Summary We review developments in cone and seed pro-duction research during the past decade. We conclude that although cone induction techniques, including hormonal and cultural procedures, have been refined, they still do not fully overcome juvenility or noninductive environmental effects. The role pollen plays in enhancing seed production and the genetic quality of seed is discussed. Techniques for handling pollen are described, in vitro viability assays are reviewed, and we also examine recent developments in pollen collection and its artificial ripening.
Keywords: flowering, fruit development, gibberellins, hor-mones, pollen, seed orchard.
Introduction
Seed orchards are widely used to produce superior seed for breeding programs and silvicultural purposes. However, clas-sical seed orchard designs suffer from many constraints includ-ing high initial investment costs with a slow incorporation of genetic gain because of the long juvenile period and inconsis-tent flowering. Even in clonal orchards established with sexu-ally mature scions, significant seed production does not normally occur for 6--8 years. The initial wide spacing of clonal orchards makes roguing difficult without severely af-fecting the distribution and parental contribution of the re-maining clones. Finally, poor or irregular flowering is often observed in orchards that are not located on good flowering sites, and even on good sites, micro-site influences are impor-tant (Sweet 1992).
In an attempt to overcome the limitations of classical seed orchards, several alternative orchard designs have been devel-oped including meadow or hedged orchards (Carson et al. 1992, Sweet et al. 1992) and indoor or container orchards. Container orchards have been used successfully for birch breeding and seed production (Tyystjarvi and Pirttila 1984) and are under development for some conifers (Greenwood et al. 1979, Ross et al. 1986, Eysteinsson et al. 1993 ). All of the alternative orchard designs have the potential for fast incorpo-ration of genetic gain and flexibility in introducing improved genotypes. The container orchards exhibit more consistent flowering than classical orchards because it is possible to accelerate maturation in them, by rapidly increasing crown
size and complexity, and to control temperature and water availability during induction, pollination and seed cone matu-ration. It should also be easier to study environmental effects on parental development (Johnsen 1989a, 1989b), reproduc-tive development and fertilization (Johnsen et al. 1995) in container orchards than in classical orchards. In addition to orchard design, specific improvements to orchard seed produc-tion include the use of inducproduc-tion treatments, supplemental mass pollination and crop protection (frost and insect control). Seed orchards are not the only delivery tool for tree improve-ment. Asexual propagation techniques, including micropropa-gation (e.g., Sutton et al. 1993 for white spruce (Picea glauca (Moench) Voss)), have been developed, but the cost per propagule is high. Both rooted cuttings (Ritchie 1991), and the more exotic emblings obtained from somatic embryogenesis (e.g., Webster et al. 1990 on Picea and Lelu et al. 1994 on Larix) are close to operational production.
Stimulation of flowering
Our ability to manipulate cone and seed production is still determined largely by juvenility and environmental condi-tions. Furthermore, the mechanisms underlying cone induction techniques remain largely unknown. A few studies have de-tailed the role of specific hormones in flowering (Moritz 1989, Pharis 1991), but the mechanism controlling the first few steps of flowering has not been elucidated (Pharis et al. 1987).
There are many examples of successful flower induction treatments in conifers (Wheeler et al. 1985, Ross and Bower 1989, Wheeler and Bramlett 1991, Ross 1991). Flower induc-tion treatments range from stem girdling, root pruning, heat treatments (within controlled environment facilities) and gen-eral fertilizer treatments to specific hormone applications (see reviews by Bonnet-Masimbert 1987, Owens 1991). Eucalyp-tus sp. exhibit a flowering response to anti-GAs, like paclobu-trazol (Cauvin 1992, Griffin et al. 1993).
It has been suggested that roots play a key role in flower induction (Bonnet-Masimbert et al. 1982, Bonnet-Masimbert and Zaerr 1987) because root pruning promotes flower induc-tion, and other flower induction treatments, including stem injection of GAs, reduce root activity (Figure 1). However, little has been done to characterize the effects of rootstock on flowering (Schmidtling 1983), a task complicated by the fact
From flower induction to seed production in forest tree orchards
M. BONNET-MASIMBERT
1and J. E. WEBBER
21 INRA, Station d’Amélioration des Arbres Forestiers, Ardon 45160, France
2 British Columbia Ministry of Forests, Glyn Road Research Station, 1320 Glyn Road, Victoria, B.C. V8W 3E7, Canada
Received August 10, 1994
that grafting stimulates flowering independently of the quality of the rootstock.
Nitrogen fertilization is widely used but we do not know whether nitrogen is important for induction, bud development, or facilitating the steps from flower to fruit or seed maturation. Daoudi et al. (1991, 1994) studied the effects of nitrate fertili-zation (N) with or without a stem injection of GA4/7 on flow-ering in 6- to 8-year-old cuttings of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco). No control trees flowered, and the maximum flowering response was observed in the N + GA4/7 treatment. As observed in previous studies, N increased total amino acids, especially arginine (e.g., Barnes and Bengston 1968, Ebell and McMullan 1970) and putrescine (e.g., Barnes and Bengston 1968, Cabanne et al. 1977, Rohozinski et al. 1986). The combined N + GA4/7 treatment had a synergistic effect on the increase in arginine, proline, putrescine, spermid-ine and spermspermid-ine content of the shoots. The increased content of nitrogenous compounds was observed for up to 10 weeks after the combined treatments were applied, including the period when flower-bud initiation occurred.
There is increasing evidence that specific GAs and their metabolites are involved in flowering (Moritz 1989, Pharis 1991). A mixture of the less polar GAs, GA4 and GA7, induces flowering in Pinaceae species, whereas GA3 is commonly used for Cupressaceae and Taxodiaceae species. Doumas et al. (un-published results, cited by Pharis 1991) observed higher con-centrations of GA4 and GA7 in shoots and primordia of flowering Douglas-fir than in similiar organs of nonflowering Douglas-fir. Primordia with a high potential to flower have 50 to 80 times more GA7 than primordia on nonflowering Douglas-fir trees (16 ng g−1 dry weight (DW) for control trees). Root pruning applied in the absence of an exogenous applica-tion of GA4/7 increased the amount of GA7 present in primordia (188 versus 831 ng g−1 DW, respectively). When both root pruning and GA4/7 treatments were applied together, a strong synergistic increase in GA7 was observed (1264 ng g−1 DW). Gibberellin treatments are normally applied in combination with other induction treatments because the combination often results in a synergistic response.
Other phytohormones, including cytokinins may also be
involved in flowering. Induction of flowering by root flooding with or without GA4/7 stem injection results in an increase in the concentration of endogenous isopentenyladenine (iPA) in Douglas-fir shoots 3--6 weeks after bud burst (Imbault et al. 1988, Pilate et al. 1990). Moreover, an exogenous application of iPA stimulates the female flowering response on lateral shoots of potted Douglas-fir trees (Imbault et al. 1988). Single stem girdling, root flooding or GA4/7 spray treatment applied to 4-year-old cuttings of Douglas-fir at bud burst decreased the iPA concentration of roots and increased the iPA concentration of lateral shoots compared to the controls (Figure 2) (Lucas et al. 1991, Hattier, unpublished observations), suggesting either modified cytokinin biosynthesis and metabolism, or modified transport.
Treatments that stimulate flowering usually increase, by 30 to 75%, the number of developing axillary meristems of all types: latent, sexual or vegetative (e.g., Bonnet-Masimbert 1988). Similar increases in axillary meristems have been ob-served between good and poor flowering years (Allen and Owens 1972). These observations support Greenwood’s (1981) conclusion that the first effect of flower induction treatments is to stimulate development of lateral apices. The second step is the growth of these apices and their conversion to sexual buds. Greenwood’s conclusion and the finding that combinations of hormonal and cultural treatments are often synergistic suggest that the hormonal and cultural treatments affect different stages of the flowering process. Some cultural treatments might create a delay between vegetative develop-ment (shoot elongation and possibly root growth) and bud development. Owens et al. (1986) observed that, in Douglas-fir, root pruning retards the rate of axillary bud development during shoot elongation. Normal bud activity resumed about Figure 1. Effects of GA4 and GA7, with or without stem girdling, on
root growth of 6-year-old Douglas-fir cuttings. Root growth is ex-pressed as the number of actively growing roots at Ti over its number at bud burst (T0). Bars with an asterisk (*) are significantly different at P < 0.05 from the control.
mid-July, and trees that exhibited the most severe retardation in bud development had the heaviest flowering.
Pollen
Pollen is a powerful tool for manipulating the genetic compo-sition of production seed lots. To exercise maximum control over contributing pollen parents, some form of pollen handling ex situ is necessary. There are several steps in handling pollen ex situ that are critical to maintaining pollen fertility (Stanley and Linskens 1974, Franklin 1981, Owens and Blake 1985, Blackmore and Knox 1990, Webber 1991, Jett et al. 1993, Bramlett et al. 1993, Webber and Painter 1995). Both stored and freshly collected pollen can be used in small-lot breeding crosses or large volume supplemental mass pollination (SMP) programs in seed orchards. However, competing pollen cloud density, application technique and pollen viability all affect SMP success (Webber 1995).
The advantages of increasing or controlling pollen supply to open pollinated orchards have been well documented (Bridg-water and Trew 1981, Bridg(Bridg-water et al. 1993). Supplemental mass pollination has been used to improve genetic efficiency (El-Kassaby and Ritland 1986a, Askew 1992, Bridgwater et al. 1993) and to reduce the effect of contaminating pollen (El-Kassaby and Ritland 1986b). However, where paternal analy-ses have been completed, the effectiveness of SMP has been generally low (Wheeler and Jech 1985, El-Kassaby et al. 1993, Eriksson et al. 1994, Webber 1995).
Pollen collection
Pollen is collected by either hand-picking cones or artificially maturing cut branches. Mass pollen collection using vacuum systems has been successfully used in Douglas-fir orchards (Copes et al. 1991). One person using this device can collect 1.5 l of pollen per hour from 10--15-m tall trees. Pollen viabil-ity of the vacuum-collected pollen is as good or better than that of naturally shed pollen. An expensive system developed by Philippe and Baldet (1992) for Larix sp. is useful for species with limited pollen production and for species for which it is difficult to force unripe male buds. The entire tree is tightly enclosed in a tank fixed to a tractor. Branches are whipped by blasting compressed air in semirigid pipes, and the pollen shaken from male strobili is sucked through a filtering system. Because the collection only takes about 1.5 min for a 4-m tall tree, several collections can be made during the pollen matu-ration phase of the tree.
Artificial pollen ripening
There are a few studies on the effects of artificial ripening on pollen yield and fertility. Colangeli and Owens (1991) studied the cytological effects on forcing western hemlock (Tsuga heterophylla (Raf.) Sarg.) pollen buds in water at room tem-perature. Forcing buds too early (i.e., before meiotic activity) resulted in reduced yields and reduced fertility potential. Ross (1988) also found lower yields per cone, increased proportions of underdeveloped pollen and poorer pollen quality when pollen cone development was hastened by 21 days by subject-ing potted Engelmann spruce (Picea englemannii Parry ex Engelm.) stock to elevated temperature and humidity in a closed polyethylene-covered greenhouse. Philipson et al. (1990) exposed potted Sitka spruce (Picea sitchensis (Bong.) Carr.) grafts to an elevated temperature when the pollen-cone buds were at their premeiotic stage and found that the tempera-ture treatment hastened cone-bud development but reduced both pollen yield and viability. They attributed the poor pollen viability to abnormalities in the latter stages of pollen develop-ment (not meiosis) and the poor seed developdevelop-ment to retarded development of the distal portion of the cones.
An important step in evaluating the effects of forcing pollen has been the development of reliable methods for assessing the amount of extracted pollen. Extraction efficiency or the ratio of extracted pollen volume to pollen-cone bud volume is the most widely used method. Extraction efficiency values range from 5--6% for larch, 4--6% for Douglas-fir, 10--15% for lodgepole pine (Pinus contorta var. latifolia Engelm. ex S. Wats.), and up to 20% for spruce (Webber, unpublished obser-vations). However, the actual volumes realized depend on the stage of elongation of the catkins or strobili. Generally, the greater the space between the microsporangia, the greater the volume extracted; however, the characteristics of pollen grains and the amount that can be expected from one male bud are species specific. Table 1, adapted from Stanley and Linskens (1974), gives examples of such variation.
When defining conditions for artificially maturing catkins to dehiscence, it is important to consider temperature, relative humidity, light, shoot water relations and nutrients, either from the shoot or within the culture solutions for cut branches (Stanley and Linskens 1974). In an experiment (summarized in Table 2) by Mellerowicz (1983), the effects of two environ-mental conditions (greenhouse and laboratory) with different values and amplitude of temperature and relative humidity were assessed in terms of pollen viability and amount of
Table 1. Characteristics of pollen size and pollen yield for some conifer and broad-leaved species (adapted from Stanley and Linskens 1974).
Species Length Width Volume of 1 million Weight of 1 million Volume of pollen per
(µm) (µm) pollen grains (10−3 ml) pollen grains (10−3 g) 100 catkins or strobili (ml)
Pinus 41.5 45.9 35.5 37.0 150
Pseudotsuga 84.8 81.1 219.2 188.8 2
Larix 76.0 72.0 180.2 176.3 0.3
Alnus 26.4 22.8 4.4 1.4 4
Betula 10.1 10.1 2.9 0.8 12
extracted pollen. The treatments were applied to cut branches of Alnus glutinosa (L.) Gaertn. that bore male catkins at either the elongation (ripening) or dehiscence phase. The more vari-able greenhouse conditions resulted in about six times more pollen being extracted than the less variable laboratory condi-tions. Some clonal variability for the response to environ-mental conditions was also observed. The treatments had little effect on pollen viability (measured by the fluorochromatic test, Heslop-Harrisson and Heslop-Harrisson 1970).
Pollen testing
In vitro pollen viability is a useful measure of the potential of pollen to set seed in a control cross-pollination. However, the in vitro pollen viability of individual pollen lots used within a pollen mix (i.e., polymix mating) cannot be used to predict mating probabilities of individual lots. The assumptions that all male parents have the same probability of mating and that paternal contributions to a polymix mating are equal are sel-dom valid (Moran and Griffin 1985, Schoen and Cheliak 1987, Wiselogel and van Buijtenen 1988). Differential male success occurs and can have both a physiological and a genetic basis (Apsit et al. 1989). Even for equally viable pollen lots, the importance of prezygotic events affecting the competitive abil-ity of pollen during germination within the micropyle, tube penetration of the nucellus and egg cells, and archegonial development must be considered (Nakamura and Wheeler 1992, Crook and Friedman 1992). Little is known about either paternal--maternal interactions or environmental effects on fer-tilization and seed development in conifers.
Pollen competition has been described in various angio-sperm species (Snow and Spira 1991), and variability in pater-nal performance can lead to nonrandom mating. Because of the pollen population size, gametophytic selection may influence traits such as tolerance to biotic and abiotic stresses and plant vigor (Ottaviano and Mulcahy 1989). It may be that pollen competition and gametophytic selection do not occur in coni-fers because few pollen grains actually compete at the mi-cropyle or archegonial level; however, when paternal genotypes compete at the tree or orchard level, the number of pollen grains involved is large.
There are numerous tests of in vitro pollen viability (see Stanley and Linskens 1974) and some tests provide a direct measure of pollen fertility (Moody and Jett 1990, Webber and Bonnet-Masimbert 1993). Assays include germination on an artificial medium, conductivity measurements of pollen leachates (Ching and Ching 1976), respiration of pollen in an
aqueous solution (Binder and Ballantyne 1975), and the fluorochromatic test (Heslop-Harrison and Heslop-Harrison 1970). For most of these tests, the pollen must be precondi-tioned (i.e., hydrated) because a minimum pollen water con-tent is critical for membrane continuity and enzyme activity. Under natural conditions, pollen hydration is essential as the first step toward germination (Heslop-Harrison 1987). Pollen prehydration is necessary before in vitro germination occurs in Douglas-fir (Charpentier and Bonnet-Masimbert 1983) or lob-lolly pine (Pinus taeda L.) (Jett and Frampton 1990). In Douglas-fir, pollen prehydration improves conductivity meas-urements (lowers response), but it has little effect on respira-tion tests (Webber and Bonnet-Masimbert 1993). Actual assay responses to prehydration effects vary with pollen water con-tent (Charpentier and Bonnet-Masimbert 1983, Jett and Frampton 1990) and are probably species specific.
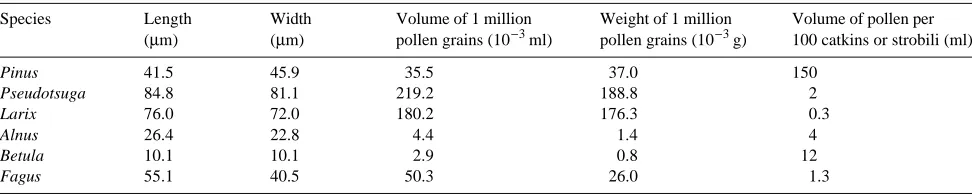
There are several difficulties associated with interpreting the results of in vitro pollen germination tests as exemplified in a study of hydrated and nonhydrated Scots pine (Pinus sylvestris L.) pollen (Figures 3A and 3B). Four germination media were used: 10% Brewbaker and Kwack (1963) solution used alone (BK10), BK10 with the addition of two concentra-tions of polyethylene glycol-4000 (P), 10% (BK10 + P10) or 20% (BK10 + P20), and BK10 with 10% sucrose (BK10 + S10). Pollen was hydrated for 4 h at 20 °C in an atmosphere with 100% relative humidity. The nonhydrated pollen had an ambient water content of about 8%. Three methods of germi-nating pollen (Stanley and Linskens 1974) in a dark incubator at 20 °C were compared (Figure 3C): (1) a spot test in which pollen was dusted on the top of a drop (75 µl) of medium on a slide placed in a petri dish, (2) a hanging drop test made by dusting the pollen on a drop as for method (1) and then inverting the slide, and (3) a hanging drop (150 µl) test in an inverted Eppendorf tube.
After 24 h, pollen germination was determined by counting those pollen grains having a tube length that was at least twice the diameter of the hydrated, ungerminated grain. The mor-phology of pollen tube growth was classified in four groups (Figure 3C): (1) pollen tubes that were straight and clear without any branching (considered normal), (2) pollen tubes that were clear but showed some branching, (3) pollen tubes that were opaque (dark grains that are likely starch in the tubes) and often had some branching, and (4) pollen tubes that were straight and clear but exhibited swelling of the subterminal part.
The results shown in Figure 3 demonstrate that the assay Table 2. Effects of environmental conditions on maturation and shedding of Alnus glutinosa pollen (from Mellerowicz 1983): G = Greenhouse (temperature variation (T) = 13.9 and 24.8 °C (average 16.8 °C), relative humidity variation (RH) = 35 to 70%), L = Laboratory (T = 14.4 to 19.2 °C (average 17.4 °C), RH = 41 to 53% (average 46.5%)), FCR+ = positive response to fluorochromatic viability test.
Conditions during Conditions during Amount of pollen Viability
catkin elongation pollen dehiscence per catkin (mg) (% FCR+)
G G 17.7 77.4
L G 20.3 77.9
G L 9.7 88.0
conditions had large effects on the in vitro germination re-sponse. There were significant interactions between germina-tion technique (the spot test was the best), pollen prehydragermina-tion (Figures 3A and 3B) and germination medium. Pollen grains in medium BK10 + S10 exhibited a high proportion of nonnor-mal tubes. Pollen germinated by the hanging drop technique exhibited normal tube growth, whereas pollen germinated in the Eppendorf tubes exhibited a high proportion of nonnormal tubes.
Based on these observations, we conclude that the in vitro germination response must be correlated to either in vivo germination or seed set before it can be used reliably to predict fertility. In loblolly pine, Moody and Jett (1990) found a high correlation (R2 = 0.82) between in vitro pollen germination on agar and in vivo pollen germination as well as a high coeffi-cient of determination (r2 = 0.79) between in vitro germination and percent filled seed per cone. Even if the germination conditions are optimized (i.e., for tube growth) for each spe-cies, the germination response may not necessarily reflect actual fertility under field conditions. Similar high correlations were found for three viability assays, including germination, and seed set for Douglas-fir (Webber and Bonnet-Masimbert 1993).
For conifers, the pollination mechanism, which can be wet or dry, active or passive (Owens and Blake 1985), must be considered when relating the results of a viability test to actual
fertility. For angiosperms, in which thousands of pollen grains can adhere to the stigma, a different approach to relating in vitro assay response to actual fertility is required than for conifers where only a few grains can be accommodated within the micropyle. Regardless of species, it should be possible to relate each assay type to seed yield and thereby establish a threshold value of pollen viability above which satisfactory seed yields are predicted.
Pollen drying and storage
Pollen of most conifer species can be stored for at least 3 years provided handling ex situ or during forcing does not adversely affect viability. Recommended protocol includes the reduction of pollen water content to 7--8%, the placement of pollen in air-tight bottles or laminated foil packages, preferably under vacuum or nitrogen (Webber 1991, Webber and Painter 1995), and the storage of pollen at temperatures below −20 °C or in liquid nitrogen (see Stanley and Linskens 1974, Owens and Blake 1985, Webber 1987, Copes 1987, Jett et al. 1993).
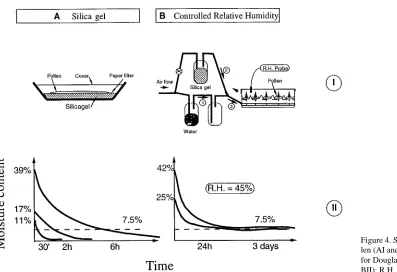
Drying pollen cone buds to a constant water content is relatively easy using internal air-conditioning systems (Eriksson 1993, Webber and Painter 1995). Drying extracted or shed pollen is more difficult. For any given species, water content can vary quickly depending on the atmosphere, the temperature and the size of the pollen lot. For small quantities of pollen, drying over silica gel (Figure 4AI) is common. However, this technique is difficult because the time to reach a desired water content (7.5%) depends on the initial water content (Figure 4AII), and the technique does not allow for continuous monitoring of water loss.
A better method is to dry pollen under controlled conditions as described in the schematic drawing shown in Figure 4B. Air is humidified by bubbling it through water (1) and then mixed with the appropriate volume of dry air (2) to obtain the desired humidity. This system allows control over both temperature and humidity (3) and was tested on various Douglas-fir pollen lots that differed in initial water content and volume. At a given temperature, the final pollen water content depends only on the relative humidity of the air provided the pollen is exposed under the set conditions long enough to equilibrate (Figure 4BII).
Table 3 shows the effects of various air relative humidities at 20 °C on Douglas-fir pollen water content. We recommend that the pollen be exposed to a relative humidity of 45% at an air temperature of 20 °C for 24 h. Extended exposure periods (i.e., 3 days) had no detrimental effect on pollen viability and did not result in increased water content.
Conclusion
The techniques for controlling flowering have been refined during the past decade, despite a lack of fundamental knowl-edge about flowering mechanisms. However, there is increas-ing concern about the genetic quality and adaptability of seed produced by seed orchards. Efforts are now being directed toward understanding the interactions between environment and genotypes at all stages of the reproductive process. Figure 3. Effects of germination medium and germination test on the
References
Allen, S. and J.N. Owens. 1972. The life history of Douglas-fir. Canadian Forest Service, Ottawa, Ontario, Inform. Can. Rep. No. Fo42-4972, 139 p.
Askew, G.R. 1992. Potential genetic improvement due to supplemen-tal mass pollination in conifer seed orchards. For. Ecol. Manage. 47:135--147.
Apsit, V.J., R.R. Nakamura and N.C. Wheeler. 1989. Differential male reproductive success in Douglas-fir. Theor. Appl. Genet. 77:681--684.
Barnes, R.L. and G.V. Bengston. 1968. Effect of fertilization, irriga-tion, and cover cropping on flowering and on nitrogen and soluble sugar composition of slash pine. For. Sci. 14:172--180.
Binder, W.D. and D.J. Ballantyne. 1875. The respiration and fertility of Pseudotsuga menziesii (Douglas-fir) pollen. Can. J. Bot. 53:819--823.
Blackmore, S. and R.B. Knox. 1990. Microspores: evolution and ontogeny. Academic Press, New York, 347 p.
Bonnet-Masimbert, M. 1987. Floral induction in conifers: a review of available techniques. For. Ecol. Manage. 19:135--146.
Bonnet-Masimbert, M. 1988. Hormones végétales et floraison chez Pseudotsuga menziesii (Mirb.) Franco. Bull. Soc. Bot. Fr. Actual. Bot. 4:7--18.
Bonnet-Masimbert, M., P. Delanzy, G. Chanteloup and J. Coupaye. 1982. Influence de l’état d’activité des racines sur la floraison induite par des gibbérellines 4 et 7 chez Pseudotsuga menziesii (Mirb.) Franco. Silvae Genet. 31:178--183.
Bonnet-Masimbert, M. and J.B. Zaerr. 1987. Hormonal control of tree growth. 2. The role of plant growth regulators in promotion of flowering. Plant Growth Regul. 6:13--35.
Bramlett, D.L., G.R. Askew, T.D. Blush, F.E. Bridgwater and J.B. Jett. 1993. Advances in pollen management. Agriculture Handbook 698, USDA Forest Service, Washington, DC, 101 p.
Brewbaker, J.G. and B.H. Kwack. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 50:859--865.
Bridgwater, F.E. and I.F. Trew. 1981. Supplemental mass pollination. In Pollen Management Handbook. Ed. E.C. Franklin. Agriculture Handbook 587, USDA Forest Service, Washington, DC, pp 52--57. Bridgwater, F.E., T.D. Blush and N.C. Wheeler. 1993. Supplemental mass pollination. In Advances in Pollen Management. Eds. E.D. Bramlett, G.R. Askew, T.D. Blush, F.E. Bridgwater and J.B. Jett. Agricultural Handbook 698, USDA Forest Service, Washington, DC, pp 69--77.
Cabanne, F., J. Martin-Tanguy and C. Martin. 1977. Phénolamides associées à l’induction florale et à l’état reproducteur de Nicotiana tabacum var. xanthi n.c. Physiol. Veg. 15:429--443.
Carson, M.J., T.G. Vincent and A. Firth. 1992. Control-pollinated and meadow seed orchards of radiata pine. In Syntheses of IUFRO Meeting on Mass Production for Genetically Improved Fast Grow-ing Forest Tree Species. AFOCEL, Paris, France, pp 100--109. Cauvin, B. 1992. Les effets du paclobutrazole applicables à la gestion
intensive des vergers à hybridation d’Eucalyptus. In Mass Produc-tion for Genetically Improved Fast Growing Forest Tree Species. AFOCEL, Paris, France, pp 117--123.
Table 3. Effect of equilibrating Douglas-fir pollen for 24 h at various relative humidities on pollen water content (on a fresh-weight basis).
Relative humidity of the air (%) Pollen water content (%)
65 12.0
60 11.3
55 10.0
50 9.2
45 7.6
Charpentier, J.P. and M. Bonnet-Masimbert. 1983. Influence d’une réhydratation préalable sur la germination in vitro du pollen de douglas (Pseudotsuga menziesii) après conservation. Ann. Sci. For. 40:309--317.
Ching, T.M. and K.K. Ching. 1976. Rapid viability tests and aging study of some coniferous pollen. Can. J. For. Res. 6:516--522. Colangeli, A.M. and J.N. Owens. 1991. Effects of accelerated
pollen-cone development on pollen cytology and fertilizing potential in western hemlock (Tsuga heterophylla). For. Ecol. Manage. 40:151--162.
Copes, D.L. 1987. Long-term storage of Douglas-fir pollen. For. Sci. 33:244--246.
Copes, D.L., N.C. Vance, W.K. Randall, A. Jasumback and R. Hall-man. 1991. Vacuum collection of Douglas-fir pollen for supplemen-tal mass pollinations. USDA Forest Service, Corvallis, OR, Research Note PNW-RN-503, 8 p.
Crook, R.W. and W.E. Friedman. 1992. Effects of pollen tube number and archegonium number on reproduction in Douglas-fir: signifi-cance for seed orchard management. Can. J. For. Res. 22:1483--1488.
Daoudi, E.H., M. Bonnet-Masimbert and J. Martin-Tanguy. 1991. Evolution des polyamines dans les bourgeons et les rameaux de Pseudotsuga menziesii (Mirb) Franco à la suite du passage de l’état végétatif à l’état floral. Ann. Sci. For. 48:367--376.
Daoudi, E.H., P. Doumas and M. Bonnet-Masimbert. 1994. Changes in amino acids and polyamines in shoots and buds of Douglas-fir trees induced to flower by nitrogen and gibberellin treatments. Can. J. For. Res. 24:1854--1863.
Ebell, L.F. and E.E. McMullan. 1970. Nitrogenous substances associ-ated with differential cone production responses of Douglas-fir to ammonium and nitrate fertilization. Can. J. Bot. 48:2169--2177. El-Kassaby, Y.A. and K. Ritland. 1986a. The relation of outcrossing
and contamination to reproductive phenology and supplemental mass pollination in a Douglas-fir seed orchard. Silvae Genet. 35:240--244.
El-Kassaby, Y.A. and K. Ritland. 1986b. Low level of pollen contami-nation in Douglas-fir seed orchard as detected by allozyme markers. Silvae Genet. 35:224--229.
El-Kassaby, Y.A., S. Barnes, C. Cook and D.A. MacLeod. 1993. Supplemental mass pollination success rate in a mature Douglas-fir seed orchard. Can. J. For. Res. 23:1096--1099.
Eriksson, U. 1993. A system for large scale extraction of Scots pine pollen. In Advances in Pollen Management. Eds. E.D. Bramlett, G.R. Askew, T.D. Blush, F.E. Bridgwater and J.B. Jett. Agricultural Handbook 698, USDA Forest Service, Washington, DC, pp 95--96. Eriksson, U., R. Yazdani, L. Wilhelmsson and Ö. Danell. 1994. Suc-cess rate of supplemental mass pollination in a seed orchard of Pinus sylvestris L. Scand. J. For. Res. 9:60--67.
Eysteinsson, T., M.S. Greenwood and J.E. Webber. 1993. Manage-ment of a prototype indoor orchard for accelerated breeding of larch. Maine Agric. Exp. Stn., Orono, ME, Misc. Rep. No. 377, 18 p.
Franklin, E.C. 1981. Pollen management handbook. Agriculture Handbook 587, USDA Forest Service, Washington, DC, 98 p. Greenwood, M.S. 1981. Reproductive development in loblolly pine.
II. The effect of age, gibberellin plus water stress and out-of-phase dormancy on long shoot growth behavior. Am. J. Bot. 68:1184--1190.
Greenwood, M.S., C.H. O’Gwynn and P.G. Wallace. 1979. Manage-ment of an indoor, potted loblolly pine breeding orchard. In Proc. 15th South. For. Tree Improve. Conf. Mississippi State University, Mississippi State, MS, pp 94--98.
Griffin, A.R., P. Whiteman, T. Rudge, I.P. Burgess and M. Moncur. 1993. Effect of paclobutrazol on flower-bud production and vegetative growth in two species of Eucalyptus. Can. J. For. Res. 23:640--647. Heslop-Harrison, J. 1987. Pollen germination and pollen-tube growth.
Int. Rev. Cytol. 107:1--78.
Heslop-Harrisson, J. and Y. Heslop-Harrisson. 1970. Evaluation of pollen viability by enzymatically induced fluorescence; intracellu-lar hydrolysis of fluorescein diacetate. Stain Technol. 45:115--120. Imbault, N., I. Tardieu, C. Joseph, J.B. Zaerr and M. Bonnet-Masim-bert. 1988. Possible role of isopentenyladenine and isopentenylade-nosine in flowering of Pseudotsuga menziesii: endogenous variations and exogenous applications. Plant Physiol. Biochem. 26:289--295.
Jett, J.B. and L.J. Frampton Jr. 1990. Effect of rehydration on in vitro germination of loblolly pine pollen. South. J. Appl. For. 14:48--51. Jett, J.B., J.B. Bramlett, J.E. Webber and U. Eriksson. 1993. Pollen collection, storage and testing. In Advances in Pollen Management. Eds. E.D. Bramlett, G.R. Askew, T.D. Blush, F.E. Bridgwater and J.B. Jett. Agricultural Handbook 698, USDA Forest Service, Wash-ington, DC, pp 41--46.
Johnsen, Ø. 1989a. Phenotypic changes in progenies of northern clones of Picea abies (L.) Karst. grown in a southern seed orchard. I. Frost hardiness in a phytotron experiment. Scand. J. For. Res. 4:317--330.
Johnsen, Ø. 1989b. Phenotypic changes in progenies of northern clones of Picea abies (L.) Karst. grown in a southern seed orchard. II. Seasonal growth rhythm and height in field trials. Scand. J. For. Res. 4:331--341.
Johnsen, Ø., T. Skrøppa, G. Haug, I. Apeland and G. Østreng. 1995. Sexual reproduction in a greenhouse and reduced autumn frost hardiness of Picea abies progenies. Tree Physiol. 15:551--555. Lelu, M.A., C. Bastien, K. Klimaszewska and P.J. Charest. 1994. An
improved method for somatic plantlet production in hybrid larch (Larix × lepteuropaea): Part 2. Control of germination and plantlet development. Plant Cell Tissue Organ Cult. 36:117--127. Lucas, I., P. Doumas and M. Bonnet-Masimbert. 1991. Comparison of
the effects of cultural treatments or gibberellin spray on endogenous cytokinins in Douglas-fir roots. In 14th Int. Conf. on Plant Growth Substances. Landbouwuniversiteit, Wageningen, Amsterdam, The Netherlands, p 131 (Abstract).
Mellerowicz, E. 1983. Pollen de Douglas, Mélèzes, Aulnes et peu-pliers. Bilan des recherches conduites en 1982--1983. Doc. Stat. Amél. Arbres Forestiers, INRA Orléans 6/83, 56 p (text) and 66 p (tables and figures).
Moody, W.R. and J.B. Jett. 1990. Effects of pollen viability and vigor on seed production of loblolly pine. South. J. Appl. For. 14:33--38. Moran, G.F. and A.R. Griffin. 1985. Non-random contribution of pollen in polycrosses of Pinus radiata D. Don. Silvae Genet. 34:117--121.
Moritz, T. 1989. Gibberellins and flower-bud differentiation in spruce. Dept. For. Genet. Plant Physiol., The Swedish Univ. Agric. Sci., Umeå, Sweden.
Nakamura, R.R. and N.C. Wheeler. 1992. Pollen competition and paternal success in Douglas-fir. Evolution 46:846--851.
Ottaviano, E. and D. Mulcahy. 1989. Genetics of angiosperm pollen. Adv. Genet. 26:1--64.
Owens, J.N. 1991. Flowering and seed set. In Physiology of Trees. Ed. A.S Raghavendra. John Wiley and Sons Inc., New York, pp 247--271.
Owens, J.N., J.E. Webber, S.D. Ross and R.P. Pharis. 1986. Interaction between gibberellin A4/7 and root-pruning on the reproductive and vegetative process in Douglas-fir. IV. Effects on lateral bud devel-opment. Can. J. For. Res. 16:211--221.
Pharis, R.P. 1991. Physiology of gibberellins in relation to floral initiation and early floral differentiation. In Symp. on 50th Anniver-sary Meeting on Isolation of Gibberellins. Eds. N. Takahashi, B.V. Phinney and J. MacMillan. Springer Verlag, Heidelberg, pp 166--178.
Pharis, R.P., J.E. Webber and S.D. Ross. 1987. The promotion of flowering in forest trees by gibberellin A4/7 and cultural treatments: a review of the possible mechanisms. For. Ecol. Manage. 19:65--84. Philippe, G. and P. Baldet. 1992. Mechanized pollen harvesting with a view to hybrid larch seed production. Ann. Sci. For. 49:297--303. Philipson, J.J., J.N. Owens and M.A. O’Donnell. 1990. Production
and development of seed and pollen in grafts of Picea sitchensis (Bong.) Carr. treated with gibberellin GA4/7 to induce coning and the effect of forcing treatments. New Phytol. 116:695--705. Pilate, G., B. Sotta, R. Maldiney, M. Bonnet-Masimbert and E.
Miginiac. 1990. Endogenous hormones in Douglas-fir trees in-duced to flower by gibberellin A4/7 treatment. Plant Physiol. Bio-chem. 28:359--366.
Ritchie, G.A. 1991. The use of conifer rooted cuttings in forestry: a world overview. New For. 5:247--275.
Rohozinski, J., G.R. Edwards and P. Hoskyns. 1986. Effect of brief exposure to nitrogenous compounds on floral initiation in apple trees. Physiol. Veg. 24:673--677.
Ross, S.D. 1988. Pre- and post-pollination polyhouse environment effects on pollen and seed development in potted Picea engelmannii grafts. Can. J. For. Res. 18:623--627.
Ross, S.D. 1991. Operations manual for container seed orchards of interior spruce. Internal Report, B.C. Ministry of Forests, Research Branch, Victoria, B.C., Canada, 55 p.
Ross, S.D., A.M. Eastham and R.C. Bower. 1986. Potential for con-tainer seed orchards. In Proc. Conifer Tree Seed in the Inland Mountain West Symp. Ed. R.C. Shearer. USDA Forest Service, Missoula, MT, Gen. Tech. Rep. INT-203, pp 180--186.
Ross, S.D. and R.C. Bower. 1989. Cost-effective promotion of flow-ering in a Douglas-fir seed orchard by girdling and pulsed stem injection of gibberellin A4/7. Silvae Genet. 38:189--195.
Schoen, D.J. and W.M. Cheliak. 1987. Genetics of the polycross. 2. Male fertility variation in Norway spruce, Picea abies (L.) Karst. Theor. Appl. Genet. 74:554--559.
Schmidtling, R.C. 1983. Rootstock influences flowering, growth and survival of loblolly pine grafts. For. Sci. 29:117--124.
Snow, A. and T.P. Spira. 1991. Pollen vigour and the potential for sexual selection in plants. Nature 352:796--797.
Stanley, R.G. and H.F. Linskens. 1974. Pollen: biology, biochemistry and management. Springer-Verlag, New York, 307 p.
Sutton, B.C.S., S.C. Grossnickle, D.R. Roberts, J.H. Russel and G.K. Kiss. 1993. Somatic embryogenesis and tree improvement in inte-rior spruce. J. For. 91:34--38.
Sweet, G.B. 1992. Seed orchard research and management in the 1990s----a New Zealand case study. In Syntheses of IUFRO Meeting on Mass Production for Genetically Improved Fast Growing Forest Tree Species. AFOCEL, Paris, France, pp 110--118.
Sweet, G.B., R.L. Dickson, B.D. Donaldson and H. Litchwark. 1992. Controlled pollination without isolation----a new approach to the management of radiata pine seed orchards. Silvae Genet. 41:95--99. Tyystjarvi, P. and V. Pirttila. 1984. Seed production of hardwoods
under plastic. Metsänjalostus-säätiö, Inform. Rep. 1/1984, 4 p. Webber, J.E. 1987. Increasing seed yield and genetic efficiency in
Douglas-fir seed orchards through pollen management. For. Ecol. Manage. 19:209--218.
Webber, J.E. 1991. Interior spruce pollen management manual. B.C. Ministry of Forests, Research Branch, Land Manage., Victoria, B.C., Canada, Report No. 70, 25 p.
Webber, J.E. 1995. Pollen management for intensive seed orchard production. Tree Physiol. 15:507--514.
Webber, J.E. and M. Bonnet-Masimbert. 1993. The response of dehy-drated Douglas-fir (Pseudotsuga menziesii) pollen to three in vitro viability assays and their relationship to actual fertility. Ann. Sci. For. 50:1--22.
Webber, J.E. and R.A. Painter. 1995. Douglas-fir pollen management manual. 2nd Edn. B.C. Ministry of Forests, Research Branch, Victoria, B.C., Canada, Work. Paper 02/1995. In press.
Webster, F.B., D.R. Roberts, S.M. McInnis and B.C.S. Sutton. 1990. Propagation of interior spruce by somatic embryogenesis. Can. J. For. Res. 20:1759--1765.
Wheeler, N.C. and K.S. Jech. 1985. Estimating supplemental mass pollination (SMP) success electrophoretically. In Proc. 20th Can. Tree Improve. Assoc. Meeting, Part 2. Quebec City, Quebec, Can-ada, pp 111--120.
Wheeler, N.C., C.J. Masters, S.C. Cade, S.D. Ross, J.W. Keeley and L.Y. Hsin. 1985. Girdling: an effective and practical treatment for enhancing seed yields in Douglas-fir seed orchards. Can. J. For. Res. 15:505--510.
Wheeler, N.C. and D.L. Bramlett. 1991. Flower stimulation in a loblolly pine seed orchard. South. J. Appl. For. 15:44--50. Wiselogel, A.E. and J.P. van Buijtenen. 1988. Probability of equal