Detection and characterization of a 36-kDa peptide in C-terminal
region of a 24-kDa vacuolar protein (VP24) precursor in
anthocyanin-producing sweet potato cells in suspension culture
Wenxin Xu
a, Kei Moriya
b, Kenji Yamada
c,1, Mikio Nishimura
c, Hidenari Shioiri
b,
Mineo Kojima
a,b, Masayuki Nozue
a,b,*
aGraduate School of Science and Technology,Shinshu Uni6ersity,3-15-1Tokida,Ueda,Nagano,386-8567,Japan bDepartment of Applied Biology,Faculty of Textile Science and Technology,Shinshu Uni6ersity,3-15-1Tokida,Ueda,Nagano,
386-8567,Japan
cDepartment of Cell Biology,National Institute for Basic Biology,Nishigonaka 38,Myodaiji,Okazaki,Aichi,444-8585,Japan
Received 13 June 2000; received in revised form 22 August 2000; accepted 28 August 2000
Abstract
A 24-kDa vacuolar protein (VP24) was found to accumulate in anthocyanin-producing sweet potato cells (Ipomoea batatas
Lam.) in suspension culture [Nozue et al., Plant Physiol. 115 (1997) 1065]. VP24 cDNA (accession No. AB025531) encodes a 96.3-kDa large precursor protein with a C-terminal propeptide which contains the eight putative transmembrane domains. The mature VP24 is probably involved in the formation of intravacuolar pigmented globules (cyanoplasts) in highly anthocyanin-con-taining vacuoles, but the biological function of the C-terminal region including the putative transmembrane domains is unknown. To confirm the expression and characterize the C-terminal region in the VP24 protein precursor in the anthocyanin-producing cells, polyclonal antibodies were developed against the fusion protein, including the C-terminal region, expressed inEscherichia coli. Western blot analysis showed that a 36-kDa peptide (VP36) localized in anthocyanin-containing vacuoles was expressed under continuous illumination, but not in darkness. The expression pattern of VP36 showed high similarity to VP24. These results suggested that VP36 may be derived from the large VP24 protein precursor; it includes several of the hydrophobic domains in the C-terminal region. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Anthocyanin; Fusion protein;Ipomoea batatas; Polyclonal antibodies; Transmembrane domain; Vacuolar protein
www.elsevier.com/locate/plantsci
1. Introduction
The central vacuole is the largest compartment in most mature plant cells, and it is the main site
of accumulation of many kinds of water-soluble secondary metabolites such as anthocyanin. We demonstrated that cultured cells of sweet potato (Ipomoea batatas Lam. cv. Kintoki) produced large amounts of anthocyanin, and formed in-tensely pigmented globules (cyanoplasts [1] or an-thocyanoplasts [2]) within the central vacuoles [3,4]. We also found that a 24-kDa protein (VP24) accumulated as a major vacuolar protein in the anthocyanin-containing vacuoles [5]. Immunocyto-chemical detection of VP24 showed that this protein was localized in cyanoplasts within the anthocyanin-containing vacuoles [6]. Expression of VP24 was induced in cultured cells upon exposure
Abbre6iations: CBB: Coomassie Brilliant Blue; 2,4-D: 2,4-dichlorophenoxyacetic acid; IPTG: isopropyl 1-thio-b-D-galactoside; MES: 2-morpholinoethanesulfonic acid; PAGE: polyacrylamide gel electrophoresis; PCR: polymerase chain reaction; PVDF: poly (vinyl-idene difluoride); rER: rough endoplasmic reticulum; SDS: sodium dodecyl sulfate.
* Corresponding author. Tel.: +81-268-215353; fax: + 81-268-215352.
E-mail address:[email protected] (M. Nozue).
1Present address; Department of Botany, Kyoto University,
Ki-tashirakawa-Oiwake-cho, Sakyo-ku, Kyoto 606-8502, Japan
to light and closely paralleled the accumulation of anthocyanin. VP24 easily combines with an-thocyanins in vitro and the greatest amount is recovered from the reddish pellet after ultracen-trifugation of the homogenate prepared from an-thocyanin-containing cells. These results suggest that VP24 probably plays a role in the intravac-uolar trapping of anthocyanins via the formation of cyanoplasts. However, whether VP24 has bio-logical roles in addition to the formation of cyanoplasts is not known. Although the non-ducing cell line (N cell line) of sweet potato pro-duces little anthocyanin and no cyanoplasts are formed, a small amount of VP24 accumulates in N cells after light irradiation [6].
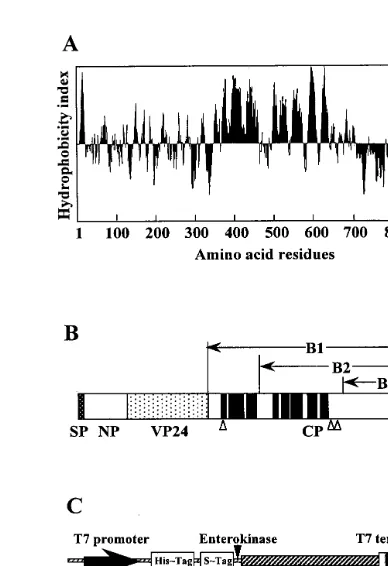
To clarify the function of VP24 in an-thocyanin-producing cells, it is important to ob-tain structural information of the VP24 protein precursor. Recently, we cloned and characterized a VP24 cDNA (accession No. AB025531) that encoded a 893-amino acid polypeptide with a predicted molecular mass of 96.3 kDa. In addi-tion, Northern blot analysis showed a single 2.9-kb VP24 mRNA which was detected only in light-irradiated cultured cells (Xu et al., unpub-lished data). VP24 may be synthesized first as a large protein processor in the endoplasmic reticu-lum (ER) reticu-lumen, then transported to the vacuole and processed into its mature form by prote-olytic cleavage. The most salient feature of the deduced precursor sequence of VP24 cDNA was the presence of a highly hydrophobic domain at the C-terminal region that contained the eight putative transmembrane domains (Fig. 1A, B) predicted by the PSORT program based on Klein’s method [7]. Homology analysis of the de-duced amino acid sequence of the transmem-brane domains showed no similarities to other known vacuolar membrane proteins.
These results obtained from sequence analysis suggest the possibility that a functional transmembrane peptide is expressed in the vac-uoles of anthocyanin-producing cells in addition to mature VP24. In order to confirm this inter-esting possibility, in the present study, we have developed polyclonal antibodies against the C-terminal region in the VP24 protein precursor and characterized a 36-kDa vacuolar protein de-tected by immunoblot analysis in anthocyanin producing sweet potato cells in suspension cul-ture.
2. Materials and methods
2.1. Cell cultures
A high anthocyanin-producing cell line (ALND) of sweet potato (I. batatas Lam. cv. Kintoki) in suspension culture was used in the present experi-ments. The ALND cells were established by visual clonal selections from callus culture that had been initiated from root tissue [8]. The ALND cell line produces large amounts of anthocyanin under continuous illumination, but little anthocyanin in darkness. The ALND callus was maintained in 25 ml of PRL-4C [9] agar medium that contained 3% sucrose and 0.3 mg/l 2,4-D in a plastic dish under continuous illumination at 25°C, with subculture
at 2-week intervals. In order to induce an-thocyanin synthesis, 7-day-old callus (2 g wet weight) was transferred to 20 ml of liquid PRL-4C medium without 2,4-D and exposed to continuous illumination as described previously [4].
2.2. Construction of bacterial expression 6ectors
Three partial cDNA sequences encoding three parts of the C-terminal propeptide (B1, B2 and B3) of the VP24 protein precursor (Fig. 1A, B) were prepared by PCR amplification. The follow-ing primers were used: Bam HI-B1 (5%
-CGCG-GATCCAACACTCTATCCCAAGGA-3%), Bam
HI-B2 (5%
-CCCCCCGGATCCCACAAGTTCT-TATCATACACA-3%) or Bam HI-B3 (5% -GC-CGCGGGATCCTATAGTTGCTGGACTGCTGA A-3%) and T7 primer, and VP24 cDNA inserted into pBluescript SK(−) was used as a template. The resulting fragments were excised using Bam HI andXhoI after cloning each PCR product into pGEM-T vector (Promega), and inserted into pET-32a (+) (Novagen) to create pET – B1, pET – B2 and pET – B3 (Fig. 1C). The sequences of the junction sites in translational fusion constructs and reading frames were confirmed by sequencing.
2.3. Expression and purification of fusion proteins from E. coli
Each C-terminal peptide-coding sequence that had been cloned in the pET-32a (+) expression vector, was expressed in BL21 (DE3) host cells as a fusion protein having N-terminal His-tag and S-tag domains according to the supplier’s recom-mendations using 1 mM IPTG to induce protein expression. Cells were grown at various tempera-tures for 1 – 3 h after addition of IPTG, then the fusion proteins were extracted from the cell pellets and purified by affinity chromatography with a His-Bind column according to the supplier’s rec-ommendations (Novagen). The expressed fusion proteins were separated by SDS-PAGE and de-tected by Western blot analysis.
2.4. Preparation of antibodies
A purified fusion protein expressed in E. coli was separated by SDS-PAGE and the CBB-visual-ized band corresponding to the estimated molecu-lar size was excised. The protein was eluted from
the gel by electroelution, then emulsified with Fre-und’s complete adjuvant (Difco) to a final volume of 1 ml. The fusion protein (500 mg) was injected subcutaneously at multiple sites on the backs of rabbits, and booster injections were administered on days 39 and 46 with 500 mg of the protein.
2.5. Preparation of 6acuoles
To isolate the anthocyanin-containing vacuoles, ALND callus (2 g wet weight) that had been cultured in 0.3 mg/l 2,4-D-containing PRL-4C agar medium for 1 week, was transferred to a 100-ml Erlenmeyer flask containing 20 ml of 2,4-D free liquid medium then cultured under continu-ous illumination. The vacuoles were isolated from 6 – 8-day-old suspension-cultured cells as described previously [4,5].
2.6. Protein extraction
Suspension-cultured cells were harvested as de-scribed previously [6]. Cultured cells (500 mg fresh weight) were homogenized on ice with a mortar and pestle in 750 ml of extraction buffer M (25 mM MES – Tris, pH 6.8, containing 0.5 M NaCl, 0.3 M sucrose and 3 mM MgCl2) with 50 mg of
Polyclar AT, 10 ml of Protease inhibitor cocktails for use with plant cell extracts (Sigma) and 500 mg of quartz sand. The homogenate was centrifuged at 3000 g for 3 min at 4°C, and the supernatant
was stored at −40°C prior to analysis. Protein content was determined as described by Bradford [10] with bovine serum albumin as the standard.
2.7. Protein detection
The fusion proteins extracted from E. coli were separated by SDS-PAGE as described by Laemmli [11] and detected by CBB staining or Western blot analysis using S-protein alkaline phosphatase con-jugate to identify the S-Tag (Novagen).
Proteins extracted from the cultured cells or vacuoles were separated by SDS-PAGE, followed by Western blot analysis with antibodies against VP24 (1:1000 dilution) and against the fusion protein B-3 (F-B3) (1:3000 dilution) as the primary antibody, as described previously [6].
analyzed by the concanavalin A/peroxidase method [12] using Lectin kit A-Per (Seikagaku Co., Japan).
3. Results
3.1. Expression of recombinant proteins in E. coli
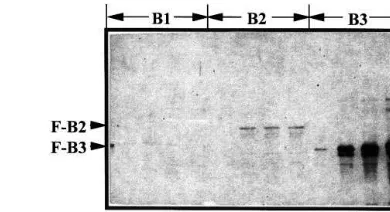
In order to develop antibodies against the C-ter-minal propeptide in the VP24 protein precursor, three parts of the C-terminal propeptide were ex-pressed as fusion proteins inE. coli. The C-termi-nal propeptide including eight putative transmembrane domains was divided into B1, B2 and B3 (Fig. 1A, B), and cDNAs encoding each part were cloned in expression vectors pET-32a (+); the resulting plasmids were called pET – B1, pET – B2 and pET – B3 (Fig. 1C), respectively. pET-B1 and pET-B2 contained the insert encoding the full length C-terminal propeptide (60.0 kDa), and the partial C-terminal propeptide including 5 putative transmembrane domains (46.4 kDa), re-spectively. PET – B3 contained the insert encoding the 19.6 kDa peptide that did not included any transmembrane domains. Expression of these re-combinant proteins inE. coli was examined under various culture conditions, then the fusion proteins were extracted and detected by Western blot analysis using S-protein alkaline phosphatase conjugate to identify the S-Tag (Fig. 2). A large amount of a fusion B3 (F-B3) protein was ex-pressed inE. coli cultured at 37°C for 1 – 3 h after addition of IPTG and obtained primarily in the soluble fraction, but little fusion B1 (F-B1) protein which contained the highly hydrophobic domains, was detected in any of the subcellular fractions examined. A small amount of fusion B2 (F-B2) protein was detected by Western blot analysis, but not by CBB staining of the gel after SDS-PAGE. Growth ofE. coli harboring pET – B1 or pET – B2 was extremely slow at the initial experimental tem-perature. Therefore, F-B3 protein which was over-expressed in E. coli after treatment with IPTG (Fig. 3 A) was purified by affinity column chro-matography (Fig. 3B). The purified fusion protein was still contaminated by a few additional bands of proteins, therefore protein further purified by SDS-PAGE was used for immunization of a rab-bit. We tried to remove the upstream domain of B3 region in the fusion protein (F-B3) by
enteroki-Fig. 2. Expression of fusion proteins in bacterial cells.E.coli, BL21 (DE3) harboring each expression vector was grown at 37°C for 1 – 3 h after addition of 1 mM IPTG. After centrifu-gation of 500ml of each culture, total protein was extracted by heating 50ml of SDS-PAGE sample buffer from each cell pellet at 70°C for 5 min. A 3.5 ml sample was subjected to SDS-PAGE with a 12.5% (w/v) polyacrylamide gel, and fu-sion proteins were detected by Western blot analysis using protein alkaline phosphatase conjugate to identify the S-Tag (Novagen). B1, B2 and B3 were the three parts of the C-terminal propeptide used for fusion constructs as shown in Fig. 1. Predicted molecular sizes of fusion proteins, F-B1, F-B2 and F-B3 were 77.8, 64. 1 and 37.3 kDa, respectively. M, Molecular mass standards. Arrow heads indicate F-B2 and F-B3.
nase (Fig. 1C), but could not obtain the target protein probably due to its structure.
High-titer antiserum was obtained 7 days after the final injection. The specificities of the resulting antibody directed against the E. coli-expressed F-B3 protein was assessed by Western blot analy-sis (Fig. 4, lane 1). A 36-kDa protein in ALND cells was found to react with the antibodies in addition to the 37.3-kDa F-B3 protein (antigen) (Fig. 4, lane 2). Only the B3 region of the F-B3 protein that had been translated in vitro using Single tube protein™ system 3 (Novagen) was detected with the antibodies (data not shown). These results suggested that the antibodies may recognize and react to the B-3 protein in the C-terminal region of VP24 protein precursor.
3.2. Light-induced expression of a 36-kDa 6acuolar protein (VP36)
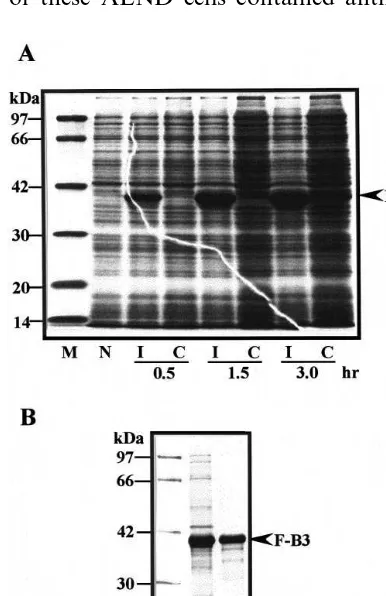
protein (VP36) in the protein extract from sweet potato suspension cells cultured under continuous illumination, but was not detected in the dark-cul-tured cells (Fig. 5A, lane 2). VP24 was also only detected in the illuminated cells (Fig. 5A, lane 1).
3.3. Vacuolar localization of VP36
To confirm the vacuolar localization of VP36, vacuoles were prepared from the anthocyanin-con-taining protoplasts of sweet potato ALND cells in suspension culture that had been cultured for 7 days under continuous illumination. More than 95% of these ALND cells contained anthocyanin
Fig. 4. Western blot analysis of total protein extract of ALND cells using polyclonal antibodies raised against the fusion protein B3 (F-B3). Total protein extract was prepared from 8-day-old ALND cells that had been cultured in 2,4-D-free liquid PRL-4C medium under continuous illumination, and then the sample (20mg of protein per lane) was subjected to SDS-PAGE (12.5% gel) and analyzed by Western blotting. Lane 1, A 37.6 kDa fusion protein B3 (F-B3) used as antigen for preparation of polyclonal antibodies; Lane 2, Crude protein extract of ALND cells. M, Molecular mass standards.
Fig. 3. Expression and purification of fusion protein, F-B3 from bacterial cells. (A) CBB stained gel after SDS-PAGE of total protein expressed in E. coli, BL21 (DE3) harboring pET – B3. Bacterial cells were incubated for 0.5, 1.5 and 3.0 h with 1 mM IPTG (I) or without IPTG (C) at 37°C. Total protein was extracted from each cell pellet by the same procedure as described in Fig. 2. A 10 ml sample was sub-jected to SDS-PAGE with a 12.5% (w/v) polyacrylamide gel. The arrow indicates the predicted molecular size of F-B3 peptide. N, Total proteins extracted from bacterial host cells; M, Molecular mass standards. (B) SDS-PAGE of F-B3 pep-tide during the purification. Crude protein extract fromE.coli
expressing fusion protein B3 (F-B3) (lane 1) was purified by affinity chromatography using a His-Bind column (lane 2). The arrow head indicates F-B3.
in the vacuoles, and little contamination by other organelles was observed in this fraction prepared by methods described previously [5]. Samples pre-pared from both vacuoles and protoplasts, which contained almost the same amount of an-thocyanin, were subjected to SDS-PAGE and Western blot analysis using anti F-B3 serum (Fig. 5B). Almost the same or slightly more VP36 was detected in the vacuole fraction than in the proto-plast fraction. This result indicated that VP36 was predominantly localized in the vacuoles of the anthocyanin-containing sweet potato cells in sus-pension culture.
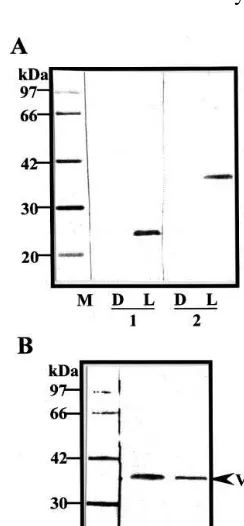
3.4. N-glycosylation of VP36
kDa. A 45-kDa protein was also detected as a minor protein by Western blot analysis and the glycoprotein-detection assay.
4. Discussion
Fusion proteins containing the putative transmembrane domains (B1 and B2) could not be expressed inE. coli. However, polyclonal antibod-ies against the hydrophilic domain (B3) in the C-terminal region of the VP24 protein precursor were developed in this study. A 36-kDa vacuolar protein (VP36) was found in ALND cells cultured under continuous illumination by Western blot
Fig. 6. Detection of glycosylated proteins in the vacuolar fraction prepared from 8-day-old ALND cells. The vacuolar protein (20mg) was subjected to SDS-PAGE (12.5% gel) and analyzed by Western blotting with antibodies against the fusion protein B3 (F-B3) (lane 1) and by the concanavalin A/peroxidase method (lane 2) as described in Section 2.7. M, Molecular mass standards. The arrow head indicates a 36-kDa protein.
Fig. 5. Expression of a 36-kDa protein in the anthocyanin-producing ALND cells. (A) Total protein extracts were pre-pared from the ALND cells that had been cultured in 2,4-D free liquid medium for 8 days under continuous illumination (L) or in darkness (D). Cell extracts (20 mg of protein per lane) were fractionated by SDS-PAGE (12.5% gel), with subsequent Western blot analysis with both antibodies against VP24 (lane 1) and the fusion protein B3 (F-B3) (lane 2). M, Molecular mass standards. (B) Detection of a 36-kDa protein in vacuoles prepared from the 7-day-old ALND cells by Western blot analysis. Vacuoles were isolated and purified by a method previously described [5]. Samples prepared from both vacuoles (lane 1) and protoplasts (lane 2) contained almost equal amounts of anthocyanin. They were subjected to SDS-PAGE (12.5% gel) and analyzed by Western blotting using polyclonal antibodies against the fusion protein B3 (F-B3). The arrow head indicates a 36-kDa protein.
analysis using these antibodies. A 45-kDa vacuolar protein was also detected as a minor protein (Fig. 6, lane 1), but whether VP36 is cleaved from VP45 in vivo or in vitro was not apparent. VP36 was expressed in the light-irradiated cells that con-tained high amounts of anthocyanin, but was not detected in the dark-cultured cells. Expression of this protein is very similar to that of VP24. A B3 peptide (19.6 kDa) prepared by an in vitro transla-tion system using a single tube protein™ system 3 (Novagen) was detected by Western blot analysis using the antibodies prepared in the present study (data not shown). This means the B3 peptide may be recognized by the antibodies, but not the N-ter-minal His-tag and S-tag domains of the fusion protein. The C-terminal region has five potential N-glycosylation sites (Fig. 1B), and it was confi-rmed that VP36 was a glycosylated protein by detection using the concanavalin A/peroxidase. These results indicate that VP36 may derive from the C-terminal region of the VP24 protein precur-sor. However, the exact position of VP36 in the C-terminal region has not yet been identified.
do-mains according to the predicted molecular size. To characterize the function of the C-terminal region of the VP24 protein precursor, and deter-mine whether other parts of the transmembrane domains are expressed in the tonoplast of an-thocyanin-producing sweet potato cells remains to be done.
The mature VP24 was detected as one of the major vacuolar proteins in the CBB-stained gel after SDS-PAGE, but the amount of VP36 ex-pressed in the vacuoles was not as large. The VP36 may be easier to degrade than VP24 in cells or during extraction.
Most vacuolar proteins in higher plants are synthesized as proprotein precursors in the rER, and then transported to the vacuoles [13]. Three different vacuolar sorting signals have been iden-tified in higher plants [14,15]. One type is localized in the N-terminal propeptide (NTPP), the second type is in the C-terminal propeptide (CTPP), while some vacuolar proteins contain an internal deter-minant in the mature protein. Although the vacuo-lar sorting signal of the VP24 protein precursor has not been identified, it is possible that the hydrophilic domain in the C-terminal region plays a role as vacuolar sorting signal for the VP24 protein precursor in sweet potato cells.
The mature VP24 protein was originally found to be a 24-kDa protein localized in cyanoplasts in the anthocyanin-containing vacuoles in sweet potato cells, and its expression was induced by exposure to light and closely paralleled the accu-mulation of anthocyanin [6]. Anthocyanins are synthesized by the cytosolic multi-enzyme complex [16,17], and effectively transported into the vac-uole through a Mg2+-ATP-dependent glutathione pump (GS-X pump) in the tonoplast [18]. The VP24 mature protein and the VP36 peptides derived from the VP24 protein precursor are local-ized in the vacuole in anthocyanin-producing cells. Therefore, they may be involved in transport or retention of anthocyanin in the vacuoles, rather than anthocyanin biosynthesis. Homology analysis of the eight transmembrane domains in the C-ter-minal region showed no similarities to other known vacuolar membrane proteins. Further anal-ysis of this C-terminal region, as well as character-ization of the vacuolar membrane proteins is necessary to clarify the biological function of the hydrophobic domains of the VP24 protein precur-sor in sweet potato.
5. Uncited reference
[19]
Acknowledgements
The authors thank Youko Ohmura, Shinshu University for her helpful assistance in the prepa-ration of vacuolar proteins. This study was carried out under the NIBB Cooperative Research Pro-gram (99-104).
References
[1] J. Politis, Cytological observations on the production of anthocyanins in certain Solanacea, Bull. Torrey Bot. Club 86 (1959) 387 – 393.
[2] R.C. Pecket, C.J. Small, Occurrence, location and devel-opment of anthocyanoplasts, Phytochemistry 19 (1980) 2571 – 2576.
[3] M. Nozue, H. Yasuda, Occurrence of anthocyanoplasts in cell suspension cultures of sweet potato, Plant Cell Rep. 4 (1985) 252 – 255.
[4] M. Nozue, H. Kubo, M. Nishimura, A. Katou, C. Hattori, N. Usuda, T. Nagata, H. Yasuda, Characteriza-tion of intravacuolar pigmented structures in an-thocyanin-containing cells of sweet potato suspension cultures, Plant Cell Physiol. 34 (1993) 803 – 808. [5] M. Nozue, H. Kubo, M. Nishimura, H. Yasuda,
Detec-tion and characterizaDetec-tion of a vacuolar protein (VP24) in anthocyanin-producing cells of sweet potato in suspen-sion culture, Plant Cell Physiol. 36 (1995) 883 – 889. [6] M. Nozue, K. Yamada, T. Nakamura, H. Kubo, M.
Kondo, M. Nishimura, Expression of a vacuolar protein (VP24) in anthocyanin-producing cells of sweet potato in suspension culture, Plant Physiol. 115 (1997) 1065 – 1072. [7] P. Klein, M. Kanehisa, C. DeLisi, The detection and classification of membrane-spanning proteins, Biochim. Biophys. Acta 815 (1985) 468 – 476.
[8] M. Nozue, J. Kawai, K. Yoshitama, Selection of a high anthocyanin-producing cell line of sweet potato cell cul-tures and identification of pigments, J. Plant Physiol. 129 (1987) 81 – 88.
[9] O.L. Gamborg, Aromatic metabolism in plants. II. En-zymes of the shikimate pathway in suspension cultures of plant cells, Can. J. Biochem. 44 (1966) 791 – 799. [10] M.M. Bradford, A rapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[11] U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
[13] T.W. Okita, J.C. Rogers, Compartmentation of proteins in the endomembrane system of plant cells, Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 (1996) 327 – 350. [14] F. Marty, Plant Vacuoles, Plant Cell 11 (1999) 587 – 599. [15] J.-M. Neuhaus, J.C. Rogers, Sorting of proteins to vacuoles in plant cells, Plant Mol. Biol. 38 (1998) 127 – 144.
[16] G. Hrazdina, G.J. Wagner, Compartmentation of plant phenolic compounds; sites of synthesis and accumula-tion, Ann. Proc. Phytochem. Soc. Europe 25 (1985) 119 – 133.
[17] I.E. Burbulis, B. Winkel-Shirley, Interactions among enzymes of the Arabidopsis flavonoid biosynthetic path-way, Proc. Natl. Acad. Sci. USA 96 (1999) 12929 – 12934.
[18] K.A. Marrs, M.R. Alfenito, A.M. Lloyd, V. Walbot, A glutathione S-transferase involved in vacuolar transfer encoded by the maize geneBronze-2, Nature 375 (1995) 397 – 400.
[19] J. Kyte, R.F. Doolittle, A simple method for displaying the hydropathic character of a protein, J. Mol. Biol. 157 (1982) 105 – 132.