CORRESPONDENCE Prof Gregory Y H Lip,
University of Birmingham Centre for Cardiovascular Sciences, City Hospital, Birmingham, United Kingdom
g.y.h.lip@bham.ac.uk
Sponsored by Bristol-Myers Squibb. The author(s) maintained full control of the content and writing of the manuscript
ISSN 2042-4884
ABSTRACT
Over the last decade, a paradigm shift is apparent in the field of stroke prevention in atrial fibrillation (AF). For more than 6 decades, warfarin has represented the mainstay of anticoagulation therapy when used in AF patients. However, warfarin has important disadvantages, which limit its use in clinical practice. The recent emergence of novel oral anticoagulant drugs (NOACs) that overcome many of limitations of warfarin has allowed the provision of effective stroke prevention for many more patients with AF, as these drugs have a favorable efficacy–safety profile but also certain pharmacokinetic and pharmacodynamic properties that render periodic anticoagulant monitoring and dose adjustments unnecessary.
The NOACs fall into 2 broad categories, the oral direct thrombin inhibitors (dabigatran) and the oral factor Xa inhibitors (rivaroxaban, apixaban).The scope of this manuscript is to review currently available data regarding NOACs and to address practical issues relating to the safe and effective use of NOACs in clinical practice.
Novel Pharmacotherapies for the Prevention of Stroke or
Systemic Embolism in Adults with Non-valvular Atrial Fibrillation -
Part 1
Christos Dresios , MD & Gregory Y H Lip, MD
University of Birmingham Centre for Cardiovascular Sciences
Received: 28/8/13, Reviewed: 28/2/14, Accepted: 28/3/14
Key words: atrial fibrillation, stroke prevention, apixaban DOI: 10.5083/ejcm.20424884.116
318 EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL III ISSUE I
INTRODUCTION
Atrial fibrillation (AF) is the most common ab-normality of the cardiac rhythm and represents a growing global health issue of epidemic pro-portions [1, 2]. There is strong evidence that AF
confers a high thromboembolic risk, and the use or oral anticoagulants (OAC) such as warfa-rin is associated with considerable reduction of the risk of stroke and thromboembolic events, as well as mortality, when compared to control
[3-6].
Despite its demonstrated efficacy, the use of warfarin in clinical practice is rendered inconve-nient by several inherent pharmacological char-acteristics. The slow onset and offset of action, the narrow therapeutic range, the necessity of periodic anticoagulant monitoring, the high inter- and intra- individual variability with day-to-day variations in the anticoagulant response as well as the multiple food and drug have led not only to the underuse of warfarin, but also to high rates of its discontinuation[7,8].
There is also a close correlation between the benefits conferred by warfarin and the time in which INR falls within the intended therapeutic range and taking in consideration the difficul-ties in achieving optimal time in therapeutic range (TTR, ideally >70% of the time within INR 2-3) in real world, it is inferred that many AF pa-tients on warfarin are suboptimally protected against thromboembolic complications [9].
The NOACs fall into two broad categories: (i) the oral direct thrombin inhibitors (dabigatran) and (ii) the oral direct factor Xa inhibitors (e.g. rivar-oxaban, apixaban, etc).In general, NOACs deliver predictable and consistent antithrombotic re-sponse allowing for a fixed-dose regimen with-out the need for rwith-outine coagulation monitor-ing. This represents an important asset because simplification and convenience help improve management of patients in clinical practice. In addition, the NOACs are generally well toler-ated, have a rapid onset of action, short half life and have few known drug-drug interactions.
Currently NOACs are based on the impressive results of well de-signed large randomised controlled trials in which were compared to warfarin. The new drugs have features in major international guidelines and represent a reliable alternative to warfarin for the management of patients with non-valvular, paroxysmal or perma-nent AF with ≥1 risk factors for stroke or systemic embolism. How-ever, despite the promising results and the great expectations that were created in the scientific community, NOAC are not 100% free of constraints and drawbacks.
The scope of this article is to review currently available data regard-ing NOACs and to address practical issues relatregard-ing to the safe and effective use of NOACs in everyday clinical practice.
METHODS
We performed a search using English-language in regard to clini-cal studies, abstracts, references of detected articles and review ar-ticles. Google scholar and, PubMed databases up to January 2013 third week were searched. The search strategies were based on the terms “atrial fibrillation”, “apixaban in atrial fibrillation’’, “dabigatran in atrial fibrillation”, “rivaroxaban in atrial fibrillation” and “novel oral anticoagulants”.
Pharmacokinetic and Pharmacodynamic characteristics of three new oral anticoagulants
The NOACs are low molecular weight synthetic molecules that in-hibit the activity of specific pathways in the coagulation process. NOACS have various favorable pharmacokinetic and pharmacody-namic properties that render its use in clinical practice very conve-nient.
Dabigatran etexilate
Dabigatran etexilate is a prodrug which is rapidly and completely converted into the main active compound, dabigatran. The genera-tion of dabigatran is carried out by esterase-catalyzed hydrolysis in the plasma and liver. Dabigatran is also further metabolized into active glucuronide products. Specifically, dabigatran exitilate is a non peptidic synthetic molecule that has been proven to be a di-rect competitive and reversible thrombin inhibitor of both free and clot-bound thrombin [10-12]. After oral administration, dabigatran
etexilate is quickly absorbed and the peak plasma concentrations are achieved within 0.5–4 hours. However, its bioavailability is low (6.5%), and this is unrelated to food intake nor drug administration
[13]
Additionally, dabigatran is characterized by low plasma protein binding and thus exhibit low likelihood of drug-drug interactions
[14].Indeed only 35% of dabigatran is protein binded. After the
rapid distribution phase, follows a relatively protracted elimination phase that results in a half-life of 17 hours after multiple doses [15].
Renal excretion of dabigatran accounts for approximately 80% of total clearance [15]. Due to the fact that in patients with impaired
re-nal function dabigatran may exhibit prolonged elimination, its use in this setting requires particular caution. According to European guidelines, dabigatran is contraindicated in patients with severe
Dabigatran exitilate does not interact with the cytochrome P450 system and thus the risk of drug – drug interaction is significantly reduced. On the other hand, dabigatran exitilate is a substrate of P-glycoprotein which is an efflux pump that exports its substrates out of the cell, and thus the concomitant use with potent P-glyco-protein inhibitors or P- glycoP-glyco-protein inducers may lead to increased risk of significant variation of its plasma concentrations. In patients who receive potent P-glycoprotein inhibitors (azole-antimycotics, immunosuppressants, and human immunodeficiency virus prote-ase inhibitors must be avoided), or P-glycoprotein inducers (potent P-gp inducers such as rifampin), the use of dabigatran is contrain-dicated (Table 2)
Which drugs matter in cardiology? Verapamil is a P-glycoprotein inhibitor and its concomitant use in patients receiving dabiga-tran may lead to increased plasma concentrations of dabigadabiga-tran. With regard to amiodarone and quinidine, no dose adjustment is required. On the other hand, dronedarone is a potent inhibitor of P-gp efflux transporter should be avoided in patients receiving dabigatran because it may leads to increased dabigatran plasma levels.
Particular care should be taken in case of concomitant use of anti-platelet agents. In addition, concurrent treatment with non-steroi-dal anti-inflammatory drugs (NSAIDs) significantly increases the risk of major bleeding and requires a careful benefit-risk assessment.
Concomitant treatment with any other anticoagulants is contrain-dicated except in case of switching therapy to or from dabigatran or when unfractionated heparin (UFH) is administrated at doses necessary to maintain the patency of central venous or arterial catheters.
The recommended dose of dabigatran is usually 150 mg twice daily. Treatment with dabigatran 110 mg b.i.d. dose is recommended for:
- Elderly patients (age >80 years),
- In patients with moderate renal impairment (CrCl 30-50 ml/min) who are at high risk of bleeding, including age 75-80 years, - Simultaneous use of P- glycoprotein inducers or inhibitors. - In patients at high bleeding risk (eg. HAS-BLED >3)
In the USA, the FDA approved the 150 mg b.i.d. dose but also ap-proved a 75 mg bid dose regimen, for patients with severe renal impairment (CrCl 15–30 ml/min), even though this does has never been tested for stroke prevention in AF. In addition to the dose of 75mg twice should be also considered in patients with moderate renal impairment who receive concomitantly dronedarone or sys-temic ketoconazole.
Rivaroxaban
320 EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL III ISSUE I
Rivaroxaban is a small-synthetic molecule and has a high bioavail-ability (80%) with rapid, absorption after oral administration. The bioavailability of rivaroxaban is dose dependent [16, 17],ranging
from 80% to 100% when administrated in the dose of 10-mg dose, while at a dose of 20mg the bioavailability is about 66%. Peak con-centrations in plasma are achieved at2.5 to 4 hours. Of note, ab-sorption rates and bioavailability are influenced by food intake, and both are reduced in fasted patients. Thus, rivaroxaban should be administrated after food intake. The half life of rivaroxaban is 5-13 hours, but this is shorter in elderly patients [16].
In contrast to dabigatran, rivaroxaban demonstrate high plasma protein binding (92%-95%), and consequently it is not dialyzable. Approximately>50% of rivaroxaban is metabolized by liver en-zymes, principally by cytochrome P450 3A4 whereas the remainder is eliminated unchanged via kidneys [17, 18].
Rivaroxaban (similar to dabigatran exitilate and apixaban) is a sub-strate of P-glycoprotein. Concurrent use of drugs which are strong inhibitors of CYP-3A4 or P-glycoprotein efflux transporter (eg, sys-temic ketoconazole, intrakonazole) lead to increased rivaroxaban anticoagulant effect and thus, the concomitant use of these drugs should be avoided [19] Additionally, potent inducers of CYP-3A4
and/or P-glycoprotein efflux transporter should be avoided due to the significant decrease in rivaroxaban plasma concentration.
Again, concomitant use of NSAIDs and platelet aggregation inhibi-tors should be undertaken with caution due to increased the bleed-ing risk by concomitant therapy. Due to the increased hemorrhagic risk, concomitant treatment with other anticoagulant drugs is con-traindicated. Notwithstanding, UFH may be administered at doses necessary to maintain an open central venous or arterial catheters, or in case of switching therapy to or from rivaroxaban.
Most of the administered dose of rivaroxaban (>66%) appears in urine and the remainder is eliminated by the faecal-biliary route. In patients with nonvalvular AF, the recommended dose of rivaroxa-ban is 20 mg once daily with the evening meal. However, taking into consideration that rivaroxaban is eliminated by the kidneys, in patients with moderate renal impairment (CrCl 30-49 mL/min), rivaroxaban should be administrated at a dose of 15mg o.d. Due to lack of evidence in relation to the efficacy-safety profile of rivaroxa-ban in patients with severe renal impairment (CrCl <30 mL/min), the European guidelines do not recommend its use in patients with a severe renal impairment (CrCl <30 mL/min). In patients with mild hepatic impairment, no dose adjustment is required.
Apixaban
Apixaban was associated with significant reduction of mortality in its pivotal Phase 3 trial, as well as superiority to warfarin in efficacy and safety. Apixaban is also well tolerated with discontinuation rates lower than warfarin. Apixaban was recently approved by Eu-ropean Commission and by the FDA for the prevention of stroke or systemic embolism in patients with non valvular AF.
Apixaban is an oral direct selective and reversible FXa inhibitor and is a small synthetic molecule, rapidly absorbed, with a bioavailabil-ity that overreach 50%. Peak plasma concentrations are achieved within 3 to 4 after oral administration and its half life is approxi-mately 12 hours [20].
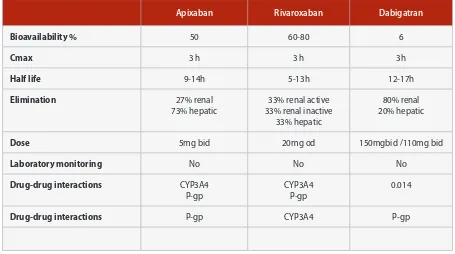
Apixaban Rivaroxaban Dabigatran
Bioavailability % 50 60-80 6
Cmax 3 h 3 h 3h
Half life 9-14h 5-13h 12-17h
Elimination 27% renal
73% hepatic
33% renal active 33% renal inactive
33% hepatic
80% renal 20% hepatic
Dose 5mg bid 20mg od 150mgbid /110mg bid
Laboratory monitoring No No No
Drug-drug interactions CYP3A4
P-gp
CYP3A4 P-gp
0.014
Drug-drug interactions P-gp CYP3A4 P-gp
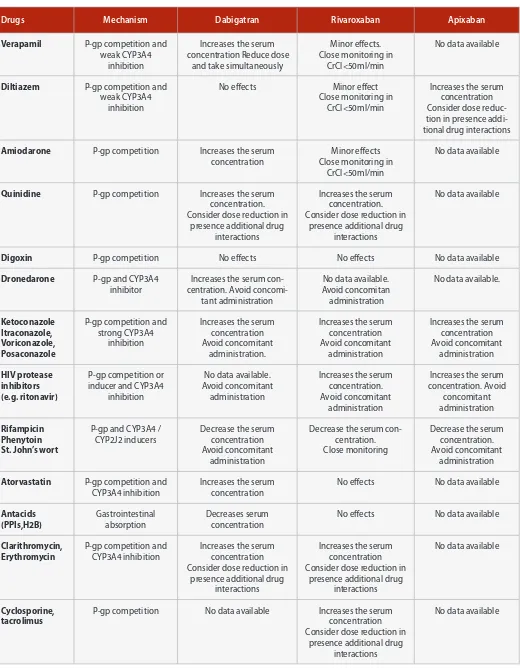
Table 2: Summary of pharmacokinetic drug-drug Interactions with NOACs (H2B: H2-blockers; PPI: proton-pump inhibitors)
Drugs Mechanism Dabigatran Rivaroxaban Apixaban
Verapamil P-gp competition and weak CYP3A4
inhibition
Increases the serum concentration Reduce dose
and take simultaneously
Minor effects. Close monitoring in
CrCl<50ml/min
No data available
Diltiazem P-gp competition and weak CYP3A4
inhibition
No effects Minor effect Close monitoring in
CrCl<50ml/min
Increases the serum concentration Consider dose reduc-tion in presence addi-tional drug interactions
Amiodarone P-gp competition Increases the serum concentration
Minor effects Close monitoring in
CrCl<50ml/min
No data available
Quinidine P-gp competition Increases the serum concentration. Consider dose reduction in
presence additional drug interactions
Increases the serum concentration. Consider dose reduction in
presence additional drug interactions
No data available
Digoxin P-gp competition No effects No effects No data available
Dronedarone P-gp and CYP3A4 inhibitor
Increases the serum con-centration. Avoid
concomi-P-gp competition and strong CYP3A4
Increases the serum concentration Avoid concomitant
administration
Increases the serum concentration
P-gp competition or inducer and CYP3A4
inhibition
No data available. Avoid concomitant
administration
Increases the serum concentration. Avoid concomitant
administration
Increases the serum concentration. Avoid
P-gp and CYP3A4 / CYP2J2 inducers
Decrease the serum concentration Avoid concomitant
administration
Decrease the serum con-centration. Close monitoring
Decrease the serum concentration. Avoid concomitant
administration
Atorvastatin P-gp competition and CYP3A4 inhibition
Increases the serum concentration
No effects No data available
Antacids
No effects No data available
Clarithromycin, Erythromycin
P-gp competition and CYP3A4 inhibition
Increases the serum concentration Consider dose reduction in
presence additional drug interactions
Increases the serum concentration Consider dose reduction in
presence additional drug interactions
No data available
322 EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL III ISSUE I
Apixaban also demonstrates a quick onset of action, of approxi-mately 3-4 hours. Plasma concentrations of apixaban are not in-fluenced by food intake and thus it can be administered with or without food.
Apixaban is metabolised in the liver mainly by CYP3A4 and to some extent via CYP-independent pathways in to inactive metabolites [20]. Apixaban does not inhibit or induce CYP enzymes
and thus the potential of drug interaction is significantly reduced. However, co-administration of drugs that are substrates for induc-ers or inhibitors of CYP3A4 may affect significantly its plasma levels. Additionally, strong inhibitors of both CYP3A4 and P-gp should be avoided due to increased plasma concentrations of apixaban. Par-ticular caution is also required in case of concomitant use of strong inducers of both CYP3A4 and P-gp that may lead to reduced plas-ma apixaban levels [21, 22].
Particular care is required if patients receive concomitantly drugs which affect hemostasis such as NSAIDs, acetylsalicylic acid (ASA), and other antiplatelets. If concomitant therapy with these drugs is considered, the risk-benefit ratio should be thoroughly assessed. Concurrent treatment with any other anticoagulants is contrain-dicated except when converting patients to or from apixaban or when UFH is administrated in order to maintain a patent central venous or arterial catheter.
The pharmacokinetic drug-drug Interactions with NOACs are sum-marised in Table 2. Similar to rivaroxaban, apixaban demonstrates a dual mechanism of elimination. Renal excretion of apixaban rep-resents an important route of apixaban elimination, accounting for approximately 25% of total excretion. On the other hand, apixaban is also excreted by the intestine and bile to the faeces. [20]
The pharmacokinetics of apixaban are not substantially affected by food consumption and thus, it can be administered without regard to food intake [23]. Similar to rivaroxaban, binding of apixaban to
plasma protein is considerably high (approximately 87%) and thus its anticoagulation effects are not reversed with haemodialysis.
The recommended dose for AF patients with normal renal function is 5 mg twice daily. Mild-moderate impairment of renal function do not significantly affect anti-factor Xa activity of apixaban and thus in the United States and Canada, no dosage amendment is recom-mended in patients with isolated mild –moderate renal dysfunc-tion. However, the dose should be reduced to 2.5 mg b.i.d in pa-tients with 2 of the following 3 criteria: Serum creatinine ≥1.5 mg/ dL, age ≥80 years or body weight ≤60 kg.
Even though apixaban is less affected by renal dysfunction than dabigatran, in individuals with severe renal impairment (CrCl: 15-29 ml/min), apixaban plasma concentrations can be significantly increased. In addition, patients with severe renal dysfunction were actually excluded from the ARISTOTLE trial. Apixaban should be avoided in patients who suffer from severe hepatic impairment.
Clinical trials of the novel oral
anticoagulant agents in atrial fibrillation
In the last few years, large randomized double blind studies have been published in relation to the role of the NOACs in the treat-ment of AF patients. The studies were designed to compared the long term efficacy and safety of NOACs with dose-adjusted war-farin (to an INR of 2.0-3.0) with the principal objective to test the hypothesis that NOACs were non inferior to vitamin K antagonists (VKAs) in the prevention of stroke and systemic thromboembolic events. The results of these trials are summarized in Table 3.
Currently the efficacy-safety profile of NOACs is well supported and the role in the management of AF patients with non valvular AF is well recognised. Moreover all three NOACs are probably superior to warfarin in patients with AF at high risk of stroke irrespective of the hemorrhagic risk, based on a modeling analysis [37]. Indeed, NOACs
have been approved by both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), and included into major international guidelines. (Table 4)
Even though the use of NOACs as alternative to warfarin is well es-tablished there is no evidence to support the recommendation for or against any of the NOACs over each other. Large scale head to head randomized studies which support one agent over the other are not yet available. Indirect comparisons of the NOACs have been published, although this method is associated with certain limita-tions which are mainly related to the differences in the pharmaco-logic properties, the study designs, and the heterogeneity of the populations studied (Table 3).
For patients at a very high risk for stroke and a low risk of hemor-rhagic complications, a more efficient and relatively less safe agent would be suitable. On the other hand for AF patients considered at moderate risk for stroke but with high bleeding risk, it is reason-able that a more safe than efficient anticoagulant agent should be preferred. Thus the patient profile could be matched for the drug characteristics, given that we now have a choice, to fit the drug to the patient.
In a recent indirect comparison study of the 3 NOACs, there no profound significant differences in efficacy between apixaban and dabigatran etexilate (both doses) or rivaroxaban. Dabigatran 150 mg BID was superior to rivaroxaban for some efficacy endpoints, whereas major bleeding was significantly lower with dabigatran 110 mg BID or apixaban[33]. As far as major bleeding are concerned,
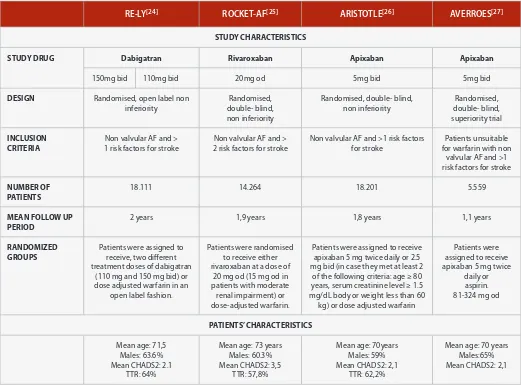
Table 3: Clinical trials of dabigatran, rivaroxaban and apixaban (Continued next page)
RE-LY[24] ROCKET-AF[25] ARISTOTLE[26] AVERROES[27]
STUDY CHARACTERISTICS
STUDY DRUG Dabigatran Rivaroxaban Apixaban Apixaban
150mg bid 110mg bid 20mg od 5mg bid 5mg bid
DESIGN Randomised, open label non inferiority
Randomised, double- blind, non inferiority
Randomised, double- blind, non inferiority
Randomised, double- blind, superiority trial
INCLUSION CRITERIA
Non valvular AF and > 1 risk factors for stroke
Non valvular AF and > 2 risk factors for stroke
Non valvular AF and >1 risk factors for stroke
Patients unsuitable for warfarin with non
valvular AF and >1 risk factors for stroke
NUMBER OF PATIENTS
18.111 14.264 18.201 5.559
MEAN FOLLOW UP PERIOD
2 years 1,9 years 1,8 years 1,1 years
RANDOMIZED GROUPS
Patients were assigned to receive, two different treatment doses of dabigatran
(110 mg and 150 mg bid) or dose adjusted warfarin in an
open label fashion.
Patients were randomised to receive either rivaroxaban at a dose of
20 mg od (15 mg od in patients with moderate renal impairment) or dose-adjusted warfarin.
Patients were assigned to receive apixaban 5 mg twice daily or 2.5 mg bid (in case they met at least 2
of the following criteria: age ≥ 80 years, serum creatinine level ≥ 1.5 mg/dL body or weight less than 60
kg) or dose adjusted warfarin
Patients were assigned to receive apixaban 5 mg twice
daily or aspirin. 81-324 mg od
PATIENTS’ CHARACTERISTICS
Mean age: 71,5 Males: 63.6% Mean CHADS2: 2.1
TTR: 64%
Mean age: 73 years Males: 60.3% Mean CHADS2: 3,5
TTR: 57,8%
Mean age: 70years Males: 59% Mean CHADS2: 2,1
TTR: 62,2%
Mean age: 70 years Males:65% Mean CHADS2: 2,1
Another indirect comparison study that focused on the secondary prevention subgroups of all three trials in an effort to obtain the minimum of heterogeneity, found that apixaban, dabigatran and rivaroxaban did not differ in efficacy for the main endpoints. Dabig-atran 110mg twice compared with rivaroxaban was associated with lower rates of haemorrhagic stroke, vascular death, major bleed-ing, and intracranial bleeding but with higher rates of myocardial infarction events. The stroke rate in the primary prevention cohort was significantly less common with dabigatran 150 mg twice daily compared with apixaban but this benefit was accompanied by an increase in major bleeding and gastrointestinal bleeding events. Apixaban compared with rivaroxaban did not differ in regard to efficacy but major bleeding was less common with apixaban than with rivaroxaban. No significant differences for safety and efficacy were found between dabigatran 150 mg twice daily and rivaroxa-ban [34]. Broadly similar findings have been seen in other similar
analyses[35].
In a recent study carried out in Canada an indirect treatment com-parison of dabigatran versus rivaroxaban was performed in relation to the efficacy and cost effectiveness. Based on the analysis of the results of RE-LY and ROCKET AF trials dabigatran was associated with a lower risk of intracranial haemorrhage. Moreover dabigatran resulted to be superior to rivaroxaban in regard to acute care and long-term follow-up costs per patient which compensate the dif-ference in drug costs. This study concluded that dabigatran is supe-rior to rivaroxaban for prevention of stroke and systemic embolism among Canadian AF patients [36].
324 EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL III ISSUE I Table 3: Clinical trials of dabigatran, rivaroxaban and apixaban (Continued)
RE-LY[24] ROCKET-AF[25] ARISTOTLE[26] AVERROES[27]
OUTCOMES(% per year)
PRIMARY
EFFICACY END POINT Stroke and systemic embolic events (%/ year; RR; 95% CI, P value)
Dabigatran 150mg vs warfarin: 1,11% vs 1,69% (0.66; 0.53–0.82; p for superiority <0.001) Dabigatran 110mg vs warfarin: 1.53% vs 1,69% (0.91,
0.74–1.11; p for non inferiority <0.001)
Rivaroxaban vs warfarin: 2,12% vs 2,42%. (0.88, 0.75–1.03;
p for non inferiority <0.001, p for superiority = 0.12)
Apixaban vs warfarin: 1,27% vs 1,6% (0.79, 0.66–0.95;
p<0.001 for non-inferiority,
p = 0.01 for superiority.
Apixaban vs aspirin: 1,6% vs 3,7%r (0.45, 0.32–0.62; p<0.001)
ISCHAEMIC STROKE Dabigatran 150mg vs warfarin: 0,92%vs 1,2%
(0.76, 0.60–0.98; p= 0.03) Dabigatran 110mg vs warfarin: 1.34% vs 1,2%
MAJOR BLEEDING Dabigatran 150mg vs warfarin: 3,11% vs 3,36%
(0.93, 0.81–1.07;p=0.31) Dabigatran 110mg vs warfarin: 2,71% vs 3,36%
(0.80, 0.69–0.93;p<0.003)
Rivaroxaban vs warfarin: 3,6% vs 3.45% (p=0.576)
Apixaban vs warfarin:
Dabigatran 150mg vs warfarin: 0.10% vs 0,38%
(0.26, 0.14–0.49; p<0.001 Dabigatran 110mg vs warfarin: 0.12% vs 0,38% (0.31,
Dabigatran 150mg vs warfarin: 20.7% vs. 16.6%
Dabigatran 110mg vs warfarin: 21.2% vs. 16.6%
Dabigatran 150mg vs warfarin: 0,81% vs 0,64%
(1.27, 0.94-1.71; p= 0.12) Dabigatran 110mg vs warfarin: 0.82% vs 0.64%(1.29, 096-1.75;p= 0.09)
Rivaroxaban vs warfarin:
Dabigatran 150mg vs warfarin: 1.51% vs 1,02%
(1.50, 1.19–1.89; p<0.0x01) Dabigatran 110mg vs warfarin: 1.12% vs 1,02%
(1.10, 0.86–1.41;p= 0.43)
Rivaroxaban vs warfarin: 3.2% vs 2,2%( p<0.001
Apixaban vs warfarin:
Dabigatran 150mg vs warfarin: 0.30%vs 0.74% (0.40, 0.27–0.60; p<0.001)
Dabigatran 110mg vs warfarin: 0.23% vs 0,74%
ALL CAUSE DEATH Dabigatran 150mg vs warfarin: 3.64% vs 4.13%
Table 4: Synopsis of clinical guidelines regarding the use of NOACs in non valvular AF.
2012 FOCUSED UPDATE OF THE ESC GUIDELINES [28]
· In patients with a CHA2DS2-VASc score >2, OAC therapy with a direct thrombin inhibitor (dabigatran) or an oral factor Xa inhibitor (e.g. rivaroxaban, apixaban) is strongly recommended, unless contraindicated (Class I, level, of evidence A). · In patients with a CHA2DS2-VASc score of 1, OAC therapy with a direct thrombin inhibitor (dabigatran) or an oral factor Xa inhibitor (e.g. rivaroxaban, apixaban) should be considered, based upon an assessment of the risk of bleeding complications and patient preferences (Class IIa, level of evidence A)
· When adjusted-dose VKA (INR 2–3) cannot be used in a patient with AF where an OAC is recommended, due to difficulties in keeping within therapeutic anticoagulation, experiencing side effects of VKAs, or inability to attend or undertake INR monitoring, one of the NOACs, either a direct thrombin inhibitor (dabigatran) or an oral factor Xa inhibitor(e.g. rivaroxaban, apixaban) is strongly recommended(Class I, level of evidence B)
FOCUSED 2012 UPDATE OF THE CANADIAN CARDIOVASCULAR SOCIETY ATRIAL FIBRILLATION GUIDELINES [29]
· When OAC therapy is indicated, most patients should receive dabigatran, rivaroxaban, or apixaban(once approved by Health Canada), in preference to warfarin (Conditional Recommendation, High-Quality Evidence).
ACCP 2012 GUIDELINES[30]
· For patients with non valvular AF, including those with paroxysmal AF that are at high (CHADS2 score>2)or at intermediate risk (CHADS2 score=1) for stroke ACCP guidelines recommend dabigatran 150 mg twice daily rather than adjusted-dose VKA therapy for primary and secondary prevention of cardioembolic stroke or TIA(Grade 2B)
SIGN 2012 [31]
· Dabigatran etexilate can be considered as an alternative to warfarin in the management of patients with atrial fibrillation with one or more risk factors for stroke (Grade A).
· Rivaroxaban can be considered as an alternative to warfarin in the management of patients with atrial fibrillation with one or more risk factors for stroke (Grade A).
2011 ACCF/AHA/HRS FOCUSED UPDATE[32]
· Dabigatran is useful as an alternative to warfarin for the prevention of stroke and systemic thromboembolism in patients with paroxysmal to permanent AF and risk factors for stroke or systemic embolisation who do not have a prosthetic heart valve or haemodynamically significant valve disease, severe renal failure (creatinine clearance <15 mL/min), or advanced liver disease (impaired baseline clotting function). (Level of Evidence: B)
SESC: European Society of Cardiology ACCP: American College of Chest Physicians
326 EUROPEAN JOURNAL OF CARDIOVASCULAR MEDICINE VOL III ISSUE I
What are the main differences between the trials?
ROCKET AF enrolled patients at much higher risk of stroke com-pared to RE-LY and ARISTOTLE [13]. In RE-LY and ARISTOTLE the
mean CHADS2 score was 2.1, patients included ROCKET AF, had a mean CHADS2 of 3.5. In addition to that 55% of the patients en-rolled in ROCKET AF had history of stroke, while the majority of the participants suffered from heart failure. Furthermore although patients with severe renal impairment (CrCl<30ml/min) were ex-cluded from the studies, more than 20% of the patients enrolled in ROCKET AF had moderate renal impairment (CrCl 30–49 mL/min) and received rivaroxaban 15mg once daily.
In ARISTOTLE, approximately 15% of patients included were classi-fied as having moderate renal impairment, but the reduced dose of apixaban (2.5mg bod) was administrated in 4.7% and 4.4% in the active and control groups, respectively. In RE-LY the percentage of patients suffering from moderate renal impairment was 19.4%. In contrast to ARISTOTLE and ROCKET-AF, in RE-LY dabigatran was not stratified on the base of the renal function. In ROCKET trial, the mean TTR obtained in the warfarin arm was much lower (57.8%) was lower in comparison to the mean TTR in RE-LY and ARISTOTLE trials in which it was 64% and 62.2% respectively. Indeed ROCKET AF enrolled more patients from low-income countries [19].
There are also important differences in regard to the design of the studies and specifically in blinding. Indeed contrary to ROCKET-AF and ARISTOTLE which were double blinded studies, RE-LY was a prospective, randomised open blinded endpoint (PROBE) designed study. Although double-blind controlled trials are considered the highest standard of quality in phase III studies, such trials may not reflect realistic clinical conditions due to their design related com-plex requirements. In particular in AF trials, the strict conditions of blind INR measurement that are required in the setting of a double-blind, double dummy trial may increase the risk of inclusion bias. On the contrary, a PROBE trial design allow for recruitment from a wider pool reflecting the real world conditions [38].
A recent study investigated what differences are present between PROBE designed and double-blind, double dummy designed trials of NOACs in AF [39]. Interestingly, the two designs did not differ in
patient populations and whilst differences in outcomes were re-vealed in this study, they do not appear to be related to the study design. Apart from higher mean mortality in ROCKET AF (a double blind study), and lower myocardial infarction rates in RELY (which was a PROBE study), both study designs had similar event rates for the primary outcome in the groups of patients assigned to receive VKA did not differ among PROBE and double-blind trials. In addi-tion, this study suggests that if adequate measures are taken to maintain blinding of outcomes, the cumbersome requirements of double blinding may not be necessary in order to evaluate antico-agulants.
REFERENCES
Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ.
Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004; 90: 286–92.
Lip GY, Tse H-F. Management of atrial fibrillation. Lancet 2007; 370: 604-18.
Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA.
Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study, JAMA 271 1994 840-844. Hnatkova K, Waktare JE, Murgatroyd FD, Guo X, Camm AJ, Malik M.
Age and gender influences on rate and duration of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 21 1998 2455-58
Kannel WB, Wolf PA, Benjamin EJ, Levy D Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation, population based estimates. Am J Cardiol 82 1998 2N-9N |
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-67.
Birman-Deych E, Radford MJ, Nilasena DS, Gage BF.
Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke 2006;37:1070-74
Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S.
Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation.
Circulation 2007;115:2689-96
Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, Bankhead C, Xu Y. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review.
Circ Cardiovasc Qual Outcomes. 2008;1:84–91
Hauel NH, Nar H, Priepke H, Ries U, Stassen JM, Wienen W.
Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem 2002; 45: 1757–1766.
Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N.
In-vitroprofile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost 2007; 98: 155–62. Van Ryn J, Hauel N, Waldmann L, Arora RR, Wienen W.
Dabigatran inhibits both clot-bound and fluid phase thrombin in vitro: Effects compared to heparin and hirudin. Blood 2007; 110: 3998.
Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate.
Clin Pharmacokinet 2008; 47: 285-295.
Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W.
The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36: 386–399 Stangier J, Rathgen K, Gansser D, Kohlbrenner V, Stassen JM.
Pharmacokinetics of BIBR 953 ZW, a novel low molecular weight direct thrombin inhibitor in healthy volunteers. Thromb Haemost 2001; 86:OC2347
Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G.
Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xainhibitor.
ClinPharmacolTher. 2005;78:412-421.
Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2009 ;37(5):1056-64.
Kubitza D, Becka M, Mueck W, Halabi A, Maatouk H, Klause N, Lufft V, Wand DD, Philipp T, Bruck H. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol 70:703–712 1 This article is continued with information about clinical
Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, Mitchell LB, Verma A, Nattel S; Canadian Cardiovascular Society Atrial Fibrillation Guidelines Committee. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control.
Can J Cardiol. 2012;28(2):125-36
You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GY; American College of ChestPhysicians.
Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-75S
Scottish Intercollegiate Guidelines Network (SIGN).
Antithrombotics: indications and management. A national clinical guideline. Edinburgh (Scotland): Scottish Intercollegiate Guidelines Network (SIGN); 2012. 66 p.(SIGN publication; no. 129).
Wann LS, Curtis AB, Ellenbogen KA, Estes NA 3rd, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM. 2011ACCF/AHA/HRSfocusedupdate on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2011 15;57(11):1330-7.
Lip GY, Larsen TB, Skjøth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012 Aug 21; 60(8):738-46.
Rasmussen LH, Larsen TB, Graungaard T, Skjøth F, Lip GY. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ. 2012 Nov 5;345:e7097.
Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost. 2012 ;108(3):476-84.
Kansal AR, Sharma M, Bradley-Kennedy C, Clemens A, Monz BU, Peng S, Roskell N, Sorensen SV. Dabigatran versus rivaroxaban for the prevention of stroke and systemic embolism in atrial fibrillation in Canada. Comparative efficacy and cost-effectiveness. Thromb Haemost. 2012 ;108(4):672-82.
Sorensen SV, Kansal AR, Connolly S, Peng S, Linnehan J, Bradley-Kennedy C, Plumb JM. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective.
ThrombHaemost2011;105:908–919.
Büller HR, Halperin JL, Bounameaux H, Prins M. Double-blind studies are not always optimum for evaluation of a novel therapy: the case of new anticoagulants. J Thromb Haemost. 2008;6(2):227-9.
O’Neil WM, Welner SA, Lip GY. Do open label blinded outcome studies of novel anticoagulants versus warfarin have equivalent validity to those carried out under double-blind conditions? Thromb Haemost. 2013 5;109(3):497-503.
De Caterina R, Husted S, Wallentin L, Andreotti F, Arnesen H, Bachmann F, Baigent C, Huber K, Jespersen J, Kristensen SD, Lip GY, Morais J, Rasmussen LH, Siegbahn A, Verheugt FW, Weitz JI; Coordinating Committee. New oral anticoagulants in atrial f ibrillation and acute coronary syndromes: ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease position paper. J Am Coll Cardio. 2012 ;59(16):1413-25.
Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, Zhang D. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009;37(1):74-81.
Zhang D, He K, Raghavan N, Wang L, Mitroka J, Maxwell BD, Knabb RM, Frost C, Schuster A, Hao F, Gu Z, Humphreys WG, Grossman SJ.
Comparative metabolism of 14C-labeled apixaban in mice, rats, rabbits, dogs, and humans. Drug MetabDispos.2009; 37:1738–48. Wang L, Zhang D, Raghavan N, Yao M, Ma L, Frost CE, Maxwell BD, Chen SY, He K, Goosen TC, Humphreys WG, Grossman SJ. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug MetabDispos.2010; 38:448–58.
Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, Reeves RA, LaCreta F. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013 Feb;75(2):476-87. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–51.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators.
Rivaroxaban versus warfarin in nonvalvular atrial fibrillation.
N Engl J Med. 2011;365:883–91.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committee and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17.
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG), Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S; Document Reviewers, Vardas P, Al-Attar N, Alfieri O, Angelini A, Blömstrom-Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R,
Heidbüchel H, Heldal M, Kristensen SD, Kolh P, Le Heuzey JY, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FW. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association.