ISSN: 1978-3019 DOI: 10.4308/hjb.20.4.151
_________________
∗Corresponding author. Phone: +62-21-7513906/7659511,
Fax: +62-21-7657950, E-mail: [email protected]
Automated Detection of Binucleated Cell and Micronuclei using
CellProfiler 2.0 Software
DWI RAMADHANI∗, SOFIATI PURNAMI
Center for Technology of Radiation Safety and Metrology,National Nuclear Energy Agency of Indonesia, Jalan Lebak Bulus Raya No. 49, Kotak Pos 7043 JKSKL, Jakarta 12070, Indonesia
Received January 1, 2013/Accepted June 24, 2013
Micronucleus assay in human peripheral lymphocytes usually used to assess chromosomal damage. Manual
scoring of micronuclei can be time consuming and large numbers of binucleated cells have to be analyzed to obtain
statistically relevant data. Automation of the micronuclei analysis using image processing analysis software can provide a faster and more reliable analysis of micronucleus assay. Here the used of CellProfiler an open access cell image analysis software for automatic detection of binucleated cells and micronuclei were reported. We aimed to know whether there was a significant difference in the number of binucleated cells and micronuclei that obtained by manual and CellProfiler counting. Wilcoxon Rank test was used for statistical analysis to test H0 hypothesis that
there was no significant difference in the number of binucleated cells and micronuclei that obtained by manual and CellProfiler counting. We analyzed 135 images for both manual and CellProfiler counting. Our results showed that
there was no significant difference between manual and CellProfiler counting for binucleated cells (P = 0.851) and
for micronuclei (P = 0.917). In conclusion, the binucleated cells and micronuclei counting using CellProfiler were comparable but not better than manual counting.
Keywords: binucleated cells, CellProfiler, micronuclei, open source, software
___________________________________________________________________________
INTRODUCTION
Micronucleus assay in human peripheral lymphocytes usually used to assess chromosomal damage that caused by exposure to different environmental, occupational or lifestyle factors and for in vitro genotoxicity testing (Patino-Garcia et al. 2006). Micronuclei (MN) are derived from chromosome fragments that arised from asymmetrical structural aberrations or represent whole chromosomes that are not incorporated into the nucleus during cell division. Acentric fragments are most often seen after irradiation of cells, whereas entire chromosomes are more frequent in spontaneously occurring MN or after induction by spindle poisons without any clastogenic treatment, as was demonstrated by anti-kinetochore antibody staining (Fenech & Morley 1989; Tucker & Eastmond 1990).
The peripheral blood lymphocyte micronucleus assay based on MN expression in short term culture
of lymphocytes was first described by Countryman
and Heddle (1976). However, in this original method no attempt was made to determine whether the cells that have been scored had actually completed nuclear division in vitro which made the assay unreliable
because chromosome damage in cells can only be expressed as micronuclei if cells divide. A more reliable approach was eventually developed based on the use of the cytokinesis inhibitor, cytochalasin-B. Fenech and Morley (1985) demonstrated that the cells that had completed one nuclear division could be accumulated using cytochalasin-B. These cells recognized as a binucleated cells (BNC). Micronuclei
could then be specifically and efficiently scored in
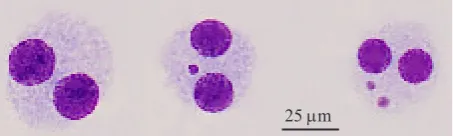
these BNC while excluding nondividing mononuclear cells that were unable to express MN in vitro (Figure 1). Consequently, the results obtained with the MN assay are not confounded by interindividual and interexperimental variation in the frequency of dividing cells, which has been shown to have a profound effect on the observed MN frequency (IAEA 2011).
Scoring of MN is usually performed in peripheral blood lymphocytes (PBL) but MN can also be
25 µm
scored relatively easy in other cell types relevant for
human biomonitoring, such as fibroblasts, exfoliated
epithelial cells (from buccal, nasal mucosa, or bladder cells in urine) and in erythrocytes. The International Collaborative Project on Micronucleus Frequency in Human Populations (the HUMN project, http:// www.humn.org) provided a detailed description of the scoring criteria for MN in PBL and used combined databases to assess intra- and interlaboratory variation in MN scoring, background MN frequencies and the influence of culture conditions, age, gender, and smoking on MN frequencies (Fenech
et al. 1999; Fenech et al. 2003; Fenech 2007). Binucleated cells and micronucleus which can be scored for micronucleus assay should have several characteristics as described elsewhere (Fenech 2007).
Manual scoring of MN can be very time consuming and large numbers of BNC have to be analyzed to obtain statistically relevant data (Decodier et al. 2009). For example in biological dosimetry in which the radiation dose assessment was done by observation of the biological changes in cells, organs or body given by ionizing irradiation, it was recommended that 1000 BNC should be scored (IAEA 2011). Therefore automation of the micronucleus assay is required to provide a faster and reliable analysis of MN frequencies with
minimization of subjective identification of MN. To
obtain reliable results, an automated system for MN
scoring should fulfill the same requirements as those
for manual scoring (the detection of MN should be based on the scoring criteria described by the HUMN project) (Decodier et al. 2009).
Several studies have been conducted to develop commercial computer softwares that allowed the application of advanced image analysis system for use in the cytokinesis-block MN test in human lymphocytes (Varga et al. 2004; Decodier et al.
2009). Metafer MNScore (MetaSystems, GmbH Altlussheim, Germany) was the first system developed and was followed by PathFinder_ Cellscan_ (IMSTAR, Paris, France) described by Decordier et al. (2009). The Metafer system was introduced by MetaSystems in 2004 (Varga et al.
2004). Nevertheless there are no studies that used open source software for automated MN scoring.
Here we reported the used of CellProfiler, an open
access cell image analysis software, for automatic detection of BNC and MN. The results obtained by
CellProfiler was later compared to manual counting. CellProfiler is a freely available modular image
analysis software capable of handling hundreds of thousands of images. The software contains
already-developed methods for many cell types and assays
and also an open-source, has flexible platform for the
sharing, testing, and development of new methods by image analysis experts. CellProfiler uses the concept of a ‘pipeline’ of individual modules. Each module processes images in several manner, and the modules are placed in sequential order to create
a pipeline usually by this order: object identification
and then measurement. Although most of the modules are automatic, CellProfiler also allows interactive modules (for example, the user clicks to outline a region of interest in each image). Modules
are mixed and matched for a specific project and
each module’s settings are adjusted appropriately. Upon starting the analysis, each image (or group of images if multiple wavelengths are available) travels through the pipeline and is processed by each module consecutively (Carpenter et al. 2006; Lamprecht et al. 2007).
MATERIALS AND METHODS
Blood Cultures. Human peripheral blood samples from a healthy donor of 48-year old were drawn by venipuncture into heparinized tubes (Vacutainer; Becton Dickinson, USA) and irradiated with 60Co
Gamma radiation at 1 Gy doses. The irradiations were done at the Secondary Standard Dosimetry Laboratory at Center for Technology of Radiation Safety and Metrology, National Nuclear Energy Agency of Indonesia. The irradiated samples were maintained at 37 oC for 1 hour to enable repairment
of chromosomal damages. Whole blood cultures (10.5 ml) were set up in 7.5 ml RPMI 1640 medium that contained HEPES and 25 mM L-Glutamine (Gibco) supplemented with 1 ml fetal bovine serum (Gibco); 0.2 ml penicillin–streptomycin (Gibco); 0.25 ml phytohaemagglutinin (PHA; Gibco) and 1 ml whole blood and cultivated at 37 oC. After
44 h, cytochalasin-B (Sigma-Aldrich, Germany) was added to the culture.
Hypotonic Shock. At 72 h, the whole-blood cultures were harvested and cells were centrifuged at 1000 rpm for 10 min at room temperature. After discarding the supernatant until 2 ml remained, cells
were resuspended by flicking the tube that contained remaining 2 ml supernatant. Prior to fixation, cells
were subjected to a cold hypotonic treatment with KCl 0.075 M and then incubated in room temperature for 3 min.
fixed with 2 ml of cold Carnoy solution that was
prepared from methanol and acetic acid (3:1). Carnoy solution was added drop by drop on vortex, followed by four additional drops of formaldehyde. After centrifugation at 1000 rpm for 10 min at
room temperature, the fixation was repeated twice
without formaldehyde. After each centrifugation, the supernatant was discarded by water pump until 2 ml remained and cells were resuspended by patting. After the last centrifugation, the supernatant was discarded until 0.5 ml remained and cells were resuspended with Carnoy until 1 ml, according to
cell density. The fixed cells were dropped on dry
slides using a micropipette on pre-marked positions, 15 mm from edges and frosted end, resulting in two clear separated spots. Two slides were prepared, and slides were dried overnight. Slides then were stained with 4% Giemsa in Sorensen’s buffer for 10 min. Image Acquisition and Standardization. A Nikon Biophot microscope attached with Nikon
D3000 digital single lens reflects (DSLR) camera
system was used to capture images of the smears.
The slides were analyzes under 40× magnification.
Images were captured at a resolution of 1936 × 1296
and saved as JPEG files.
Automated Counting of Binucleated Cells and
Micronuclei. An open access cell image analysis
software CellProfiler 2.0 r10997 that developed by
Broad Institute was used for an automated counting
of binucleated cells and micronuclei. CellProfiler
(CP) runs on Microsoft Windows XP SP 2 32-bit platform. Processor type of the computer was AMD Athlon(tm) 64 X2 Dual Core 5000+ with 1.87 Gb memory (RAM). A pipelines was developed to do an automatic detection of binucleated cells
containing micronuclei (Figure 2). CellProfiler can be downloaded at CellProfiler webpage (http://www. cellprofiler.org/index.shtmL).
Statistical Analysis. Total of BNC and MN obtained by manual or automated counting were compared using Wilcoxon test with H0 hypothesis
that there was no significant difference in variation
between manual and automated counts and H1
hypothesis that there was a significant difference
between manual and automated counts. Significant level used in this research was 0.05 (5%).
RESULTS
Automated and Manual Counting. A total of 135 images were collected and subjected to automated counting, as well as manual counting by
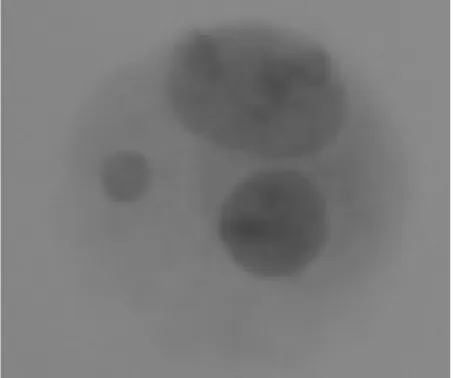
an experienced scorer. Total number of binucleated cells found using CellProfiler from 135 images were 65 and total number of micronuclei were 57. Meanwhile total number of binucleated cells found manually was 64 and total number of micronuclei was 43. A variation of result between manual and automated counts of BNC was found in the image that has metaphase cell. Our pipelines tend to overestimate the number of BNC. Factor that may lead this phenomenon was the presence of cell that had a two nucleus but did not have equal size and imperfect oval nucleus shape. This cell also detected as a BNC because our pipelines were not able to measure whether the area of both the nucleus is approximately equal or not (Figure 3). The presence of metaphase cell also can caused overestimation of BNC. Metaphase cell that contains chromosomes also counted as a nucleus inside the cell, because the
Figure 3. The apperance of cell that also determine as a binucleated cells.
watershed algorithm separated chromosome into two or more parts and as a consequence the pipelines will identify this cell as a BNC (Figure 4).
Statistical Analysis to Compare Between Automated and Manual Counting Results. Statistical analysis using Wilcoxon Rank test showed
that there was no significant difference between
manual and automated detection of binucleated cells (P = 0.851). For micronuclei Wilcoxon Rank test
also showed that there was no significant different
between manual and automated detection (P = 0.917).
DISCUSSION
In this research, Giemsa staining was chosen even though for the automation of MN assay it was
not suitable because many debris will appear in the
slide and can be identified as MN. The reason to used
Giemsa because it do not have to be protected from
the light which allows a more efficient acquisition as compared to fluorescent dyes that need protection
from light. Moreover, this staining also provides the advantage that the slides can be easily reexamined visually if necessary without loss of quality of the staining (Decodier et al. 2009).
In order to determined which cell was the BNC, a
parent and child relationship concept in CellProfiler
between the cells and the nucleus was used. The cells (as a parent) should determine as the BNC if it has two nucleus (as a child) inside it. Cells will not count as a BNC if it has one or more than two nucleus. Our pipelines also tend to overestimate the number of MN. This was happened because in several images after applying Li threshold several small areas were appeared and as a consequence it will determine as a MN by our pipelines. For example like shown in Figure 5 there were seven small areas that considered as MN but in the real images we saw that there was no MN inside the BNC (Figure 5).
Eventhough our pipelines had succeeded to detect the BNC and MN inside it, several disadvantages also appeared in our research. First the pipelines were not able to measure whether the area of both the nucleus in BNC was approximately equal or not. Our pipelines also could not measure staining pattern and staining intensity of the two nuclei inside the BNC. Second for MN our pipelines could not determine the MN that in touch with the nucleus. The pipelines also Figure 4. The metaphase cell that also determine as a binucleated
cells.
Figure 5. Seven small areas (red circle) that defined as micronuclei (left) and real binucleated cell picture that show there were no
could not follow the HUMN scoring criteria for MN that the MN diameter in human lymphocytes usually varies between 1/16 and 1/3 of the diameter of main nuclei in BNC.
Overall our pipelines failed to followed several HUMN scoring criteria for binucleated cells and micronuclei. These problems were also found in other research. Automatic MN assays system developed by Castelain et al. (1993) also addressed inability to follow the HUMN scoring criteria for BNC and MN. Only a system developed by Decodier et al.
(2011) succeeded to follow the HUMN scoring criteria and used a Giemsa staining for automatic MN assay. Unfortunately, all the system described in the literature is the commercial system and usually proprietary or bundled with dedicated analysis equipment.
In our research a minor modification has been
done in the slide preparation protocol. Resuspention of cells in a higher volume of fixative before spreading onto slides was done in our research. Decodier et al. (2011) also resuspended the cell in
a higher volume of fixative as compared to the one
that used for the standard protocol before spreading onto slides to obtain an optimal spreading of the cells without too much overlapping.
Further development of our pipelines for automated detection of the BNC and MN is needed to improve the accuracy especially for micronuclei detection. We hope that the improvement of
CellProfiler as open source biological cell image
analysis software can also improve the accuracy of our pipelines for detection of the BNC and MN in MN
assays. A modification of slide preparation procedure
also must be done in our next research to get a better images quality that can increase the accuracy of our pipelines. A development of automated capturing system and stage movement using our equipment (Nikon Biophot and Nikon D3000 DSLR) with minimal cost also must be consider to get a full automatic system for automated detection of the BNC and MN in MN assays. Overall it can be concluded that in our research automated detection of BNC
and MN for the CBMN assays with CellProfiler are
comparable but not better than manual detection.
ACKNOWLEDGEMENT
The authors are greatly obliged to thank Mukh Syaifudin, Center for Technology of Radiation Safety
and Metrology, National Nuclear Energy Agency of Indonesia for the critical reading of the manuscript. The valuable technical assistance of Yanti Lusiyanti is gratefully acknowledged.
REFERENCES
Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. 2006. CellProfiler: image analysis software for identifying and quantifying cell phenotypes.
Genome Biology 7:1-11.
http://dx.doi.org/10.1186/gb-2006-7-1-r1
Castelain P, Van Hummelen P, Deleener A, Kirsch-Volders M. 1993. Automated detection of cytochalasin-B blocked binucleated lymphocytes for scoring micronuclei. Mutagenesis 8:285-293. http://dx.doi.org/10.1093/mutage/8.4.285
Countryman PI, Heddle JA. 1976. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res 41:321-331. http://dx.doi. org/10.1016/0027-5107(76)90105-6
Decordier I, Papine A, Loock KV, Plas G, Soussaline F, Kirsch-Volders M. 2011. Automated image analysis of micronuclei by IMSTAR for biomonitoring. Mutagenesis 26:163-168. http://dx.doi.org/10.1093/mutage/geq063
Decodier I, Papine A, Plas G, Roesems S, Loock KV, Palomo JF, Cemeli E, Anderson D, Fucic A, Marcos R, Soussaline F, Volders MK. 2009. Automated image analysis of cytokinesis-blocked micronuclei: an adapted protocol and a validated scoring procedure for biomonitoring. Mutagenesis 24:85-93. http://dx.doi.org/10.1093/mutage/gen057
Fenech M. 2007. Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084-1104. http://dx.doi.org/10.1038/ nprot.2007.77
Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. 2003. Human Micronucleus Project. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65-75. http://dx.doi. org/10.1016/S1383-5718(02)00249-8
Fenech M, Holland N, Chang WP, Zeiger E, Bonassi S. 1999. The human micronucleus project—an international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat Res 428:271-283. http:// dx.doi.org/10.1016/S1383-5742(99)00053-8
Fenech M, Morley AA. 1985. Measurement of micronuclei in lymphocytes. Mutat Res 147:29-36. http://dx.doi. org/10.1016/0165-1161(85)90015-9
Fenech M, Morley AA. 1989. Kinetochore detection in micronuclei: an alternative method for measuring chromosome loss. Mutagenesis 4:98-104. http://dx.doi. org/10.1093/mutage/4.2.98
International Atomic Energy Agency [IAEA]. 2011. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies. International Atomic Energy Agency. Vienna.
Lamprecht MR, Sabatini DM, Carpenter AE. 2007. CellProfiler:
Patino-Garcia B, Hoegel J, Varga D, Hoehne M, Michel I, Jainta S, Kreienberg R, Maier C, Vogel W. 2006. Scoring variability of micronuclei in binucleated human lymphocytes in a case-control study. Mutagenesis 21:191-197. http://dx.doi. org/10.1093/mutage/gel018
Tucker JD, Eastmond DA. 1990. Use of an antikinetochore antibody to discriminate between micronuclei induced by aneuploidogens and clastogens. Prog Clin Biol Res 340B:275-284.
Varga D, Johannes T, Jainta S, Schuster S, Schwarz-Boeger U, Kiechle M, Garcia BP, Vogel W. 2004. An automated scoring procedure for the micronucleus test by image analysis.
Mutagenesis 19:391-397. http://dx.doi.org/10.1093/mutage/