the risk of porcelain fracture. Orthodontic Waves 2010; 69: 110–16. 9. O’Brien WJ. Dental materials and their selection. 4th
ed. London: Quintessence Publishing; 2008. p. 212–5. 10. Hatrick CD, Eakle WS, Bird WF. Dental materials clinical
applications for dental assistants and dental hygienists. 2nd ed. St. Louis: Elsevier; 2011. p. 99–101.
11. Trakyah G, Malkondu O, Kazazoglu E. Effects of different silanes and acid concentrations on bond strength of brackets to porcelain surfaces. Eur J Orthod 2009; 31: 402–6.
12. Eliades G, Watts DC, Eliades T. Dental hard tissue and bonding. New York: Springer; 2005. p. 163–5.
13. Anusavice KJ, Philips RW. Phillip’s science of dental materials. 12nd ed. St. Louis: Elsevier; 2012. p. 137–8.
14. Powers JM, Farah JW, O’Keefe KL. Guide to all ceramic bonding. Texas: University of Texas School of Dentistry; 2012. p. 7–8.
15. Powers JM, Wataha JC. Dental materials properties and manipulation. 10th ed. St. Louis: Elsevier; 2013. p. 187–93.
16. Vatarugegrid S, Viteporn S. Shear-peel bond strength of metal bracket to porcelain surface treated with 1.23% acidulated phosphate fluoride gel. CU Dent J 2010; 33: 109–18.
17. Sarac YS, Kulunk T, Turk SE. Effects of surface-conditioning methods on shear bond strength of brackets bonded to different all-ceramic materials. Eur J Orthod 2011; 33: 667–72.
18. Sideridou I, Karabela M. Effect of the amount of 3-methacyloxypropyltrimethoxysilane coupling agent on physical properties of dental resin nanocomposites. Dent Mater 2009; 25: 1315–24.
19. McCabe JF, Walls AWG. Applied dental materials. 9th ed. Iowa: Blackwell Publishing; 2008. p. 23–5.
20. Turk T, Sarac D, Sarac Y. Effects of surface conditioning on bond strength of metal brackets to all-ceramic surfaces. Eur J Orthod 2006; 28: 450–56.
21. Kukiattrakoon B, Thammasitboon K. The effect of different etching times of acidulated phosphate fluoride gel on the shear bond strength of high-leucite ceramics bonded to composite resin. J Prosthet Dent 2007; 98: 17–23.
22. Sastroasmoro S. Dasar-dasar metodologi penelitian klinis. Edisi ke-4. Jakarta: Sagung Seto; 2011. p. 348–81. 23. Monticelli F, Toledano M. Effect of temperature on the
silane coupling agent when bonding core resin to quartz fiber posts. Dent Mater 2006; 22: 1024–8.