ORIGINAL ARTICLE
Behavioral Effects of Omega-3 Fatty Acid Supplementation in Young
Adults with Severe Autism: An Open Label Study
Pierluigi Politi,
aHellas Cena,
bMario Comelli,
cGaetano Marrone,
cChiara Allegri,
bEnzo Emanuele,
aand Stefania Ucelli di Nemi
aaDepartment of Health Applied Sciences, Section of Psychiatry bDepartment of Health Applied Sciences, Section of Human Nutrition
cDepartment of Health Applied Sciences, Section of Statistics, University of Pavia, Pavia, Italy
Received for publication April 14, 2008; accepted June 30, 2008 (ARCMED-D-08-00158).
Background. Pilot findings seem to suggest a potential beneficial effect of omega-3 fatty acid (FA) supplementation on behavioral alterations in children with autism. However, data on the potential benefits of omega-3 supplements in young adults with severe autism are lacking. In the present study, we sought to explore this issue in an open label study. Methods. Nineteen young adults with severe autism (CARS O40), aged 18e40 years,
received two fish oil capsules per day [0.93 g of eicosapentaenoic acid (EPA) and doco-sahexaenoic acid (DHA) plus 5 mg of vitamin E to avoid lipid peroxidation] for 6 weeks. Subjects were assessed with anad hoc caregiver questionnaire, the Rossago Behavioral Checklist, for the assessment of behavioral anomalies.
Results. No significant improvements were observed with regard to the severity and fre-quency of problematic behaviors either during the active treatment period or during the post-treatment 6-week observation period. Moreover, no effect on the number of episodes and severity of behavior aberrations was observed.
Conclusions. Our negative findings do not point toward a major effect of omega-3 FA supplementation on behavioral abnormalities in adults with severe autism. Further studies on larger sample sizes are warranted to shed more light on this important issue. Ó2008
IMSS. Published by Elsevier Inc.
Key Words:Autism, Behavior, Omega-3 fatty acids.
Introduction
Autism is characterized by impairments in social interaction, difficulty with communication, and restrictive and repetitive behaviors (1). This condition is traditionally considered as a stable neuropsychiatric disorder and improvements in core autistic features are uncommon. However, preliminary evi-dences mainly derived from clinical studies seem to suggest that specific pharmacological or nutraceutical interventions may improve behavioral problems in this patient group(2e5).
There is growing evidence that deficiencies of omega-3 fatty acids (FA) may be involved in numerous psychiatric disorders(6). Interestingly, a recent double-blind study by
Amminger et al.(7) found that omega-3 FA supplements were superior to placebo for reducing hyperactivity and ste-reotypy in autistic children. However, there were significant shortcomings in this study including the small sample size (n512), the large baseline differences between patients with autism and controls, the fluctuating nature of the out-comes, and problems inherent in the effect sizes(8).
To our knowledge, there are no published data on the effi-cacy of omega-3 FA supplementation in young adults with severe autism. The issue of pharmacological treatment of adult autistic patients is not trivial inasmuch as severe cases of au-tism usually persist into adulthood, and the majority of cases diagnosed in childhood continue to meet the criteria for the disorder in early adult life(9,10). We therefore conducted this open label trial to examine the safety and behavioral ef-fects of omega-3 FA supplementation in this patient group. Address reprint requests to: Pierluigi Politi, MD, PhD, Department of
Health Applied Sciences, Section of Psychiatry, University of Pavia, Via Bassi 21, Ie27100, Pavia, Italy; E-mail:pierluigi.politi@unipv.it
0188-4409/08 $esee front matter. CopyrightÓ2008 IMSS. Published by Elsevier Inc. doi: 10.1016/j.arcmed.2008.06.005
Materials and Methods
Study Participants
This open label trial was conducted from April 2007 to June 2007 in a single farm community center specifically designed for individuals with autism (Cascina Rossago, Pontenizza, Pavia, Italy). The sample included 19 adults (15 males, 4 females) aged 18 to 40 years (meanSD: 28.94.9 years) with severe autistic disorder (CARSO40)(11). The sam-pling design followed previously established selection criteria: (a) a physician and a psychologist, both experienced in autism, confirmed that the subject diagnosis met the DSM-IV-Text Re-vised; (b) the patients manifested moderate to profound mental retardation as assessed by standardized tests of intelligence, including the Wechsler Adult Intelligence Scale(12); (c) all subjects had a positive history of severe maladaptive behav-iors; (d) all subjects had no medical contraindications.
Design
An open label design extending over three 6-week periods was used. All patients initially took part in a prebaseline period (from February to March 2007). During this interval, behaviors targeted for observation were selected and opera-tionalized. Inter-rater reliability was established at or above 90%. The prebaseline period was followed by a 6-week pre-treatment period (from March 14, 2007 to April 24, 2007) when subjects did not receive any unusual treatment. During the following 6-week treatment period (from April 25, 2007 to June 5, 2007) all subjects were given two gelatin capsules of fish oil supplements containing 0.93 g of eicosapentaenoic acid (EPA) and dososahexaenoic acid (DHA). Moreover, all subjects received two multimineral and vitamin supplements containing 5 mg of vitamin E in order to avoid lipid peroxi-dation. During the post-treatment 6-week period (from June 6, 2007 to July 17, 2007) the experimental treatment was stopped. Throughout all phases, all subjects were assessed daily with the Rossago Behavioral Checklist (see below).
Outcome Measure
In the present study, anad hoccaregiver questionnaire, the Rossago Behavioral Checklist (personal communication) was used (Table 1). This instrument consists of a 22-item rat-ing scale focusrat-ing on behavioral problems. Each item was rated on a 5-point Likert scale from 0 (none) to 4 (extremely severe). In this study, we analyzed problematic behaviors assessed during an 18-week period (6 week pretreatment ob-servational period, 6-week treatment period, 6-week posttreat-ment period). Primary endpoint of the trial is the severity score as measured by the Rossago Behavioral Checklist.
Data Analysis
The whole sample averages and standard deviations of the severity score and the number of problematic behaviors
computed for the three study periods were plotted. Individ-ual pretreatment severity score means were compared with their treatment period counterparts using the Wilcoxon test for paired observations.
Statistical packages R (2.5.1; Foundation for Statistical Computing, Vienna, Austria) and SPSS (15.0; SPSS Inc., Chi-cago, IL) were used for all statistical analyses. A two-tailed pvalue of!0.05 was considered statistically significant.
Results
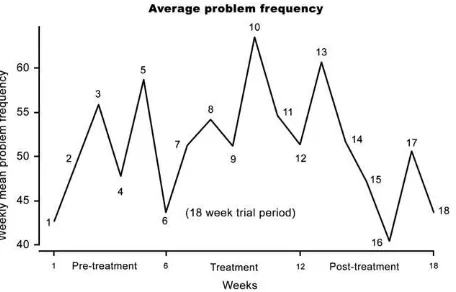
Severity and frequency of problematic behaviors during the study periods are depicted inFigures 1 and 2. Between the pretreatment period (mean severity score: 18.73, SD513.06) and the treatment period (mean severity score: 19.75, SD511.58), the severity of symptoms worsened slightly but not significantly (p50.054). During the post-treatment period the mean severity score decreased to 14.46 Table 1.Rossago Behavioral Checklist
1. Plays with or eats mucus, saliva, stools, or earth (includes picacism) 2. Excessive or deficient hygiene
3. No sphincteric control
4. Urinates or defecates in inappropriate places 5. Excessive or deficient food intake
6. Improper sexual behavior 7. Undresses him/herself in the public
8. Shuts him/herself in the room or bathroom. Refuses to go out 9. Absconds or runs away from the caregiver
10. Damages objects
11. Cries, shouts, sings, or laughs in an excessive manner or with no reason 12. Disturbs others
13. Picks at healing wounds and scars 14. Hits, pinches, bites, scratches him/herself 15. Hits, pinches, bites, scratches others 16. Irritates, quarrels verbally with others 17. Disobeys caregivers’ orders 18. Throws objects at others
19. Obsessive, repetitive, compulsive behaviors 20. Intense fear or anxiety without cause 21. Steals food or objects
22. Does not sleep and disturbs others
Figure 1.Evolution of the weekly average severity score. 683
(SD57.55). The patients’ weekly average frequency of problematic events did not show any improvement between pretreatment and treatment period (results not shown). No clear trend was detectable when severity and frequency of problematic behaviors were computed taking into account only the hyperactivity-related items of the Rossago Behav-ioral Checklist (data not shown).
Discussion
Epidemiological data have shown the beneficial effects that a diet rich in fish oils can have on several psychiatric disor-ders(13,14). Pilot findings by Amminger et al.(7)seemed to suggest the effectiveness of fish oil supplementation to reduce hyperactivity in children with autism. The primary outcome measure used in the study was the change in the Aberrant Behavior Checklist (ABC) at 6 weeks (6). How-ever, there are caveats in the study of Amminger et al.(7)that merit consideration. The sample size was small (n512) and the authors’ use of repeated measures statistical analysis may have led to misleading interpretation of results related to ste-reotypy and hyperactivity scores(8). We thus designed this study to test the hypothesis that omega-3 FA supplementation could exert beneficial behavioral effects in adult patients with severe autism.
Our results failed to convincingly demonstrate a benefi-cial effect of omega-3 FA supplementation in a group of young adults with severe autism. In an attempt to confirm the effect of omega-3 FA on several behavior problems in persons with autism, we performed a comprehensive anal-ysis of several aberrant behaviors commonly found in autis-tic individuals. Not only were differences in outcome between the intervention groups in our study small, but also the results of our study were inconsistent with those of Am-minger et al.(7)and do not support such a positive effect on hyperactivity reduction. Thus, overall effect of supplemen-tation with omega-3 PUFAs on the incidence of behavioral problems in this population of adult patients with severe au-tism seems more modest than that observed for hyperactiv-ity in a trial conducted in children.
Although our open label study and the trial by Amminger et al.(7)differed with respect to the study population, we do not think that this explains the apparent divergence of results. We chose a treatment time and dosage of omega-3 PUFAs similar to the dose in the previous trial(6), which showed sig-nificant effects on hyperactivity scores. Furthermore, the length of our treatment and the follow-up schedule is such that we were able to perform a sufficient number of clinical observations to judge the potential effect of treatment.
There may be some methodological concerns about this study in adult patients. With respect to limitations of this investigation, we must consider two issues. Firstly, this is a single-center study and the number of patients is small. This limited sample size may have obscured a possible ef-fect of omega-3 FA on aberrant behaviors. Second, behav-ioral problems were evaluated with a different instrument compared with the study by Amminger et al.(7). Strengths of our study rely on our assessment of multiple measures of behavior and on close follow-up assessments.
In conclusion, in this open label trial we did not find ev-idence of strong behavioral effects of intake of omega-3 PUFAs from fish oil against problematic behaviors in adult patients with autism. Fluctuations of the behavioral scores as observed during our study are likely to represent random fluctuations, not real treatment effects. In light of our results and based on the methodological limitations of the study by Amminger et al., we conclude that caution should be exer-cised on the potential usefulness of omega-3 supplementa-tions to reduce hyperactivity or other problematic behaviors in autistic individuals.
Acknowledgments
We are grateful to Sigma Tau Italia and Wyeth Italia for their kind gift of omega-3 capsules and multivitamin supplementation. We gratefully acknowledge the excellent nursing assistance of Rob-erto Albasi.
References
1. Sigman M, Spence SJ, Wang AT. Autism from developmental and neuropsychological perspectives. Annu Rev Clin Psychol 2006;2: 327e355.
2. Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. J Child Neurol 2007;22:574e579. 3. Dosman CF, Brian JA, Drmic IE, Senthilselvan A, Harford MM,
Smith RW, et al. Children with autism: effect of iron supplementation on sleep and ferritin. Pediatr Neurol 2007;36:152e158.
4. Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psy-chiatry 2008;17:1e8.
5. McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142e1148. Figure 2.Evolution of the weekly average problem frequency.
6. Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006;67: 1954e1967.
7. Amminger GP, Berger GE, Scha¨fer MR, Klier C, Friedrich MH, Feucht M. Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Bi-ol Psychiatry 2007;61:551e553.
8. Gilbert DL. Regarding ‘‘omega-3 fatty acids supplementation in chil-dren with autism: a double-blind randomized, placebo-controlled pilot study’’. Biol Psychiatry 2008;63:e13.
9. Billstedt E, Gillberg IC, Gillberg C. Autism in adults: symptom pat-terns and early childhood predictors. Use of the DISCO in a commu-nity sample followed from childhood. J Child Psychol Psychiatry 2007;48:1102e1110.
10. Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004;10:234e247.
11. Rellini E, Tortolani D, Trillo S, Carbone S, Montecchi F. Childhood Autism Rating Scale (CARS) and Autism Behavior Checklist (ABC) correspondence and conflicts with DSMeIV criteria in diagnosis of autism. J Autism Dev Disord 2004;34:703e708.
12. Hawkins KA, Sullivan TE, Choi EJ. Memory deficits in schizophrenia: inadequate assimilation or true amnesia? Findings from the Wechsler Memory Scaleerevised. J Psychiatry Neurosci 1997;22:169e179. 13. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments
for mental illness: which disorder and which fatty acid? Lipids Health Dis 2007;6:21.
14. Peet M, Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 2005;65:1051e1059.
685