This ar t icle was dow nloaded by: [ Canakkale Onsekiz Mar t Univer sit esi] On: 09 Decem ber 2013, At : 09: 09
Publisher : Taylor & Francis
I nfor m a Lt d Regist er ed in England and Wales Regist er ed Num ber : 1072954 Regist er ed office: Mor t im er House, 37- 41 Mor t im er St r eet , London W1T 3JH, UK
Journal of Applied Aquaculture
Publ icat ion det ail s, incl uding inst ruct ions f or aut hors and subscript ion inf ormat ion:ht t p: / / www. t andf onl ine. com/ l oi/ wj aa20
Chickweed (Stellaria media) Leaf
Meal as a Feed Ingredient for Tilapia
(Oreochromis mossambicus)
Sevdan Yıl maz a & Sebahat t in Ergün a
a
Depart ment of Aquacul t ure, Facul t y of Marine Sciences and Technol ogy , Çanakkal e Onsekiz Mart Universit y , Çanakkal e , Turkey Publ ished onl ine: 09 Dec 2013.
To cite this article: Sevdan Yıl maz & Sebahat t in Ergün (2013) Chickweed (St el l aria media) Leaf Meal
as a Feed Ingredient f or Til apia (Oreochromis mossambicus), Journal of Appl ied Aquacul t ure, 25: 4, 329-336
To link to this article: ht t p: / / dx. doi. org/ 10. 1080/ 10454438. 2013. 851531
PLEASE SCROLL DOWN FOR ARTI CLE
Taylor & Francis m akes ever y effor t t o ensur e t he accuracy of all t he infor m at ion ( t he “ Cont ent ” ) cont ained in t he publicat ions on our plat for m . How ever, Taylor & Francis, our agent s, and our licensor s m ake no r epr esent at ions or war rant ies w hat soever as t o t he accuracy, com plet eness, or suit abilit y for any pur pose of t he Cont ent . Any opinions and view s expr essed in t his publicat ion ar e t he opinions and view s of t he aut hor s, and ar e not t he view s of or endor sed by Taylor & Francis. The accuracy of t he Cont ent should not be r elied upon and should be independent ly ver ified w it h pr im ar y sour ces of infor m at ion. Taylor and Francis shall not be liable for any losses, act ions, claim s, pr oceedings, dem ands, cost s, expenses, dam ages, and ot her liabilit ies w hat soever or how soever caused ar ising dir ect ly or indir ect ly in connect ion w it h, in r elat ion t o or ar ising out of t he use of t he Cont ent .
Copyright Taylor & Francis Group, LLC ISSN: 1045-4438 print/1545-0805 online DOI: 10.1080/10454438.2013.851531
Chickweed (
Stellaria media
) Leaf Meal
as a Feed Ingredient for Tilapia
(
Oreochromis mossambicus
)
SEVDAN YILMAZ and SEBAHATTIN ERGÜN
Department of Aquaculture, Faculty of Marine Sciences and Technology, Çanakkale Onsekiz Mart University, Çanakkale, Turkey
A study was undertaken to determine the effect of the inclusion of chickweed (Stellaria media) leaf meal (CLM) on growth per-formance, feed utilization, nutrition retention, and whole body composition of tilapia,Oreochromis mossambicus. Five isonitroge-nous (35% crude protein) and isoenergetic diets were formulated to contain chickweed leaf meal at levels of 0%, 2.5%, 5%, 10%, and 20%. A 45-day feeding trial was carried out on triplicate groups of 225 mixed sex fish in 140-L fiberglass tanks. There were no particular differences in protein retention, fat retention, energy retention, whole body dry matter, and ash levels of fish fed experi-mental diets (P>0.05). However, 20% chickweed supplementation significantly decreased final fish weight, weight gain, feed conver-sion ratio, whole body protein, and fat levels (P<0.05), probably as a result of oxalic acid toxicity. Inclusion of CLM can be used at 10% in tilapia diets without significant reduction in growth.
KEYWORDS Tilapia, chickweed leaf meal, growth, feed utilization, whole body composition
We would like to thanks Necla TÜRK and AGROMEY for amino acid analysis. The authors also thank Mr. Yahya Kaya, Mr. Eray Alabak, and Mr. Mert Yücesan for their assistance while conducting of the experiment.
Address correspondence to Sevdan Yılmaz, Department of Aquaculture, Faculty of Marine Sciences and Technology, Çanakkale Onsekiz Mart University, Çanakkale 17100, Turkey. E-mail: sevdanyilmaz@comu.edu.tr
329
330 S. Yılmaz and S. Ergün
INTRODUCTION
Oreochromis sp. feed on low trophic levels (El-Sayed 2006; Nguyen and Davis 2009). Tilapias have long coiled intestines, and it is known that O. mossambicus is a functional omnivore (Doupé and Knott 2010), indi-cating that plant protein is useful in its diet. Previous studies showed that vegetable protein sources such as jack bean (Martinez-Palacios et al. 1988), alfalfa (Olvera-Novoa et al. 1990), rapeseed (Davies et al. 1990), and biogas-plant effluent (Gopal et al. 1996) are readily accepted by O. mossambicus. Plant sources are cheaper than fishmeal but generally include anti-nutritional contents.
Chickweed (Caryophyllaceae, Stellaria media) is a cosmopolitan weed (Allen and Hatfield 2004). Many animals eat their shoots and seeds, both domesticated and wild, with no reported deleterious effects (Sobey 1981), but high oxalic acid content may be a problem (Guil et al. 1996, 1997). Chickweed is readily eaten and digested by chaffinches. Derrick et al. (1993) reported that sheep readily digested chickweed. It is used as a leaf veg-etable, often raw in salads by people (Slavokhotova et al. 2011). Chickweed is reported to have probiotic effects including: inflammation, anti-viral, anti-itch, anti-pyretic, anti-rheumatic, demulcent, depurative, digestive, diuretic, emmenagogue, emollient, expectorant, lactagogue, and vulnerary (Zhu 1998; Duke et al. 2002).
One of the main problems in utilization of plant protein sources is defi-ciency of certain essential amino acids (Houlihan et al. 2001). Chickweed has a total of 16 free amino acids including threonine, valine, methionine, isoleucine, leucine, phenylalanine, lysine, histidine, and arginine (Shan et al. 2010), which are essential for fish health and growth. Chickweed appears to be a useable fish food, but its inclusion level in the diet ofO. mossambicus needs to be evaluated.
MATERIALS AND METHODS
Healthy cultured Oreochromis mossambicus (weight average ∼20 g) were produced at Çanakkale Onsekiz Mart University, Faculty of Fisheries. The physical qualities of fresh water during the experiment were as follows: tem-perature, 28.7 ± 0.2◦C; pH, 7.3 ± 0.5; dissolved oxygen, 7.10 ± 0.6 mg
/L; conductivity, 520 ± 15 uS; total ammonia, 0.09 ± 0.01 mg/L; nitrite, 0.03 ± 0.01 mg/L; and nitrate, 1.1 ± 0.1 mg/L. Temperature, dissolved oxygen, and conductivity were measured once a day using a YSI-85 digital temperature/oxygen/conductivity/hand-held meter, while pH, total ammo-nia, nitrite, and nitrate levels were monitored twice per week using a HANNA C 200 (HI 83200) spectrophotometer.
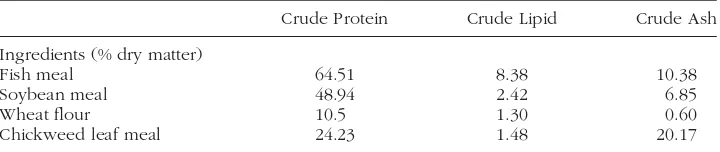
TABLE 1 Chemical composition of dietary ingredients.
Crude Protein Crude Lipid Crude Ash
Ingredients (% dry matter)
Fish meal 64.51 8.38 10.38
Soybean meal 48.94 2.42 6.85
Wheat flour 10.5 1.30 0.60
Chickweed leaf meal 24.23 1.48 20.17
Two hundred twenty-five (225) mixed sex fish were kept in indoor 140-L fiberglass tanks (15 fish/tank) with filtered, recirculating, well-aerated tap water. About 5% of the water was exchanged daily. The experiment lasted 45 days. During the experiment, fish were fed ad libidum with the experimental diets three times a day.
Chickweed (Stellaria media) leaf was collected around Çanakkale, Turkey, dried (45◦C, in a drying cabinet), and crushed for use in the
experi-ment. Chickweed leaf meal (CLM) was added to a laboratory-prepared feed at a rate of 2.5%, 5%, 10%, and 20%. The control group was fed diets with-out supplementation (0%). Chemical composition of dietary ingredients are presented in Table 1. The ingredients (Table 2) were mixed in a blender and pressed through a 2-mm die in a pelleting machine, and the pellets were dried in a drying cabinet (40◦
C) until moisture dropped to around 10%. It was then stored in bags and frozen in the deep freezer at−20◦C until used.
Growth performance, feed utilization, and nutrient retention were calculated according to the following formulae:
● Weight gain=100 (final fish weight - initial fish weight)/initial fish weight; ● SGR (specific growth rate)= 100 (ln final fish weight (g)) - (ln initial fish
weight (g))/experimental days;
● FCR (feed conversion ratio)=feed intake (g)/weight gain (g); ● PER (protein efficiency ratio)=weight gain (g)/protein intake (g);
● PR (protein retention) = [(final protein concentrations× final fish weight (g))-(initial protein concentrations × initial fish weight (g))] / [(protein content of the diet × FCR × (final fish weight (g)-initial fish weight (g)))]×100;
● FR (fat retention)=[(final fat concentrations×final fish weight (g))-(initial fat concentrations × initial fish weight (g))] / [(fat content of the diet × FCR×(final fish weight (g)-initial fish weight (g)))]× 100;
● ER (energy retention) = [(final energy concentrations × final fish weight (g))-(initial energy concentrations × initial fish weight (g))] / [(energy content of the diet × FCR × (final fish weight (g)-initial fish weight (g)))]×100.
Proximate analyses of the diets were performed using standard methods (AOAC 1998). Dry matter was analyzed by drying at 105◦C in an oven to
332 S. Yılmaz and S. Ergün
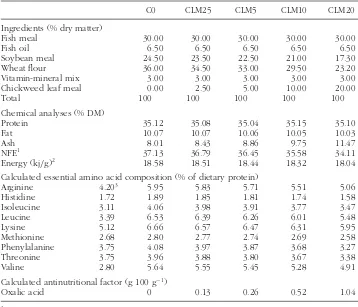
TABLE 2 Percentage and proximate composition of the experimental diets containing supplement of different chickweed leaf meal rate.
C0 CLM25 CLM5 CLM10 CLM20
Ingredients (% dry matter)
Fish meal 30.00 30.00 30.00 30.00 30.00
Fish oil 6.50 6.50 6.50 6.50 6.50
Soybean meal 24.50 23.50 22.50 21.00 17.30
Wheat flour 36.00 34.50 33.00 29.50 23.20
Vitamin-mineral mix 3.00 3.00 3.00 3.00 3.00
Chickweed leaf meal 0.00 2.50 5.00 10.00 20.00
Total 100 100 100 100 100
Chemical analyses (% DM)
Protein 35.12 35.08 35.04 35.15 35.10
Fat 10.07 10.07 10.06 10.05 10.03
Ash 8.01 8.43 8.86 9.75 11.47
NFE1 37.13 36.79 36.45 35.58 34.11
Energy (kj/g)2 18.58 18.51 18.44 18.32 18.04
Calculated essential amino acid composition (% of dietary protein)
Arginine 4.203 5.95 5.83 5.71 5.51 5.06
Histidine 1.72 1.89 1.85 1.81 1.74 1.58
Isoleucine 3.11 4.06 3.98 3.91 3.77 3.47
Leucine 3.39 6.53 6.39 6.26 6.01 5.48
Lysine 5.12 6.66 6.57 6.47 6.31 5.95
Methionine 2.68 2.80 2.77 2.74 2.69 2.58
Phenylalanine 3.75 4.08 3.97 3.87 3.68 3.27
Threonine 3.75 3.96 3.88 3.80 3.67 3.38
Valine 2.80 5.64 5.55 5.45 5.28 4.91
Calculated antinutritional factor (g 100 g−1)
Oxalic acid 0 0.13 0.26 0.52 1.04
1Nitrogen-free extracts (NFE)=matter - (crude lipid+crude ash+crude protein). 2Energy was calculated according to 23.6 kJ/g protein, 39.5 kJ/g lipid, and 17.0 kJ/g NFE. 3EAA requirements for tilapia (Santiago and Lovell 1988).
a constant weight, crude protein by the Kjeldahl method, and crude ash by incineration at 525◦C in a muffle furnace for 12 h. Crude fat was
ana-lyzed by methanol/chloroform extraction (Folch et al. 1957). Oxalic acid was determined via conductometric titration using NH3. Amino acid content was provided by the local fish feed manufacturer (AGROMEY, Turkey).
Statistical significance determined by one-way analysis of variance (ANOVA) followed by a TUKEY multi comparison test with the SPSS 17.0 packaged software (Logan, 2010). Statistical significance was established at P<0.05.
RESULTS
The fish equally accepted the five diets, and there was no mortality or dis-ease in any treatment. The tilapia fed the CLM25 and CLM5 chickweed diets
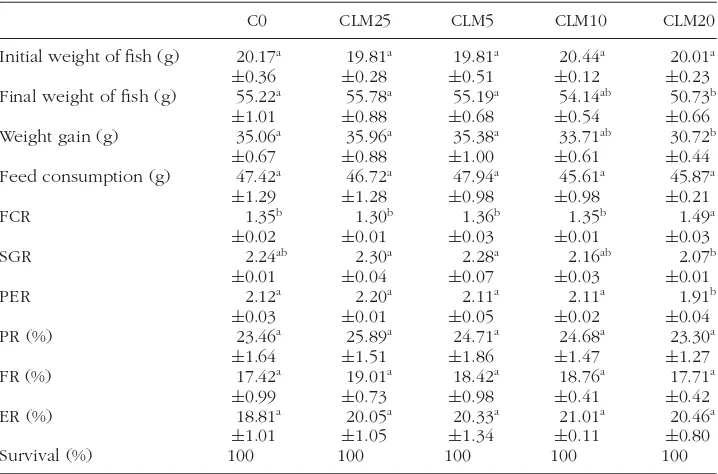
TABLE 3 Fish performance, feed utilization, and nutrient retention for tilapia fed diets containing different levels of chickweed leaf meal supplements for 45 days.
C0 CLM25 CLM5 CLM10 CLM20
Initial weight of fish (g) 20.17a 19.81a 19.81a 20.44a 20.01a
±0.36 ±0.28 ±0.51 ±0.12 ±0.23
Final weight of fish (g) 55.22a 55.78a 55.19a 54.14ab 50.73b
±1.01 ±0.88 ±0.68 ±0.54 ±0.66
Weight gain (g) 35.06a 35.96a 35.38a 33.71ab 30.72b
±0.67 ±0.88 ±1.00 ±0.61 ±0.44
Feed consumption (g) 47.42a 46.72a 47.94a 45.61a 45.87a
±1.29 ±1.28 ±0.98 ±0.98 ±0.21
Survival (%) 100 100 100 100 100
Values are mean± SEM (n= 3). Different letters in same line indicate significant differences within groups (P<0.05).
had significantly higher specific growth rates (SGR) compared with the 20% chickweed diet, while the C0 and CLM20 diets were not significantly differ-ent. However, 20% chickweed supplementation significantly decreased final fish weight, weight gain, and feed conversion ratio (FCR) when compared to control group (Table 3).
There were no particular differences (P > 0.05) in protein retention (PR), fat retention (FR), and energy retention (ER). Nevertheless, PR and FR slightly decreased with increase in plant protein diet.
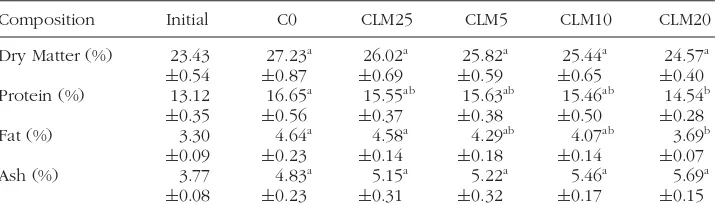
Whole-body proximate composition of fish at the beginning and end of the experiment are presented in Table 4. There was no significant difference in dry matter and ash between the experimental group and the control group. However, protein and fat decreased with an increase in inclusion level of chickweed leaf meal and was significantly reduced in CLM20 diet (P<0.05).
DISCUSSION
Growth performance decreased with increasing levels of chickweed leaf meal but did not significantly change up to an inclusion rate of 10%. Chickweed depressed growth performance at an inclusion rate of 20% of
334 S. Yılmaz and S. Ergün
TABLE 4 Whole-body proximate composition of tilapia fish fed diets with different levels of chickweed leaf meal for 45 days.
Composition Initial C0 CLM25 CLM5 CLM10 CLM20
Dry Matter (%) 23.43 27.23a 26.02a 25.82a 25.44a 24.57a
±0.54 ±0.87 ±0.69 ±0.59 ±0.65 ±0.40
Protein (%) 13.12 16.65a 15.55ab 15.63ab 15.46ab 14.54b
±0.35 ±0.56 ±0.37 ±0.38 ±0.50 ±0.28
Fat (%) 3.30 4.64a 4.58a 4.29ab 4.07ab 3.69b
±0.09 ±0.23 ±0.14 ±0.18 ±0.14 ±0.07
Ash (%) 3.77 4.83a 5.15a 5.22a 5.46a 5.69a
±0.08 ±0.23 ±0.31 ±0.32 ±0.17 ±0.15
Values are mean± SEM (n= 9). Different letters in same line indicate significant differences within groups (P<0.05).
the diet, probably attributable to high (3–5.4 mg/100g DM) oxalic acid lev-els (Table 2) (Guil et al. 1996, 1997). Oxalic acid binds calcium and forms calcium oxalate, adversely affecting absorption and utilization of calcium in the animal body (Guil et al. 1996; Akande et al. 2010). In addition, oxalates react with proteins to form complexes that have an inhibitory effect in peptic digestion (Akande et al. 2010).
In this study, PR and FR slightly decreased with an increase in chickweed diets, indicating that the final whole body protein and fat the experimental fish was lower for the diets with CLM, presumably as oxalic acid increased with increasing CLM in the diet, which reduces diet digestibil-ity and growth performance. In contrast, sesame seed meal also has high oxalic acid (3.9 mg/100 g) but does not significantly affect growth per-formance in tilapia (up to 16%) and African catfish (up to 25%–30%) (Fagbenro et al. 2010; Guo et al. 2011; Jimoh and Aroyehun 2011). These differences might be explained by the different amino acid compositions between the two plants. Methionine, phenylalanine, and threonine were deficient EAAs in the CLM20 diet. High oxalic acid concentration coupled with amino acid deficiency most probably explains the poor performance of the CLM20 diet. Research has reported similar trends with plant pro-teins such as spirulina (Olvera-Novoa et al. 1998), sunflower meal (Fagbenro et al. 2010), and groundnut (Agbo et al. 2011) in tilapia without amino acid supplementation.
REFERENCES
Agbo, N. W., D. Adjei-Boateng, and K. Jauncey. 2011. The potential of ground-nut (Arachis hypogaea L.) by-products as alternative protein sources in the diet of Nile Tilapia (Oreochromis niloticus). Journal of Applied Aquaculture 23:367–378.
Akande, K. E., U. D. Doma, H. O. Agu, and H. M. Adamu. 2010. Major antinutrients found in plant protein sources: their effect on nutrition. Pakistan Journal of Nutrition9:827–832.
Allen, D. E., and G. Hatfield. 2004.Stellaria media(Linnaeus) Villars. InMedicinal plants in folk tradition an ethnobotany of Britain and Ireland, edited by D. E. Allen and G. Hatfield, 91–92. Cambridge, MA: Timber Press.
Association of Official Analytical Chemists (AOAC). 1998.Official methods of analysis of AOAC International. Gaithersburg, MD: AOAC.
Davies, S. J., S. Mcconnell, and R. I. Bateson. 1990. Potential of rapeseed meal as an alternative protein source in complete diets for tilapia (Oreochromis mossambicusPeters).Aquaculture87:145–154.
Derrick, R. W., G. Moseley, and D. Wilman. 1993. Intake by sheep, and digestibility of chickweed, dandelion, dock, ribwort, and spurrey, compared with perennial ryegrass.Journal of Agricultural Science120:51– 61.
Doupé, R. G., and M. J. Knott. 2010. Rapid digestion of fish prey by the highly invasive ‘detritivore’Oreochromis mossambicus. Journal of Fish Biology 76:1,019–1,024.
Duke, J. A., M. J. B. Godwin, J. Cellier, and P. A. K. Duke. 2002. Chickweed (Stellaria media (L.) Vill.). In Handbook of Medicinal Herbs, edited by J. A. Duke, M. J. B. Godwin, J. Cellier and P. A. K. Duke, 183–184. New York, NY: CRC Press. El-Sayed, A.-F. M. 2006.Tilapia culture. Wallingford, UK: CAB International.
Fagbenro, O. A., E. O. Adeparusi, and W. A. Jimoh. 2010. Nutritional evaluation of sunflower and sesame seed meal in Clarias gariepinus: an assessment by growth performance and nutrient utilization. African Journal of Agricultural Research5:3,096–3,101.
Folch, J., M. Lees, and G. H. A. Sladane-Stanley. 1957. Simple method for the iso-lation and purification of total lipids from animal tissue.Journal of Biological Chemistry226:497–509.
Gopal, V., S. Prabakaran, and P. R. Balasubramanian. 1996. Effect of a biogas-plant effluentbased pelleted diet on the growth ofOreochromis mossambicus fingerlings.Bioresource Technology 58:315–317.
Guil, J. L., M. E. Torija, J. J. Giménez, I. Rodríguez-García, and A. Giménez. 1996. Oxalic acid and calcium determination in wild edible plants.Journal of Agricultural and Food Chemistry44:1,821–1,823.
Guil, J. L., I. Rodríguez-García, and E. Torija. 1997. Nutritional and toxic factors in selected wild edible plants.Plant Foods for Human Nutrition51:99–107. Guo, Y.-X., X.-H. Dong, B.-P. Tan, S.-Y. Chi, Q.-H. Yang, G. Chen, and L. Zhang.
2011. Partial replacement of soybean meal by sesame meal in diets of juvenile Nile tilapia,Oreochromis niloticusL.Aquaculture Research42:1,298–1,307. Jimoh, W. A., and H. T. Aroyehun. 2011. Evaluation of cooked and mechanically
defatted sesame (Sesamum indicum) seed meal as a replacer for soybean meal in the diet of African Catfish (Clarias gariepinus). Turkish Journal of Fisheries and Aquatic Sciences11:185–190.
Logan, M. 2010. Worked examples of real biological data sets. InBiostatistical design and analysis using R: A practical guide, edited by M. Logan, 265–268. London: Wiley-Blackwell.
336 S. Yılmaz and S. Ergün
Houlihan, D., T. Boujard, and M. Jobling. 2001. Feed types, manufacture and ingredi-ents. InFood Intake in Fish, edited by M. Jobling, E. Gomes, and J. Dias, 25–48. London, UK: Blackwell Science.
Martinez-Palacios, C. A., R. G. Cruz, M. A. Olvera, and C. Chavez-Martinez. 1988. The use of jack bean (Canavalia ensiformis Leguminosae) meal as a partial substi-tute for fishmeal in diets for tilapia (Oreochromis mossambicus). Aquaculture 68:165–175.
Nguyen, T., and D. A. Davis. 2009. Evaluation of alternative protein sources to replace fish meal in practical diets for juvenile Tilapia,Oreochromisspp.Journal of the World Aquaculture Society40:113–121.
Olvera-Novoa, M. A., G. Silvia-Campos, G. Mirna Sabido, and C. A. Martínez-Palacios. 1990. The use of alfalfa leaf protein concentrates as a protein source in diets for Tilapia(Oreochromis mossambicus). Aquaculture90:291–302.
Olvera-Novoa, M. A., L. J. Dominguez-Cen, L. Olivera-Castillo, and C. A. Martínez-Palacios. 1998. Effect of the use of the microalga Spirulina maxima as fish meal replacement in diets for tilapia, Oreochromis mossambicus (Peters), fry. Aquaculture Research29:709–715.
Santiago, C. B., and R. T. Lovell. 1988. Amino acid requirements for growth of Nile Tilapia.Journal of Nutrition118:1,540–1,546.
Shan, Y., J. Zhou, H. Guang Zhao, X. Feng, Y. Dong, and B. Xia. 2010. Amino-acid and mineral composition ofStellaria media. Chemistry of Natural Compounds 46:667–668.
Slavokhotova, A. A., T. I. Odintsova, E. A. Rogozhin, A. K. Musolyamov, Y. A. Andreev, E. V. Grishin, and T. A. Egorov, 2011. Isolation, molecular cloning and antimicrobial activity of novel defensins from common Chickweed (Stellaria mediaL.) seeds.Biochimie93:450–456.
Sobey, D. G. 1981. Biological flora of the british isles. Stellaria media (L.) Vill. Journal of Ecology69:311–335.
Zhu, Y. P. 1998.Chinese materia medica: Chemistry, pharmacology and applications. Amsterdam: Harwood Academic Publishers.