O R I G I N A L C O M M U N I C A T I O N
Non-convulsive status epilepticus after ischemic stroke: a
hospital-based stroke cohort study
Vincenzo Belcastro
•Simone Vidale
•Gaetano Gorgone
•Laura Rosa Pisani
•Luigi Sironi
•Marco Arnaboldi
•Francesco Pisani
Received: 10 May 2014 / Revised: 19 July 2014 / Accepted: 11 August 2014 ÓSpringer-Verlag Berlin Heidelberg 2014
Abstract
To evaluate in the setting of a stroke unit ward
the usefulness of a prolonged (
[
6 h) video-EEG recording
(PVEEG) in identifying non-convulsive status epilepticus
(NCSE) in patients with an acute ischemic stroke.
Predic-tors of NCSE were also evaluated. Patients with an acute
ischemic stroke, referred to our unit, were included in this
prospective observational study. A PVEEG recording was
implemented after stroke in all patients during the first
week: (a) promptly in those exhibiting a clear or suspected
epileptic manifestation; (b) at any time during the routine
activity in the remaining patients. After the first week, a
standard EEG/PVEEG recording was hooked up only in
presence of an evident or suspected epileptic manifestation
or as control of a previous epileptic episode. NCSE was
identified in 32 of the 889 patients (3.6 %) included in the
study. It occurred early (within the first week) in 20/32
(62.5 %) patients and late in the remaining 12. Diagnosis
was made on the basis of a specific clinical suspect
(
n
=
19, 59.4 %) or without any suspect (
n
=
13, 40.6 %).
In a multivariate analysis, a significant association of
NCSE was observed with NIHSS score, infarct size and
large atherothrombotic etiology. NCSE is not a rare event
after an acute ischemic stroke and a delayed diagnosis
could worsen patient prognosis. Since NCSE can be
diffi-cult to be diagnosed only on clinical grounds,
implemen-tation of a prompt PVEEG should be kept available in a
stroke unit whenever a patient develop signs, although
subtle, consistent with NCSE.
Keywords
Non-convulsive status epilepticus
Post-stroke seizures
Prolonged video-EEG recording
Introduction
Stroke is the most common cause of symptomatic epilepsy
in older adults and status epilepticus (SE) can be a
pre-senting clinical feature of an acute stroke [
1
–
6
]. Values
concerning the incidence of post-stroke seizures and
development of epilepsy after stroke show a great
vari-ability in the literature. This varivari-ability is due to different
reasons: small sample sizes, different study designs,
het-erogeneous terminology, different periods of follow-up, no
specification on the ischemic/hemorrhagic nature of the
lesion. Incidence values of ‘‘early’’ (i.e., within the first
7 days) seizures range from 3 to 15 % [
1
,
7
–
11
], but values
[
30 % have been also reported in the literature [
5
]. ‘‘Late’’
seizures, i.e., those after the first week, have been reported
to occur in a percentage ranging from 3 to 9 % with a
gradual increase by increasing the time period after stroke,
the higher values being observed at 10 years [
1
,
4
,
5
].
Similarly, post-stroke epilepsy has been seen to develop in
6.4 % of patients as a mean value, ranging from 1.5 % at
3 months to 12.4 % at 10 years after stroke [
2
].
Concern-ing the incidence of SE, it has been estimated to range from
0.2 to 1.4 % [
9
–
14
] with very small differences observed
between ischemic and hemorrhagic stroke (0.2 vs. 0.3 %)
[
14
]. Additionally, most of these data regard convulsive
V. Belcastro (&)S. VidaleL. SironiM. Arnaboldi
Neurology Unit, S. Anna Hospital, Como, Italy e-mail: vincenzobelcastro@libero.it
G. Gorgone
Neurology Unit, Treviglio-Caravaggio Hospital, Treviglio, Italy L. R. Pisani
IRCCS Centro Neurolesi ‘‘Bonino-Pulejo’’, Messina, Italy F. Pisani
Department of Neurosciences, University of Messina, Messina, Italy
generalized SE (CGSE) and only few observations have
been specifically reported on non-convulsive SE (NCSE)
after stroke [6,
15]. In particular, in comatose patients with
NCSE, 20 % of patients had an ischemic stroke [15].
NCSE has been defined as a state of ongoing (or
non-recovery between) seizures without convulsions, usually
for more than 30 min [16]. It is a clinical entity very
dif-ficult to be detected in acute and critically ill patients,
including those with stroke, because of its subtle,
non-convulsive manifestations associated with transient
con-sciousness disturbances [16–20].
Using a hospital-based stroke cohort, the first aim of the
present study was to evaluate in the setting of a stroke unit
ward the usefulness of a prolonged, namely lasting for at
least 6 h, video-EEG recording (PVEEG) in identifying
episodes of NCSE after an acute ischemic stroke.
Predic-tors of NCSE were also evaluated.
Patients and methods
The Institutional Review Board of the S. Anna Hospital,
Como, Italy, approved the study and granted a waiver of
informed consent. The present investigation is a
prospec-tive observational study.
Patient population
Patients, referred to the Stroke Unit of S. Anna Hospital of
Como between January 2010 and September 2013, were
included in this study according to the following inclusion
criteria: (1) acute ischemic stroke defined by the
occur-rence of acute neurologic signs and symptoms of vascular
origin lasting 24 h or longer and documented through brain
CT/MRI; (2) no other concomitant causes potentially
responsible of acute seizures (for example: alcohol
with-drawal, psychotropic drugs, electrolyte disturbance,
previ-ous brain lesions). A direct interview was required, except
for aphasic or uncooperative patients, for whom the
inter-view was released by his relatives.
Patients with: (1) a previous history of seizures; (2)
recurrent stroke; (3) i.v. or p.o. treatment with antiepileptic
(AEDs) or sedative drugs, including barbiturates,
benzo-diazepines and propofol, prior to video-EEG execution; (4)
hemorrhagic transformation of the ischemic area were
excluded from analysis.
EEG recording and epilepsy characterization
Given that
[
50 % of epileptic seizures/SE occur in the
first days after stroke [4,
5,
7–10,
13], a PVEEG
recording, including ECG tracing, was implemented in all
patients admitted to our Stroke Unit within the first week
after stroke onset: it was hooked up as soon as an
epi-leptic activity was clear or suspected or at any time
during the routine activity in the remaining patients.
Additional conventional (i.e., of
*
30 min) EEG
record-ings, or even PVEEG when clinically judged more
appropriate, were made after the first week only in
pre-sence of clear or suspected epileptic seizures/SE or as
control of a previous epileptic episode. EEG was
recor-ded digitally using caps with 21 fixed gel electrodes
placed according to the International 10–20 system. EEGs
were
immediately
evaluated
by
board-certified
electroencephalographers.
Seizures were divided into convulsive and
non-con-vulsive. Convulsive seizures (CSz) were described as
‘‘tonic–clonic,’’ ‘‘clonic’’ or ‘‘tonic’’ (including also
syn-onyms like ‘‘jerking’’ or ‘‘twitching’’). SE was reported
as convulsive (CSE) for CSz lasting longer than 5 min or
if 2 or more fits occurred without a return to baseline in
between. Non-convulsive seizures (NCSz) were
consid-ered those characterized by subtle movements like slow
facial twitching and eye deviation. NCSE was defined as
30 min of continuous seizure activity without major
motor phenomena and according to the criteria of Young
et al. [16]. At least one of the following three primary
EEG criteria and at least one of the four secondary EEG
criteria had to be fulfilled. Primary criteria were (1)
repetitive focal or generalized spikes, sharp waves,
spike-and-wave or sharp-and-slow wave complexes at a
fre-quency of
[
3/s; (2) repetitive focal or generalized spikes,
sharp waves, spike-and-wave or sharp-and-slow wave
complexes at a frequency of
[
3/s and secondary
signifi-cant improvement in clinical state or baseline EEG after
administration of AEDs; or (3) sequential rhythmic waves
and secondary criteria (1), (2), (3) with or without
sig-nificant improvement in clinical state or baseline EEG
after administration of AEDs. Secondary EEG criteria
were the following discharge patterns with a duration of
[
10 s: (1) incrementing onset with increase in voltage
and/or increase or slowing of frequency; (2) decrementing
offset with decrease in voltage or frequency; (3)
post-discharge slowing or voltage attenuation; or (4)
signifi-cant improvement in clinical state or baseline EEG after
administration of AEDs.
Patients with EEG showing only bilateral periodic
dis-charges or stimulus-induced rhythmic, periodic or ictal
discharges (SIRPIDs) were excluded. The presence of
periodic lateralized epileptiform discharges (PLEDs), an
EEG pattern usually observed in patients with acute stroke,
was not the reason for exclusion.
screened to determine clinical correlates for NCSE and
episodes of electroencephalographic seizures. Level of
consciousness, evaluated before pharmacological treatment
and upon EEG initiation, was categorized on clinical
grounds
as
alert/somnolent/stuporous/comatose.
Each
patient was monitored through video-EEG recording for at
least 6 h following SE resolution, and a further
conven-tional EEG recording was made after 24 h.
Antiepileptic drug treatment
When a true diagnosis of SE was made, pharmacological
treatment was promptly started and its effect evaluated
through both EEG and clinical monitoring. As a standard
therapeutic approach, i.v. administered drugs were:
lor-azepam (0.1 mg/kg within 1–2 min), dilor-azepam (10 mg,
5 mg/min) or phenytoin (PHT) (18 mg/kg, 5 mg/kg/min).
According to the recent literature suggestions [21],
lev-etiracetam (LEV) 25 mg/kg over 15 min or lacosamide
(LCM) as initial bolus dose of 400 mg over 30 min was
used in patients with concomitant medical conditions in
whom the use of traditional AEDs was judged potentially
unsafe because of their adverse effects (hypotension, QT
prolongation,
arrhythmias,
respiratory
complications).
During i.v. treatment, patients were monitored through
video-EEG, ECG, heart rate and blood pressure
mea-surements. SE was considered fully controlled only after
both clinical and electrographic resolution.
Stroke characterization
Based on history and the results of diagnostic studies
including brain imaging, Doppler sonography,
echocar-diograms, and electrocarechocar-diograms, patients were classified
according to stroke syndrome, etiology and severity.
Clinical syndrome classification was assessed using the
Oxfordshire Community Stroke Project (OCSP) criteria
[22]. Stroke etiology was classified using the TOAST
(Trial of ORG 10172 in Acute Stroke Treatment) criteria
[23]. Stroke severity was assessed at admission using the
National Institutes of Health (NIH) stroke scale (NIHSS)
score [24]. Neuroimaging data (CT or MRI) were evaluated
according to the arterial territory circulation involved
(anterior or posterior) and lesion size (large, medium,
small, or lacunar). A large lesion was required to have a
diameter
[
3 cm on neuroimages; a small lesion a diameter
\
1 cm; a lacunar lesion was a CT hypodense area or T2
MRI hyperintense area
\
1 cm in maximum diameter
dee-ply located in the cerebral hemispheres or in the brainstem.
Finally, functional disability was obtained using the
mod-ified Rankin scale (mRS) [25] and mortality was assessed
during hospitalization.
Statistical analysis
Distribution of gender, vascular risk factors, functional
disability, OCSP and TOAST criteria were summarized as
frequencies and percentages and comparisons were made
using the Pearson Chi-square test or Fisher exact test, as
appropriate. Age and NIHSS score were indicated as
median and interquartile range, and were investigated using
the Mann–Whitney
U
test. Univariate and multivariate
logistic regression models were used to evaluate the
fol-lowing potential determinants of NCSE: stroke syndrome,
stroke etiology, size of infarction, NIHSS score, age,
gender, vascular risk factors. In the analysis, the size of
infarction was expressed as a three-level ordinal variable,
according to the classification above mentioned.
Multi-variate models were constructed using all variables resulted
significant in the univariate analysis. Measures of
associ-ation were odds ratios (ORs) with 95 % confidence
inter-vals. Each covariate was tested independently and with the
main interaction terms. Finally, the ROC analysis was
performed to check the goodness of fit of the implemented
logistic regression final model. Statistical significance was
chosen at the 5 % level. All statistics were implemented
using STATA version 12 (StataCorp, USA).
Results
Cohort characteristics
A total of 1,362 patients were admitted to our Stroke Unit
between January 2010 and September 2013: 1,034 (76 %)
have an ischemic stroke; 227 (16.6 %) have an
intra-cerebral hemorrhage (ICH); 101 (7.4 %) have a
subarach-noid hemorrhage. Of the 1,034 patients with ischemic
stroke, 72 (7 %) were excluded from the analysis because
of a hemorrhagic transformation of the ischemic area.
Moreover, 73 patients were also excluded from the analysis
because of a previous history of seizures, sedative
medi-cation prior to PVEEG recording, a history of recurrent
stroke or EEG recordings showing only bilateral periodic
discharges. The final cohort consisted of 889 patients. The
characteristics of the sample are summarized in Table
1.
Seizures and status epilepticus
SE (n
=
4) during the period of hospitalization. Two
patients exhibited for the first time tonic–clonic Sz during
the third week after stroke. Complex partial seizures,
although suspected in eight additional patients on the basis
of apparently not oriented motor manifestations and/or
level of consciousness oscillations, were not firmly
dem-onstrated through EEG recording. Similarly, electrical
seizures not associated to clinical manifestations were not
intercepted in our population of patients during
hospital-ization. In our cohort, NCSE was identified in 32/889
(3.6 %) patients: it occurred early in 20/32 (i.e., 62.5 %)
and late in the remaining 12 patients. Early NCSE
devel-oped as continuation of a CSE in five patients and during
the post-ictal phase of a tonic–clonic seizure in two
patients. Late NCSE was diagnosed in 12 patients: during
the second week in eight and during the third week in the
remaining four. Seven of these 12 patients had already had
early tonic–clonic seizures and two early focal motor SE;
the remaining three patients developed NCSE without any
previously identified epileptic activity. Impairment of
consciousness and agitation were the main manifestations
in 22/32 (68.8 %) patients and aphasia in the remaining 10
(31.2 %). Noteworthy, NCSE was diagnosed on the basis
of a specific clinical suspect in 19 patients (59.4 %) and
without any clinical suspect in the remaining 13 patients
(40.6 %). In 6 out of these 13 patients, it was intercepted in
the first week during PVEEG recording implemented as
part of the routine activity, and in the other seven patients
during PVEEG made to monitor the time course of a tonic–
clonic seizure (1 patient in the first week), a CSE (3
patients in the first week), or made just as a control in 3
patients (1 in the second week and 2 in the third week) who
had had previous CSzs.
EEG features
PVEEG was recorded for a mean duration of 9 h 22 min,
range 6 h 15 min–13 h 27 min.
PVEEG recording was implemented within the first
3 days in the majority of patients, namely 605, and
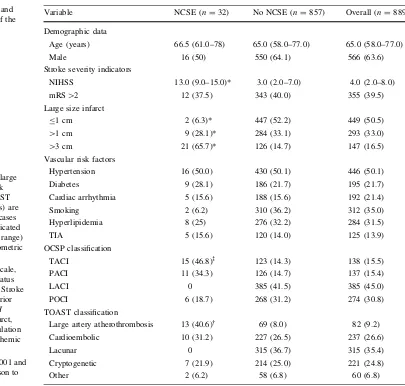
Table 1 Demographic andclinical characteristics of the screened population
Discrete variables (sex, large size infarct, vascular risk factors, OCSP and TOAST ischemic stroke subtypes) are indicated as number of cases (percentage). Age is indicated as median (interquartile range) and NIHSS score as geometric mean (standard error)
mRSModified Rankin Scale, NCSEnon-convulsive status epilepticus,NIHSSNIH Stroke Scale,PACIpartial anterior circulation infarct,POCI posterior circulation infarct, TACItotal anterior circulation infarct,TIAtransient ischemic attack
*p\0.0001, p=0.0001 and à
p=0.001 in comparison to No NCSE patients
Variable NCSE (n=32) No NCSE (n=857) Overall (n=889)
Demographic data
Age (years) 66.5 (61.0–78) 65.0 (58.0–77.0) 65.0 (58.0–77.0)
Male 16 (50) 550 (64.1) 566 (63.6)
Stroke severity indicators
NIHSS 13.0 (9.0–15.0)* 3.0 (2.0–7.0) 4.0 (2.0–8.0)
mRS[2 12 (37.5) 343 (40.0) 355 (39.5)
Large size infarct
B1 cm 2 (6.3)* 447 (52.2) 449 (50.5)
[1 cm 9 (28.1)* 284 (33.1) 293 (33.0)
[3 cm 21 (65.7)* 126 (14.7) 147 (16.5)
Vascular risk factors
Hypertension 16 (50.0) 430 (50.1) 446 (50.1)
Diabetes 9 (28.1) 186 (21.7) 195 (21.7)
Cardiac arrhythmia 5 (15.6) 188 (15.6) 192 (21.4)
Smoking 2 (6.2) 310 (36.2) 312 (35.0)
Hyperlipidemia 8 (25) 276 (32.2) 284 (31.5)
TIA 5 (15.6) 120 (14.0) 125 (13.9)
OCSP classification
TACI 15 (46.8)à 123 (14.3) 138 (15.5)
PACI 11 (34.3) 126 (14.7) 137 (15.4)
LACI 0 385 (41.5) 385 (45.0)
POCI 6 (18.7) 268 (31.2) 274 (30.8)
TOAST classification
Large artery atherothrombosis 13 (40.6) 69 (8.0) 82 (9.2)
Cardioembolic 10 (31.2) 227 (26.5) 237 (26.6)
Lacunar 0 315 (36.7) 315 (35.4)
Cryptogenetic 7 (21.9) 214 (25.0) 221 (24.8)
between the forth and the seventh day in the remaining 284
patients.
Ictal EEG features showed: (a) high-voltage, theta
rhythmic activity with intermingled spikes over the
temp-oro-occipital regions in eight patients; (b) high-voltage
theta activity intermingled with sharp waves over frontal
regions in seven patients; (c) bilateral, over frontal regions,
continuous spike- and slow-wave discharges in six patients.
Continuous focal sharp waves with change in amplitude,
frequency and spatial distribution were observed in 11
patients.
Treatment and epilepsy outcome
Initial treatment of NCSE was efficacious in 21 patients in
a time ranging from 30 to 60 min after i.v. administration:
lorazepam (
n
=
4), diazepam (
n
=
9), LCM (
n
=
5) and
PHT (
n
=
3). Eight patients did not show any response to
initial diazepam treatment and were treated with PHT
(
n
=
6) or LEV (
n
=
2) introduced as second-line
treat-ment. None of these patients relapse. In three patients,
NCSE was refractory to diazepam followed by PHT and
LCM and a transfer to the intensive care unit was needed.
A chronic treatment with AEDs was started in all SE
patients. The following drugs were given to patients with
NCSE: LEV in 24 patients, oxcarbazepine in six and
phenobarbital in the remaining two patients. The present
follow-up for 28 of these patients ranges from 2 to
43 months. In the control visits, usually planned every
6–8 weeks, a global neurological evaluation, seizure
fre-quency recordings through ad hoc calendars, therapy
adjustments and standard EEG execution have been
per-formed. Of these patients, 25 developed post-stroke
epi-lepsy with seizure frequency of 3–8 fits per month (18
patients) and
\
1 per month (7 patients). Three patients are
seizure free (present follow-up 5, 13 and 16 months).
Detailed data concerning long-term outcome of both
epi-lepsy and stroke in our population will be object of a
forthcoming article. No information has been obtained on
the remaining four patients after discharge from hospital
and until the preparation of the present manuscript.
Stroke features and outcome
Of the 32 patients with NCSE, a total anterior circulation
infarct (TACI) was diagnosed in 15, a partial anterior
cir-culation infarct (PACI) in 11 and a posterior circir-culation
infarct (POCI) in 6 patients. Three patients with early
NCSE received intravenous recombinant tissue
plasmino-gen activator (t-PA). None of the patients developing
NCSE died during the period of hospitalization, and all
were discharged to rehabilitation or to home within
14–30 days after stroke onset. No differences in outcome
of stroke between NCSE patients and the other patients
were detected through the NIHH score and other functional
assessment scales used (Barthel index, modified Rankin
Scale). As above mentioned, detailed data on the long-term
outcome of our population will be illustrated in a
forth-coming study.
Statistical significance
Univariate analysis showed significant differences between
patients with NCSE and those without NCSE with regard
to etiology, clinical presentation and size of ischemic area
(Table
1
). Regarding stroke etiology, large artery
athero-thrombosis showed a different distribution between NCSE
and non-NCSE groups (Table
1
). In the multiple logistic
regression models, we considered as predictors of the
NCSE those variables resulting significant from the
uni-variate analysis (Table
1
). A significant association of
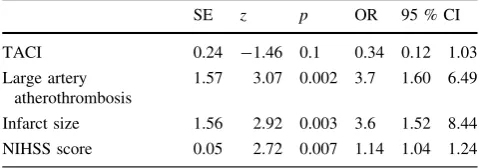
NCSE was observed with NIHSS score, infarct size and
large atherothrombotic etiology (Table
2
). No significant
differences between the expected and the observed
regression matrix were observed (area under ROC
curve
=
0.87).
Discussion
The results of the present study indicate that NCSE is not a
rare event after an acute ischemic stroke and occurs in a
percentage of 3–4 % of patients. In our cohort, significant
predictors of NCSE were a large size infarct, large artery
atherothrombosis and NIHSS score at admission.
With regard to infarct area, it has been proposed that the
ischemic penumbra of a stroke can contain electrically
irritable tissue that provides a focus for seizure activity
[
26
]. Enhanced release of glutamate, ionic imbalances
associated to breakdown of membrane phospholipids,
release of free fatty acids, reduced GABAergic function
Table 2 Independent predictors of NCSE in patients with ischemic stroke
SE z p OR 95 % CI
TACI 0.24 -1.46 0.1 0.34 0.12 1.03
Large artery atherothrombosis
1.57 3.07 0.002 3.7 1.60 6.49
Infarct size 1.56 2.92 0.003 3.6 1.52 8.44 NIHSS score 0.05 2.72 0.007 1.14 1.04 1.24 Variables not in equations: gender, age, hypertension, diabetes mel-litus, cardiac arrhythmia, smoking, hyperlipidemia, TIA, PACI, POCI, other TOAST classification aetiologies
with functional and structural impairment of GABAergic
interneurons have been described to occur in the ischemic
area after an acute insult [27,
28]. Cortical location and
large size infarct have been constantly found to be
asso-ciated with post-stroke seizures [2,
9,
11], a finding that has
emerged also from the present study. On the basis of the
cascade of synaptic and intracellular events shared by
seizure and vascular brain injuries, it can be hypothesized
that post-stroke NCSE could be triggered by acute cellular
biochemical disturbances occurring with acute ischemic
stroke.
In the present study, large artery atherothrombosis was
another significant predictor of NCSE.
Large artery atherothrombosis is more common in
cor-tical than lacunar infarcts, implying that an embolic
etiol-ogy would be more common in TACIs and PACIs than in
LACIs [29]. Notably, in our NCSE group, TACI was
diagnosed in 15/32 (46.8 %) and PACI in 11/32 (34.3 %)
patients while lacunar infarct was completely absent. These
observations reinforce the crucial role of cortical
involve-ment in seizure genesis.
A clinically relevant aspect is the possible impact of
NCSE on outcome of patients with ischemic stroke.
Pre-vious studies have shown that NCSE is a risk factor for the
development of refractory SE, poor outcome, and increased
mortality independently of the underlying pathology [16,
30]. In one of these studies, for example, the mortality rate
in an intensive care unit was high: more than half of the
patients with NCSE, 13/23 (57 %) died [16]. Specific data
on NCSE after an ischemic stroke are difficult to be
extrapolated from published studies because of a number of
reasons, including no reported distinction between CSE
and NCSE in the analysis, combination of both patients
with ischemic stroke and ICH, focus mainly on EEG
activity, generic report on patients with acute insults as
seen in medical intensive care units [8,
16–18,
20,
31]. In
our cohort, cases of death were not observed during
hos-pitalization and until the present follow-up. Additionally,
NCSE patients did not show, as compared to patients
without NCSE, differences concerning outcome and
com-plications until the present follow-up.
The NIHSS score was a significant predictor of NCSE.
Interpretation of NIHSS score is difficult since the
impair-ment of consciousness, which is a hallmark of NCSE, could
have increased itself NIHSS score at admission. In fact,
early NCSE occurred in 62.5 % of our 32 NCSE patients,
this value being in agreement with that reported in critically
ill patients, in whom seizures activity was observed in the
first 24 h after the insult in a large majority of patients
(88 %) [20]. Additionally, as above mentioned, SE has been
seen to be a possible presenting clinical feature of an acute
stroke [1–3]. On this basis, it can be hypothesized that a
percentage of our patients might have at admittance a
condition of NCSE which has not been detected. In patients
with ischemic stroke, values concerning the incidence of SE
range between 1 and 10 % [20] and NCSE has been
reported to occur in 30 % of patients developing post-stroke
SE [13]. In our cohort, 5 out of 20 patients developed early
NCSE as continuation of CSE and in two patients it
occurred in the post-ictal phase of a tonic–clonic seizure.
These values are very close to those reported in other
studies [17,
32] and emphasize the importance to have the
possibility in a stroke unit to implement a VEEG recording
longer than a conventional one, which lasts about
20–30 min on average. In a population of 110 patients, the
first seizures were detected in slightly more than half of the
cases within the first hour of recording [33].
The second relevant finding deriving from the present
study, concerning EEG, is that the diagnosis of NCSE has
been made during a routine EEG recording and without any
clinical suspect or any previous epileptic manifestation in a
considerable proportion of patients. This result, in line with
a large body of literature observations [18,
31,
32],
pro-vides further support in favor of the view that the incidence
of NCSE after an ischemic stroke is underappreciated and
that this pathology is more frequent than thought in the
past. As above mentioned, in fact, NCSE is a clinical entity
which goes easily unnoticed especially in elderly patients
and in patients with acute brain disorders in whom
impairment of consciousness is frequent as a consequence
of various pathologies [32–36]. In particular, in patients
with an acute ischemic stroke diagnosis of NCSE might be
made difficult because of various factors: (1) NCSE occurs
more frequently in the first days after stroke onset when
impairment of consciousness is a common expression of
stroke itself, (2) NCSE can occur just after CSz/CSE and
might be easily interpreted as a post-ictal state, (3)
mani-festations of NCSE can be so subtle as no to raise the
suspect of an epileptic activity.
Our study has different limitations: (1) the
single-insti-tution approach makes the study vulnerable to bias; (2) the
relatively small sample size of NCSE group does not
necessarily represent all the characteristics of patients
suffering from acute stroke and developing NCSE; (3)
cases of NCSE before and after PVEEG implementation
could have been easily missed. The latter limit, in
partic-ular, has been also emphasized by various authors [36,
37].
Conclusions
recording increases the possibility to diagnose NCSE. An
increased awareness that NCSE is a possible, not rare
event after an ischemic stroke and that it can most
fre-quently occur with subtle signs can contribute to speed a
diagnosis of NCSE. A quick diagnosis of this condition is
crucial to implement a prompt treatment and hence to
avoid possible clinical complications in patients with
ischemic stroke [
33
,
37
].
Acknowledgments We would like to acknowledge the contribution of Neurology EEG technologists of S. Anna Hospital in connecting these patients to the PVEEG at all odd hours.
Conflicts of interest None.
References
1. So EL, Annegers JF, Hauser WA, O’Brien PC, Whisnant JP (1996) Population-based study of seizure disorders after cerebral infarction. Neurology 46:350–355
2. Graham NS, Crichton S, Koutroumanidis M, Wolfe CD, Rudd AG (2013) Incidence and associations of post-stroke epilepsy: the prospective South London stroke register. Stroke 44:605–611 3. Ryvlin P, Montavont A, Nighoghossian N (2006) Optimizing
therapy of seizures in stroke patients. Neurology 67:S3–S9 4. Lossius MI, Ronning OM, Slapo GD, Mowinckel P, Gjerstad L
(2005) Post-stroke epilepsy: occurrence and predictors: a long-term prospective controlled study (Akershus Stroke Study). Epilepsia 46:1246–1251
5. Camilo O, Goldstein LB (2004) Seizures and epilepsy after ischemic stroke. Stroke 35:1769–1775
6. Afsar N, Kaya D, Aktan S, Aykut-Bingol C (2003) Stroke and status epilepticus: stroke type, type of status epilepticus, and prognosis. Seizure 12:23–27
7. Beghi E, D’Alessandro R, Beretta S et al (2011) Incidence and predictors of acute symptomatic seizures after stroke. Neurology 77:1785–1793
8. Pezzini A, Grassi M, Del Zotto E et al (2013) Complications of acute stroke and the occurrence of early seizures. Cerebrovasc Dis 35:444–450
9. Labovitz DL, Hauser WA, Sacco RL (2001) Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 57:200–206
10. Velioglu SK, O¨ zmenoglu M, Boz C, Alioglu Z (2001) Status epilepticus after stroke. Stroke 32:1169–1172
11. Procaccianti G, Zaniboni A, Rondelli F, Crisci M, Sacquengna T (2012) Seizures in acute stroke: incidence, risk factors and prognosis. Neuroepidemiology 39:45–50
12. Kilpatrick CJ, Davis SM, Tress B et al (1990) Epileptic seizures in acute stroke. Arch Neurol 47:157–160
13. Rumbach L, Sablot D, Berger E, Tatu L, Vuillier F, Moulin T (2000) Status epilepticus in stroke: report on a hospital-based stroke cohort. Neurology 54:350–354
14. Bateman BT, Claassen J, Willey JZ et al (2007) Convulsive status epilepticus after ischemic stroke and intracerebral haemorrhage: frequency, predictors, and impact on outcome in a large admin-istrative dataset. Neurocrit Care 7:187–193
15. Towne AR, Waterhouse EJ, Boggs JG et al (2000) Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology 25:340–345
16. Young GB, Jordan KG, Doig GS (1996) An assessment of non-convulsive seizures in the intensive care unit using continuous
EEG monitoring: an investigation of variables associated with mortality. Neurology 47:83–89
17. De Lorenzo RJ, Waterhouse EJ, Towne AR et al (1998) Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 39:833–840
18. Bottaro FJ, Martinez OA, Pardal MM, Bruetman JE, Reisin RC (2007) Nonconvulsive status epilepticus in the elderly: a case– control study. Epilepsia 48:966–972
19. Ney JP, van der Goes DN, Nuwer MR, Nelson L, Eccher MA (2013) Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005–2009. Neurology 81:2002–2008
20. Sutter R, Stevens RD, Kaplan PW (2013) Continuous electro-encephalographic monitoring in critically ill patients: indications, limitations, and strategies. Crit Care Med 41:1124–1132 21. Shorvon S (2011) The treatment of status epilepticus. Curr Opin
Neurol 24:165–170
22. Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C (1994) Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke 25:333–337 23. Goldstein LB, Jones MR, Matchar D et al (2001) Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. Stroke 32:1091–1098
24. Brott T, Adams HP Jr, Olinger CP et al (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20:864–870
25. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19:604–607
26. Heiss WD, Huber M, Fink GR et al (1992) Progressive derangement of periinfarct viable tissue in ischemic stroke. J Cereb Blood Flow Metab 12:193–203
27. Luhmann HJ, Mudrick-Donnon LA, Mittmann T, Heinemann U (1995) Ischemia-induced long-term hyperexcitability in rat neo-cortex. Eur J Neurosci 7:180–191
28. Belcastro V, Pierguidi L, Tambasco N (2011) Levetiracetam in brain ischemia: clinical implications in neuroprotection and pre-vention of post-stroke epilepsy. Brain Dev 33:289–293 29. Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP
(2000) How well does the Oxfordshire community stroke project classification predict the site and size of the infarct on brain imaging? J Neurol Neurosurg Psychiatry 68:558–562
30. Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF (2002) Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 59:205–210 31. Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ
(2004) Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 62:1743–1748 32. Meierkord H, Holtkamp M (2007) Non-convulsive status
epi-lepticus in adults: clinical forms and treatment. Lancet Neurol 6:329–339
33. Claassen J, Lokin JK, Fitzsimmons BF, Mendelsohn FA, Mayer SA (2002) Predictors of functional disability and mortality after status epilepticus. Neurology 58:139–142
34. Jordan KG (2004) Emergency EEG and continuous EEG moni-toring in acute ischemic stroke. J Clin Neurophysiol 21:341–352 35. Carrera E, Michel P, Despland PA et al (2006) Continuous assessment of electrical epileptic activity in acute stroke. Neu-rology 67:99–104
36. Sutter R, Fuhr P, Grize L, Marsch S, Ru¨egg S (2011) Continuous video-EEG monitoring increases detection rate of nonconvulsive status epilepticus in the ICU. Epilepsia 52:453–457