Research report
A neuroendocrine study of serotonin function in depressed stroke
patients compared to non depressed stroke patients and healthy
controls
a ,
*
b c cRajamannar Ramasubbu
, Alastair Flint , Gregory Brown , George Awad ,
c
Sidney Kennedy
aThe Department of Psychiatry, University of Ottawa, Royal Ottawa Hospital, Ottawa, Canada
b
The Department of Psychiatry, University of Toronto, The Queen Elizabeth and The Toronto Hospital, Toronto, Canada
c
The Clarke Institute of Psychiatry, University of Toronto, Toronto, Canada Received 5 February 1998; received in revised form 6 March 1998; accepted 6 March 1998
Abstract
Objectives: We employed a neuroendocrine challenge paradigm to study serotonergic abnormalities associated with poststroke depression. Method: Twelve depressed stroke patients (major depression N55, minor depression N57), 8 nondepressed stroke patients and 12 healthy volunteers completed a single-blind, placebo-controlled, challenge tests. Baseline cortisol (CORT) and prolactin (PRL) values, and these hormonal responses to 30 mg of oral d-FEN and placebo over a 4 hour period were measured in the three groups. Results: There were intergroup differences for baseline adjusted PRL responses (change scores from baseline) to d-FEN (group effect F54.38, df52,29, p50.02) while these responses to placebo were comparable between groups (group effect F51.82, df52,29, p50.18). Peak PRL responses (post d-FEN maximal PRL change from baseline scores) in depressed stroke patients were significantly greater than in nondepressed patients ( p50.005) but comparable to healthy normals ( p50.47). However, these responses between major and minor depression were not significant ( p50.34). There was a trend suggesting a negative correlation between peak PRL response and severity of depression ( p50.056). Depressed patients were younger than the controls ( p50.054). Also, the depressed group was more functionally impaired ( p50.04) and more likely to have right-sided lesions ( p50.009) compared with the nondepressed group. Differences in baseline adjusted PRL changes between depressed and nondepressed groups became non significant when the influence of laterality of lesions was covaried, whereas covariation of functional scores and age did not alter the significance. CORT responses did not show intergroup differences. Limitations: The study group was small and was heterogenous in lesion characteristics, time since stroke and type of depression. A fixed-order design was used in the challenge test paradigm. Conclusions: When laterality of stroke lesion was taken into account, depressed and nondepressed stroke patients did not differ in PRL responses to d-FEN. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Serotonin; Poststroke depression; d-Fenfluramine; Prolactin; Cortisol
*Corresponding author.
1. Introduction sions are associated with disturbances in the serotonergic system (Ferrarise et al., 1986; Mrsulja Depression is a common mood disturbance in et al., 1976). Further, the demonstration of a negative stroke patients with a prevalence of 30–50% at the correlation between severity of depression and 5HT2
initial evaluation (Robinson and Starkstein, 1990). receptor binding in the left temporal cortex in left Despite growing recognition of its clinical impor- hemispheric stroke (Mayberg et al., 1988), low tance, the nature and etiology of depression associ- levels of CSF 5H1AA, the principal metabolite of ated with stroke remains uncertain. There is a 5HT in PSD (Bryer et al., 1992) and proven efficacy controversy regarding the etiology of poststroke of serotonergic antidepressants such as citalopram depression (PSD). Some have described the depres- and fluoxetine in treatment of PSD (Anderson et al., sion as an understandable emotional response to 1994; Stamenkovic et al., 1996) provide evidence multiple deficits associated with stroke (Charatan linking the serotonergic system and PSD. However,
and Fisk, 1978). However, investigators who specific 5HT receptor binding studies and CSF
attempted to relate severity of depression to func- studies may not be reliable indicators of net physio-tional, cognitive and social impairment have found logical responsiveness of 5HT systems.
only weak positive correlations in some studies Among several strategies that have been employed (Ebrahim et al., 1987; Robinson et al., 1984a) and no to examine brain 5HT, neuroendocrine challenge correlation in other studies (Feibel and Springer, tests provide a reliable measure of physiological 1982; Robinson and Szetela, 1981). Further, there responsiveness of the central 5HT systems. These have been suggestions that depression may contrib- tests have been designed on the basis of abundant ute to functional (Ramasubbu et al., in press) and evidence substantiating the stimulatory role of 5HT cognitive impairments (Robinson et al., 1986). in the release of PRL, ACTH, and CORT in animals Moreover, trials involving psychosocial interventions and humans (Van de Kar, 1991). Hormonal respon-have failed to show any significant improvement in sivity to fenfluramine (FEN) challenge has been poststroke depression compared to routine care widely used as a measure of central 5HT function to
(Friedland and McColl, 1992). study serotonergic dysfunction in depressive
dis-The evidence in support of an organic hypothesis orders. FEN releases 5HT, inhibits 5HT reuptake and comes from studies reporting a significant associa- stimulates post-synaptic receptors directly and in-tion between major depression and left frontal corti- directly (Costa et al., 1971; Fuex et al., 1975). This cal or left basal ganglia lesions (Robinson et al., provides a measure of net pre-synaptic and post-1984b; Astrom et al., 1993; Hermann et al., 1993). synaptic serotonergic activity. The racemic com-Focal vascular brain lesions cause direct damage to pound of fenfluramine d,l-FEN is a mixture of d-specific brain structures, altering not only local isomer (d-FEN) and l-isomer (l-FEN). The d-isomer
neurochemical and physiological systems but also has been reported to be a more specific 5HT
those of distant brain regions (Andrews, 1991) releasing agent than the d,l-isomer since the l-isomer involved in neural organisations that subserve emo- has additional influences on the catecholomine sys-tions, resulting in mood disturbances (Robinson and tem (Invernizzi et al., 1989). Hence, hormonal Bloom, 1977). Hence, the pathogenesis of depression responses induced by d-FEN might be assumed to be after stroke is better understood in terms of disrup- mediated solely by stimulation of the 5HT system. tion to neurotransmitter systems rather than by d-FEN induces a dose dependent increase in plasma traditional clinical pathological correlations. In addi- PRL levels which has been shown to be diminished tion, since clinical approaches are inadequate in by pretreatment with the 5HT2A / 2C receptor antago-recognising the presence of depression in stroke nist Ritanserin (Goodall et al., 1993) or the 5HT1A
patients with severe communication and comprehen- receptor antagonist pindolol (Palazidou et al., 1995), sion deficits, identification of biological markers suggesting that this response is mediated by a might improve diagnosis (Ramasubbu and Kennedy, serotonergic mechanism. The cortisolemic effect of
1994a,b). A growing body of experimental and d-FEN in humans has also been reported to be
(Feeney et al., 1993). Furthermore, studies indicate 1989) period, stroke subjects were allowed to partici-that d-FEN and its main metabolite d-nor-FEN are pate in the study only after one or more months had more potent than racemic d,l-FEN and d,l-nor-FEN elapsed poststroke. Stroke subjects who scored 16 or (Garattini et al., 1986; Invernizzi et al., 1986). And higher on The Center for Epidemiological Studies also 30 mg of oral d-FEN is well tolerated in healthy Depression (CES-D) Scale, a self-report instrument, subjects without any spontaneously reported adverse were considered to be depressed (Radloff, 1977). effects (O’Keane and Dinan, 1991) as opposed to 60 Using a score of 16 or higher, CES-D has been mg of oral d,l-FEN. Thus d-FEN appears to be a found to have a sensitivity of 0.86, specificity of more specific, potent and safe serotonergic releasing 0.90, and a positive predictive validity of 0.80 in the agent than d,l-FEN. Further, given the growing stroke population (Parikh et al., 1988). Further, evidence that the primary 5HT abnormality in de- severity of depression was evaluated in depressed pression may be presynaptic (Anand et al., 1994), stroke subjects using the Hamilton Rating Scale for d-FEN which exerts a specific excitatory influence Depression (HRSD) (Hamilton, 1960). This instru-primarily on presynaptic neurotransmission is a ment has been shown to be useful in assessing the preferred challenge agent to study serotonergic ab- severity of depression in previous studies of stroke
normalities of depression. subjects (Robinson et al., 1984a). The Hamilton
To our knowledge this is the first study to Rating Scale for Anxiety (HRSA) was used to assess
investigate the central 5HT system in depressed the presence of co-existing anxiety symptoms
stroke patients using d-FEN in a neuroendocrine (Hamilton, 1959). The Schedule for Affective Dis-challenge paradigm. The purpose of this study was to orders and Schizophrenia, a diagnostic interview, test the hypothesis that there will be a significant was used to qualify the symptoms of depression and decrease in PRL and CORT responses to d-FEN to derive affective disorder diagnosis according to among depressed stroke patients compared to non- the Research Diagnostic Criteria (RDC, Spitzer and depressed stroke patients and healthy controls. Endicott, 1979). The Barthel Index was administered to evaluate the functional abilities of stroke patients (Mahoney and Barthel, 1965). CT scan findings were
2. Methods obtained from chart analysis.
The exclusion criteria were as follows: (a) patients
2.1. Subject selection who were unable to provide informed consent in
English and who had poor communications skills in A group of twelve depressed patients (8 women, 4 English; (b) subjects with severe cognitive deficits as men) with a mean age of 61.75615.37 years (S.D), 8 determined by the Mini Mental State Examination nondepressed stroke patients (4 women, 4 men) with (score ,15) (Folstein et al., 1975); (c) severe
a mean age of 7367.55 years and 12 healthy impairment in comprehension and expressive
lan-volunteers (9 women, 3 men) with a mean age of guage; (d) severe essential hypertension (diastolic 71.0866.77 years participated in this study. De- blood pressure$120 mm Hg) (Williams, 1994); (e) pressed and nondepressed stroke subjects were re- uncontrolled diabetes mellitus (fasting blood sugar$ cruited from the Stroke Rehabilitation Unit of The 8 mmol); (f) patients with myocardial infarction Queen Elizabeth Hospital, Toronto and also through within the last 2 months; (g) hypothyroidism; (h) local distribution of posters and media advertise- neurological illnesses other than stroke; (i) history of
ments. alcohol or substance abuse in the last six months; (j)
thyroxine, cimetidine, metaclopropamide, antidepres- inserted into the anterior cubital vein and a
physio-sants and antipsychotics. logical saline drip was commenced. Baseline blood
Healthy normals were recruited by media adver- samples were drawn through a threeway stop cock in tisement and word of mouth. Subjects were free of the intravenous line 30 minutes after insertion, and present or past psychiatric illnesses as determined by then 30 minutes after that (Time 0) before the oral a clinical psychiatric interview. These healthy nor- administration of 30 mg of d-FEN or placebo mals were further screened for current severe medi- capsule. Thereafter, blood samples were drawn at 60, cal illnesses, substance abuse and also for a family 120, 180, and 240 minutes post-challenge for hor-history of mental disorders. Subjects were excluded monal assays. Vital signs (B.P/ Pulse) and self rated if they had medical disorders such as diabetes behavioral responses on a Visual Analogue Scale mellitus requiring insulin or hypoglycemic agents, were also recorded at these time points. The Visual moderate and severe hypertension (diastolic blood Analouge Scale (10 cm scale) was used to measure pressure$105 mm Hg), (Williams, 1994) coronary changes in 4 parameters (drowsy, anxiety, sad, high). heart disease, hypothyroidism, serious liver diseases, The sampling period was limited to 4 hrs because the cancer, stroke, epilepsy, or any other severe medical peak RPL response seemed to occur at 4 hrs after or neurological conditions. Subjects receiving any of d-FEN administration (Quattrone et al., 1983) and the aforementioned medications that could alter also to reduce the inconvenience of prolonged test-serotonin function or PRL secretion were also ex- ing. A fixed dose of 30 mg of oral d-FEN was cluded. Physical examination and appropriate labora- chosen as this might be equivalent to the dose of tory tests were performed to rule out any of the d-isomer in 60 mg of oral d,l-FEN (Silverstone et al., above mentioned conditions. Healthy normals who 1987). An additional blood sample was taken at 3 hrs participated in the study were ambulatory and well after administration of d-FEN for assays of FEN and functioning with normal independent existence in the nor-FEN levels. Maximal plasma levels of d-FEN
community. and d-nor-FEN metabolites were found at 2–4 hrs
post d-FEN (Campbell, 1991).
2.2. d-FEN challenge test
2.3. Assays The challenge tests were conducted at the Clinical
Investigation Unit of The Toronto Hospital. Subjects Each blood sample was centrifuged and plasma attended the test centre at 08:00 hours after an was stored at 2258C before assays. Each sample overnight fast. On each test day, they were served a was assayed for PRL and CORT by quantitative low tryptophan breakfast (apple sauce, jello, orange enzyme immuno assay using transferable solid phase juice) to prevent hormonal changes secondary to technology. The kits were supplied by Sychron
hypoglycemia which could occur as a consequence Enzyme Linked Immunosorbent Assay
mg / ml was 3.7% and 5.4% respectively. The lower significance of all tests was set at p50.05 (two
detection limit was 2 mg / ml. tailed).
2.4. Data analysis 3. Results
Hormonal responses were calculated for 3 out- 3.1. Sample characteristics come measures: (a) Mean baseline (mean of 230, 0
time) adjusted net changes (change scores from Among the twelve depressed stroke patients, 7 had baseline), (b) peak hormonal response concentration minor depression and 5 had major depression accord-(baseline values subtracted from the maximum in- ing to RDC criteria. Since these two groups did not crease in post d-FEN), (c) area under the response significantly differ in hormonal response to d-FEN or curve (AUC), calculated using the trapezoid rule, for in demographic statistics, they were combined into
both PRL and CORT responses to d-FEN and one group for analyses. Their mean scores on
CES-placebo from time 0 until time 240 minutes. Mean D, HRSD and HRSA were 22.6, 23.1 and 14
baseline values for each of the hormones were respectively. As shown in Table 1, the depressed compared between placebo and d-FEN sessions by group did not significantly differ from control groups
2
using student t-tests and between depressed and in sex (x 51.33, df52, p50.51), body weight comparison groups using ANOVA. Baseline adjusted (F51.38, df52,29, p5.27) or plasma concen-net changes in placebo and drug conditions were tration of d-FEN and d-nor-FEN (t52.15, df52,26, compared between groups using a threeway analysis p5.13). The d-FEN and d-nor-FEN levels of three of variance for repeated measures (ANOVA). A subjects were not available due to technical difficul-comparison between groups for peak hormonal re- ties. Further, there were no differences in time since sponses was performed using ANOVA and student t stroke (t5 2.27, df518, p5.79) or in Mini tests as appropriate. Tests of differences in charac- Mental Status Examination Scores (t5 21.26, df5
teristics between groups for continuous and categori- 18, p5.22) between the depressed and nondepres-cal variables were performed with student t tests and sed stroke group. However, the depressed group was
2
x tests, respectively. AUC values were compared younger than the control groups (F53.24, df52,29, using ANOVA and student t tests where significant. p5.054) and differed significantly from the nondep-All results are expressed as means and standard ressed group in laterality of lesions and in functional deviations unless stated otherwise. Data were ana- impairment. The majority of depressed patients with lysed using the computer program SPSS. Statistical stroke had right-sided lesions (81.8%) while
non-Table 1
Sample characteristics
a
Variables Depressed Non-depressed Healthy normals P-value
N512 N58 N512
Sex (MF) 4 / 8 4 / 4 3 / 9 P50.51
Age (years) 61.75615.37 73.0067.56 71.0866.78 P50.054
Weight (Kg) 68.20612.49 75.14616.19 68.0168.70 P50.27
Plasma concentration of d-FEN1d-nor-FEN (ng / ml) 24.63613.32 19.0067.64 24.64610.85 P50.13
MMSE 26.2562.73 24.7562.38 2 P50.22
Barthel Scores 58.75622.17 80.63620.43 2 P50.04
Laterality of lesions right sided 81.8% left sided 83.3% 2 P50.009
Time since stroke 18.79617.08 22.14614.88 2 P50.79
a
depressed stroke patients had left-sided lesions ( p5 to placebo, a two way ANOVA with repeated
2
83.3%) (x 56.80, df51, p5.009). Depressed measures was performed (Fig. 1). There were no patients expressed greater functional impairment than differences in baseline adjusted PRL responses to nondepressed patients (t52.23, df518, p5.04). placebo in these three groups (group effect F51.82, df52,29, p50.18: group3time F51.06, df5
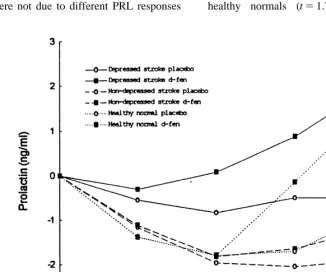
3.2. Prolactin responses 6,87, p5.39) while baseline adjusted PRL changes
to d-FEN were significant between groups (group The baseline PRL values in drug and placebo effect F54.38, df52,29, p5.02: group3time conditions did not differ between groups. A three F52.52, df56,87, p5.03). Further, peak PRL way analysis of variance for repeated measures responses of depressed stroke patients were greater (ANOVA) yielded a main effect of group (F53.84, than nondepressed patients (t53.38, df511.98, p5
df52,29, p5.033), drug (F510.99, df51,29, .005) while the differences in these responses
be-p5.002), time (F58.47, df53,87, p5.00), tween depressed stroke and healthy normals were not group3time interaction (F52.74, df56,87, p5 statistically significant (t5 2.73, df533, p5.47). .017), and drug3time interaction (F58.39, df5 Similarly, AUC 60–240 (area under the curve re-3,87, p5.00) on baseline adjusted PRL responses sponse from time point 60–240 minutes) PRL re-for drug and placebo condition indicating intergroup sponse post d-FEN was significantly greater in the differences in PRL responses. In order to determine depressed stroke group than the nondepressed stroke that the observed group effect and group3time (t53.09, df517.18, p5.007) and comparable with interaction were not due to different PRL responses healthy normals (t51.75, df518.45, p50.1).
When laterality of lesion was entered into an analysis (r5 2.33, p5.29), and Barthel scores (r5.07, of co-variance, the differences in baseline adjusted p5.81).
PRL responses between depressed and nondepressed
patients became non significant (group effect F5 3.4. Behavioral and cardiovascular responses .82, df51,14, p5.381; group3time interaction
F52.14, df53,45, p5.11). However, covariation None of the subjects reported unpleasant side of Barthel scores did not alter the significance of effects or showed any change in self rated anxiety, main effect of group (depressed Vs nondepressed) sad affect, mood elevation or alertness following (F54.66, df51,17 p5.045) and group3time d-FEN. There were no significant differences in interaction (F52.75, df53,54, p5.05). When age, diastolic blood pressure (group3time3drug inter-was added as a co-variate, there inter-was no change in action; f51.15, df58,100, p5.33) or heart rate the significance of group effect (F54.54, df52,29, (group3time3drug interaction f5.77, df58,100,
p5.020) or group3time interaction (F52.74, df5 p50.63). However, ANOVA yielded significant 6,87, p5.017) in net PRL responses. The differ- differences in systolic blood pressure (group3
ences in peak PRL responses between patients with time3drug; f52.25, df58,100, p5.03). major and minor depression did not reach statistical
significance (t51.01, df510, p5.34). However,
the level of significance of the differences in peak 4. Discussion
PRL responses between stroke patients with
depres-sion and nondepressed patients (t52.72, df56.22, The main finding of this study is that PRL
p5.033) was greater than the level of significance responses to acute administration of 30 mg of oral in PRL differences between patients with and non- d-FEN in depressed stroke patients who had a depressed patients (t52.44, df54.45, p5.064). preponderance of right sided lesions were compar-And also there was a trend suggesting a negative able with healthy normals while these responses correlation between peak PRL response and severity were attenuated in nondepressed stroke patients who of depression (HRSD) (r5 2.56, p5.056). A were predominantly affected by left sided lesions. significant negative correlation was found between Our findings indicate that differences in lateralised peak PRL response and anxiety symptoms (HRSA) lesions could account for differential PRL respon-(r5 2.57, p5.05). There was no correlation be- sivity between depressed and nondepressed stroke tween peak PRL response and MMSE scores (r5 patients. Furthermore, there was a trend indicating a
2.26, p5.41), or Barthel scores (r5 2.28, negative correlation between peak PRL responses
p5.23). and severity of depression.
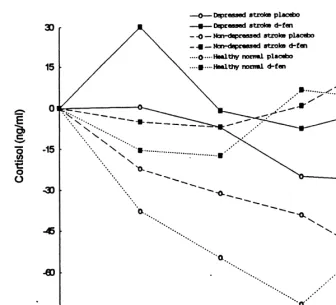
3.3. Cortisol responses 4.1. Methodological limitations
The baseline CORT values on the two challenge Before discussing these findings further, several days did not differ between and within groups. methodological shortcomings concerning patient Although there was a main effect of drug (F518.82, selection and the neuroendocrine challenge paradigm df51,29, p5.00) for baseline adjusted CORT need to be addressed. Despite aggressive recruitment changes, there was no group effect (F5.94, df5 efforts, it was extremely difficult to identify post-2,29, p5.40) or group3drug (F51.06, df52,29, stroke depressed subjects who met the rigorous entry
p5.36), or group3time (F5.58, df56,87, p5 criteria. Similar difficulties in recruitment have been .74), or group3drug3time interaction (F5.60, reported in a previous challenge study involving df56,87, p5.73) indicating an absence of inter- depressed stroke patients (Barry and Dinan, 1990a). group differences in d-FEN induced CORT re- As a result of small sample size we were not able to sponses (Fig. 2). There was no significant correlation control for important variables such as laterality, size between CORT responses and scores of HRSD, (r5 and location of lesion and medication. Furthermore,
left-Fig. 2. Comparison of baseline adjusted cortisol responses in depressed stroke patients with non-depressed stroke and healthy normals. There was a main effect of drug for baseline adjusted cortisol responses whereas group effect or group3drug or group3time or group3drug3
time were not significant (ANOVA).
sided lesions who fulfilled entry criteria were unable results and hence caution should be exercised before to take part in the challenge tests due to agitation and drawing any clear conclusions. Future research catastrophic reactions in response to communication should address these methodological difficulties deficits. This results in a bias towards inclusion of more adequately.
moderately depressed stroke patients with a
pre-ponderance of right-sided vascular lesions. Another 4.2. Prolactin responses between depressed and major drawback is that the stroke patients participat- non-depressed stroke patients
ing in this study were heterogenous in age and
and hemispheric lesion location within 5 days fol- in 5HT receptors compared to left-sided lesions in2
lowing stroke. There are several possible explana- rats (Mayberg et al., 1990). In contrast, 5HT uptake tions for the discrepancies in these findings. In our into platelets did not differ between those patients study, the majority of depressed stroke patients had with left and right sided lesions (Barry et al., 1990b). right-sided lesions (9 of 12) and suffered from minor However, the interpretation of platelets studies is depression (7 of 12) in contrast to those of the CSF complicated by lack of normative data concerning study who were predominantly affected by left sided the influence of potential physiological factors on lesions and had major depression (3 of 4). Further- platelet 5HT uptake (Hrdina, 1994). In summary, the
more, 5HIAA concentration in CSF was measured findings of our study, and the above mentioned
during the acute phase of infarction (5 days after studies, suggest that there may be a relative increase stroke) while stroke patients participated in this in 5HT function in the right hemisphere compared to challenge test at least one month following stroke. the left hemisphere and left-sided lesions may cause Therefore, it is likely that laterality of lesions, time a greater depletion of 5HT than right-sided lesions. since stroke and type of depression might influence
the nature of serotonergic abnormalities associated 4.3. Prolactin responses between depressed stroke with poststroke depression. There were no differ- and healthy normal
ences in time since stroke between depressed and
nondepressed groups and hence the observed differ- Another important finding is that PRL responsivity ences in PRL responsivity between groups could not to d-FEN was unable to distinguish stroke depressed be accounted for by this variable. Due to the small patients from healthy controls. Even taking into sample size, we were unable to match hemispheric account the negative findings (Park et al., 1996; lesions in order to examine whether the type of Maes et al., 1991) most of the studies to date seem to depression may influence PRL responses. To further point to the presence of a diminished serotonergic explore the possibility that the relative increase in responsivity in functional major depression (Siever PRL response in the depressed stroke group could be et al., 1984; O’Keane and Dinan, 1991; Cleare et al., a stress response to limited physical functioning, the 1996; Mann et al., 1995). Although we do not have relationship between Barthel scores and PRL were an exact answer to explain our negative findings, examined. There was no correlation between Barthel there are several possibilities. First, it is possible that scores and peak PRL response and covarying Barthel a type II error occurred owing to small sample size. scores did not alter the PRL responses between Second, the preponderance of minor depression (7 of
groups. 12) among the depressed stroke patients might have
(1991); Park et al. (1996); Maes et al. (1991). Third, were not examined in this study it is not possible to since PRL responsivity is inversely correlated with rule out the peripheral mechanisms in the mediation age (McBride et al., 1990) and there is an age-related of d-FEN induced CORT release. Third, hypercor-decline in post-synaptic 5HT receptors, (Marcusson tisolism has been shown to be associated with stroke et al., 1984a,b) it could be argued that the low lesions (Olsson et al., 1989). Hence, due to heig-therapeutic dose used in this study (normal range htened HPA axis in our elderly stroke sample, subtle 30–60 mg) might have masked the subsensitivity of differences in CORT responses to d-FEN between 5HT receptors in depressed stroke patients compared depressed and controls might have gone undetected. to healthy normals. However, mean body weight and
plasma concentration of d-FEN and d-nor-FEN were
comparable between groups and the weight adjusted 4.5. Conclusions dose used in this study (0.44 mg / kg) was higher
than the recommended therapeutic dose (0.2–0.3 The influence of lateralised lesions on PRL re-mg / kg) (Guy–Grand et al., 1989), suggesting that sponsivity and a probable negative correlation of the findings concerning PRL responses could not be PRL responses with severity of depression seem to attributed to pharmacokinetic aspects of d-FEN but fit nicely with the hypothetical model proposed by might be related to receptor mechanisms. Thus, the Mayberg et al. (1988). According to this model, absence of deductible differences between depressed depletion of 5HT following right-sided lesions might and healthy controls may be due to an age-related lead to upregulation of 5HT receptors while there decline in post-synaptic receptor responsivity as might be a failure in upregulation following left-reported in previous studies (Mann et al., 1995; sided lesions. This might explain why major depres-McBride et al., 1990). Fourth, the validity of blunted sion is commonly associated with left anterior le-PRL responses to d-FEN reported in previous studies sions while right anterior lesions are frequently is questionable as these studies failed to examine associated with undue cheerfulness or emotional pharmacokinetic aspects of d-FEN and also omitted a indifference (Robinson et al., 1984a). In addition, placebo-controlled condition (O’Keane and Dinan, different mechanisms might be responsible for depre-1991; Cleare et al., 1996; Lopez–ibor et al., 1989). ssive illnesses associated with right-sided lesions and Fifth, the association of blunted PRL responses to left-sided lesions (Starkstein et al., 1989). Further a d-FEN and major depression may depend on per- recent PET study has shown an increase in metabolic sonality variables such as aggression and impulsivity response in left prefrontal, and temporo-parietal (Coccaro et al., 1989). We did not measure personali- cortical areas and decrease in the right prefrontal ty in this study and so we were not able to examine cortex to 60 mg of oral d,l-FEN challenge in healthy
its impact on hormonal responses. subjects compared to patients with major depression.
This suggests that, unlike patients with major
depres-4.4. Cortisol responses sion, inhibitory effects of the right hemisphere are
Bryer, J.B., Starkstein, S.E., Votypka, V., Parikh, R.M., Price, T.R.,
the emergence of depressive disorders in stroke
Robinson, R.G., 1992. Reduction of CSF monoamine
metabo-patients.
lites in poststroke depression: a preliminary report. J. Neuro-psychiatry Clin. Neurosci. 4, 440–442.
Campbell, D.B., 1991. Dexfenfluramine: an overview of its mechanisms of action. Rev. Contemp. Pharmacother. 2, 93– Acknowledgements
113.
Charatan, F.B., Fisk, A., 1978. The mental and emotional results
Part of this study was presented at the
Internation-of stroke. New York State Journal Internation-of Medicine 78, 1403–1405.
al Congress in Neuropsychiatry at Seville 1996 and Cleare, A.J., Murray, R.M., O’Keane, V., 1996. Reduced prolactin the Biological Psychiatry Conference at San Diego, and cortisol response to d-fenfluramine in depressed compared to healthy matched control subjects.
Neuropsychopharmacolo-1997. This research is supported by a grant from the
gy 14, 349–354.
Canadian Psychiatric Research Foundation. Authors
Coccaro, E.F., Siever, L.J., Klar, H.M., Maurer, G., Cochrane, K.,
thank Dr. Hajek and Dr. Ruderman and the staff of
Cooper, T.B., Mohs, R.C., Davis, K.L., 1989. Serotonergic
the stroke unit at the Queen Elizabeth Hospital, staff studies in patients with affective and personality disorders. of the Clinical Investigation Unit, the Toronto Hospi- Arch. Gen. Psychiatry 46, 587–599.
tal, Toronto for their co-operation and assistance in Cordes, M., Henkes, H., Roll, D., Eichstadt, H., Christe, W., Langer, M., Felix, R., 1989. Subacute and chronic cerebral
recruitment and performing challenge tests. We
infarctions: SPECT and gadolinium-DAPT enhanced MR
would also like to thank Servier for supplying
d-imaging. J. Comput. Assist. Tomogr. 13, 567–571.
Fenfluramine and placebo capsules and Mr. Thomas
Costa, E.A., Groppetti, A., Refuelta, A., 1971. Action of
fen-Cooper, Nathan Kline Institute of Psychiatric Re- fluramine on monoamine stores of rat tissue. Br. J. Pharmacol. search, New York State for measuring d-fenfluramine 41, 57–64.
Ebrahim, S., Barer, K.D., Nouri, F., 1987. Affective illness after
and d-nor-fenfluramine metabolites. Dr. David
stroke. Br. J. Psychiatry 151, 52–56.
Streiner provided statistical consultation and Mr.
Feeney, S., Goodall, E., Silverstone, T., 1993. The effects of d-and
Paul Miceli assisted with statistical analysis. We also
l-Fenfluramine (and their interactions with d-amphetamine) on
acknowledge Mary Kinckle for technical assistance cortisol secretion. Int. Clin. Psychopharmacol. 8, 139–142. and Sangeetha Ramasubbu for administrative sup- Feibel, J.H., Springer, C.J., 1982. Depression and failure to
port. resume social activities after stroke. Arch. Phys. Med. Rehabil.
63, 276–278.
Ferrarise, C., Bassie, S., Frattola, L., Locatelli, P., Piolti, R., Trabucchi, M., 1986. Different patterns of CSF neurotrans-References mitter metabolism in patients with left or right hemispheric
stroke. Acta. Neurol. Scand. 73, 581–585.
Folstein, M.F., Folstein, S.E., McHugh, P.R., 1975. Mini mental Anand, A., Charney, D.S., Delgado, P.L., Mcdongle, C.J.,
Hening-state: a practical method for grading the cognitive state of er, G.R., Price, L.H., 1994. Neuroendocrine and behavioural
patients for the clinician. J. Psychiatr. Res. 12, 189–198. responses to intravenous m-chlorophenyl piperazine (mcpp) in
Friedland, J.F., McColl, M., 1992. Social support intervention depressed patients and healthy subjects. Am. J. Psychiatry 151,
after stroke: results of a randomized trial. Arch. Phys. Med. 1626–1630.
Rehabil. 73, 573–581. Andrews, R.J., 1991. Transhemispheric diaschisis: a review and
Fuex, K., Farnebo, L.O., Hamberger, B., Ogren, S.O., 1975. On comment. Stroke 22, 943–949.
the in vivo and in vitro actions of fenfluramine and its Anderson, G., Vestergaard, K., Lauritzen, L., 1994. Effective
derivatives on central monoamine neurons, especially 5 hy-treatment of poststroke depression with the selective serotonin
droxytryptamine neurons and their relation to the anorectic reuptake inhibitor citalopram. Stroke 25, 1009–1104.
activity of fenfluramine. Postgrad. Med. J. 1, 35–40. Arato, M., Tekes, K., Palkovits, M., Demeter, E., Falues, A.,
Garattini, S., Mennini, T., Bendotti, C., Invernizzi, R., 1986. 1987. Serotonergic split brain and suicide. Psychiatry Res. 21,
Neurochemical mechanism of action of drugs which modify 355–356.
feeding via the serotonergic system. Appetite 7, 15–38. Astrom, M., Adolfsoson, R., Asplund, K., 1993. Major depression
Goodall, E.M., Cowen, P.J., Franklin, M., Silverstone, T., 1993. in stroke patients: a 3 year longitudinal study. Stroke 24,
Ritanserin attentuates anorectic, endocrine and thermic re-976–982.
sponse to d-fenfluramine in human volunteers. Psycho-Barry, S., Dinan, T.G., 1990. Alpha-2 adrenergic receptor function
pharmacology 112, 461–466. in post-stroke depression. Psychol. Med. 20, 305–356.
Hamilton, M.A., 1960. A rating scale for depression. J. Neurol. Mitchell, P., Smythe, G., 1990. Hormonal responses to fen-Neurosurg. Psychiatry 23, 56. fluramine in depressed and control subjects. J. Affect. Disord.
19, 43–51. Hamilton, M.A., 1959. The assessment of anxiety states by rating.
Br. J. Med. Psychol. 32, 50. Mrsulja, B.B., Mrsulja, B.J., Spatz, M., Klatzo, I., 1976. Brain serotonin alter experimental vascular occlusion. Neurology 26, Hermann, M., Bartles, C., Wallesh, C.W., 1993. Depression in
785–787. acute and chronic aphasia. J. Neurol. Neurosurg. Psychiatry 56,
672–678. O’Keane, V., Dinan, T.G., 1991. Prolactin and cortisol responses to d-fenfluramine in major depression: evidence for diminished Hrdina, P.D., 1994. Platelet serotonergic markers in psychiatric
responsivity of central serotonergic function. Am. J. Psychiatry disorders: use, abuse and limitations. J. Psychiatr. Neurosci. 19,
148, 1009–1015. 87–90.
Olsson, T., Astrom, M., Ericksson, S., Forssell, A., 1989. Hy-Invernizzi, R., Berettera, C., Garattini, S., Samanin, R., 1986. D
percortisolism revealed by the Dexamethasone Supression Test and L-isomer of fenfluramine differ markedly in their
inter-with acute ischaemic stroke. Stroke 20, 1685–1690. action with brain serotonin and catecholomines in the rat. Euro.
J. Pharmacol. 120, 9–15. Palazidou, E., Stephenson, T., Butler, J., Coskeran, P., Chambers, S., McGregor, A., 1995. Evidence for 5HT1A receptor in-Invernizzi, R., Bertorelli, R., Consolo, S., Garattini, S., Samanin,
volvement in the control of prolactin in man. Psycho-R., 1989. The effects of the l-isomer of fenfluramine and
pharmacology 119, 311–314. dopamine mechanism in rat brain. Eur. J. Pharmacol. 164,
241–248. Park, S.B.G., Williamson, D.J., Cowen, P.J., 1996. 5-HT neuroen-docrine function in major depression: prolactin and cortisol Kerbs, H.A., Cheng, L.K., Wright, G.S., 1984. Determination of
response to D-fenfluramine. Psychol. Med. 26, 1191–1196. fenfluramine and nor-fenfluramine in plasma using a nitrogen
sensitive detector. J. Chromato. Biomed. Appl. 310, 412–413. Parikh, R.M., Eden, D.T., Robinson, R.G., 1988. The sensitivity and specificity of the centre of the centre for epidemiological Lopez–ibor, J.J., Saiz–Ruiz, J., Iglesias, L.M., 1989.
Neuroen-studies depression scale in screening for poststroke depression. docrine challanges in the diagnosis of depressive disorders. Br.
Int. J. Psychiatry. Med. 18, 169–181. J. Psychiatry 154, 73–76.
Quattrone, A., Tedeschi, G., Aguglia, U., Scopacasa, F., Di Renzo, Maes, M., D’Hondt, P., Suy, E., Minner, B., Vandavost, C., Raus,
G.F., Annunziato, L., 1983. Prolactin secretion in man: a useful J., 1991. HPA axis hormones and prolactin responses to dextro
tool to evaluate the activity of drugs on central 5-hydroxy-fenfluramine in depressed patients and healthy controls. Prog.
tryptaminergic neurones. Studies with fenfluramine. Br. J. Clin. Neuropsychopharmacol. Biol. Psychiat. 15, 781–790.
Pharmac. 16, 471–475. Mann, J.J., McBride, P.A., Malone, K.M., DeMeo, M., Keilp, J.,
Radloff, L.S., 1977. The CES-D scale: a self report depression 1995. Blunted serotonergic responsivity in depressed
inpati-scale for research in the general population. Applied Psycho-ents. Neuropsychopharmacology 13, 53–64.
logical Measurement 1, 385–401. Mann, J.J., Malone, K.M., Dienl, D.J., Cooper, T.B., Mintum,
M.A., 1996. Demonstration in vivo of reduced serotonin Ramasubbu, R., Robinson, R.G., Flint, A.J., Kosier, T., Price, responsivity in brain of untreated patients. Am. J. Psychiatry T.R., in press. Functional impairment associated with acute 153, 174–182. post stroke depression: the stroke data bank study. J.
Neuro-psychiatry. Clin. Neurosci. Mahoney, F.I., Barthel, D.W., 1965. Functional evaluation: the
Barthel index. Md. Med. J. 14, 61–65. Ramasubbu, R., Kennedy, S.H., 1994. Factors complicating the diagnosis of depression in cerebrovascular disease, Part 1 – Marcusson, J., Oreland, L., Winbald, B., 1984. Effect of age on
Phenomenological and nosological issues. Can. J. Psychiatry human brain serotonin (SI) binding sites. J. Neurochem. 43,
39, 596–600. 1699–1705.
Ramasubbu, R., Kennedy, S.H., 1994. Factors complicating the Marcusson, J., Morgan, D.G., Winbaild, B., Finch, C.E., 1984.
diagnosis of depression in cerebrovascular disease, Part 11 – Serotonin-2 binding sites in human frontal cortex and
hip-Neurological deficits and various assessment methods. Can. J. pocampus: selective loss of S-2A sites with age. Brain Res.
Psychiatry 39, 601–607. 311, 51–56.
Robinson, R.G., Bloom, F.E., 1977. Pharmacologic treatment McBride, P.A., Tierney, H., Demco, M., Chen, J.S., Mann, J.,
following experimental cerebral infarction: implications for 1990. Effect of age and gender on CSN serotonergic
re-understanding psychological symptoms of human stroke. Biol. sponsivity in normal adults. Biol. Psychiatry 27, 1143–1155.
Psychiatry 12, 669–680. Mayberg, H.S., Robinson, R.G., Wong, D.F., Parikh, R., Bolduc,
Robinson, R.G., Szetela, B., 1981. Mood change following left P., Starkstein, S.E., Price, T., Dannals, R.F., Links, J.M.,
hemispheric brain injury. Ann. Neurol. 9, 447–453. Wilson, A.A., Ravert, H.T., Wagner, H.N., 1988. PET imaging
of cortical: S2 serotonin receptors after stroke: laterlized Robinson, R.G., Starr, L., Lipsey, J.R., Rao, K., Price, T.R., 1984. changes and relationship to depression. Am.J. Psychiatry. 145, A two year longitudinal study of post-stroke mood disorders: 937–943. dynamic changes in associated variables over the first six
months of follow-up. Stroke 15, 510–517. Mayberg, H.S., Moran, T.H., Robinson, R.G., 1990. Remote
lateralised changes in cortical (3H) spiperone binding follow- Robinson, R.G., Kubos, K.L., Starr, L.B., Rao, K., Price, T.R., ing focal frontal cortex lesions in the rat. Brain Res. 515, 1984. Mood disorders in stroke patients: Importance of location
Robinson, R.G., Bolla, Wilson, K., Kaplan, E., Lipsey, J.R., Price, Spitzer, R.L., Endicott, J., 1979. Schedule for affective disorders T.R., 1986. Depression influence intellectual impairment in and schizophernia. New York State Psychiatric Institute. stroke patients. Br. J. Psychiatry. 148, 541–547. Stamenkovic, M., Schindler, S., Kasper, S., 1996. Poststroke Robinson, R.G., Starkstein, S.E., 1990. Current research in depression and fluoxetine. Am. J. Psychiatry 153, 446–447.
affective disorders following stroke. J. Neuropsychiatry Clin. Starkstein, S.E., Robinson, R.G., Hoing, M.A., Parikh, R.M., Neurosci. 2, 1–14. Joselyn, R., Price, T.R., 1989. Mood changes after right Siever, L.J., Murphy, D.L., Slater, S., de la Vega, E., Lipper, S., hemispheric lesion. Br. J. Psychiatry 155, 79–85.
1984. Plasma prolactin changes following fenfluramine in Van de Kar, L.D., 1991. Neuroendocrine pharmacology of depressed patients compared to controls: an evaluation of serotonergic (5-HT) neurons. Annu. Rev. Pharmacol. Toxicol. central serotonergic responsivity in depression. Life Sci. 3–4, 31, 289–320.
1029–1039. Williams, G.H., 1994. Hypertensive vascular disease. Harrison’s Silverstone, T., Smith, G., Richards, R., 1987. A comparative Principles of Internal Medicine Thirteenth Edition. In: Issel-evaluation of dextrofenfluramine and dl-fenfluramine. In: Ben- bacher, K.J., Braunwald, E., Wilson, J.D., Martin,J.V., Fauci, der, A.F., Brooks, L.J. (Eds.), Bodyweight control: The Physi- A.S., Kasper, D.L. (Eds.), McGraw Hill, inc U.S.A pp. 1116– ology, Clinical Treatment and Prevention of Obesity. Churchill 1131.