Identification of light- and nitrate-responsive regions of the nitrate
reductase promoter from birch
Tim Strater, Wolfgang Hachtel *
Botanisches Institut,Uni6ersita¨t Bonn,Kirschallee1,D-53115Bonn,Germany
Received 8 February 1999; received in revised form 19 August 1999; accepted 10 September 1999
Abstract
The regulation of nitrate reductase (NR) gene expression in higher plants involves both internal and external factors. In birch (Betula pendula Roth), the transcription of the NR gene depends on light and nitrate in both leaves and roots. In order to investigate the transcriptional regulation of the birch NR gene, a 1.6 kb 5% flanking sequence of the NR gene was isolated, sequenced, and the transcription start site was determined. The 1.6 kb sequence and eight 5%deleted versions of the promoter
sequence were each fused with theb-glucuronidase (GUS) reporter gene (uidA) and introduced into Nicotiana plumbaginifolia. GUS expression was examined in primary transformants that were transferred from dark to light or from a medium without nitrate to a nitrate-containing medium. A detailed analysis revealed the presence of light- and nitrate-responsive promoter regions, respectively. Several promoter regions conferred a differential expression of the GUS gene between leaves and roots. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Birch (Betula pendula); Light response; Nitrate response; Nitrate reductase; Promoter analysis
www.elsevier.com/locate/plantsci
1. Introduction
Nitrate reductase (NR) catalyses the reduction of nitrate to nitrite, the first step of nitrate assimi-lation. Nitrite is subsequently reduced to ammo-nium by nitrite reductase, and ammoammo-nium is then incorporated into amino acids. The expression of higher plant NR genes is regulated by a number of factors including nitrate, light, circadian rhythms, carbohydrate levels, nitrogen metabolites, and phytohormones [1]. Because nitrate assimilation requires both a high input of energy and carbon skeletons, the complex regulation of NR gene expression can be viewed most simply as a balanc-ing device between nitrate assimilation and carbon fixation [2].
Despite the extensive study of NR gene expres-sion, little is known about DNA sequences involved in the regulation of higher plant NR genes [3]. Moreover, molecular approaches to study nitrate reduction have focused on herbaceous angiosperms while the molecular physiology of nitrate assimila-tion in woody species virtually has been ignored for a long time. On the other hand, nitrogen has been recognised to be a critical factor in forest ecosystems and world wide observed forest decline. For these reasons we decided to investigate NR gene expres-sion in the European white birch Betula pendula
Roth. cDNA clones encoding NR were isolated from birch [4] and used for quantitative Northern analyses. When young birch plants were grown on ammonium as the nitrogen source, NR mRNA was found to be very rare in leaves and undetectable in roots. Upon transfer of the plants to a nitrate-con-taining medium, levels of NR mRNA and activity dramatically increased in leaves and roots in a strictly light dependent manner. In darkness, no transcripts and activities were found [5].
The nucleotide sequence data reported will appear in the EMBL, GenBank and DDBJ Nucleotide Sequence Databases under the ac-cession number AJ001725.
* Corresponding author. Tel.: +49-228-735-584; fax: + 49-228-735-502.
E-mail address:[email protected] (W. Hachtel)
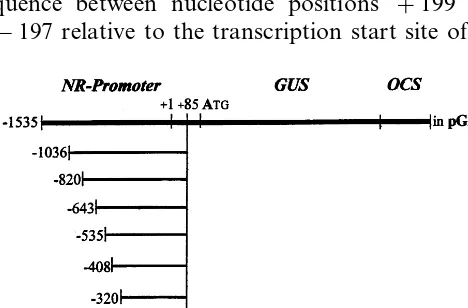
In order to identify promoter regions involved in the regulation of the birch NR gene expression, the 1535 bp sequence 5% flanking this gene, and eight sequences obtained by sequential 5% deletion were fused to the b-glucuronidase (GUS) reporter gene. The fusion constructs were introduced into
Nicotiana plumbaginifolia via agrobacteria. Trans-formants harbouring a distinct NR promoter GUS fusion either were grown on a medium containing nitrate, and GUS activity was determined at the end of both prolonged darkness (3 days) and a 3-h light period thereafter, or GUS activity was com-pared between nitrate-grown and ammonium-fed plants.
2. Materials and methods
2.1. DNA and RNA analysis
DNA was sequenced by the dideoxy chain-ter-mination method. DNA for PCR analysis was isolated from leaves of transgenicN. plumbaginifo
-lia according to Cheung et al. [6]. RNA was isolated from leaves of young birch plants accord-ing to de Vries et al. [7], and the transcription start of the NR gene was mapped by nuclease SI pro-tection and primer-extension analysis [8].
2.2. Construction and screening of a birch genomic library
DNA was extracted from leaves of hydroponi-cally grown birch plants according to Murray and Thompson [9] and partially digested with MboI. Fragments between 9 and 23 kb were ligated into theBamHI cloning site of the EMBL4 vector, and the DNA was packaged using an in vitro Lambda Packaging System (Promega). Recombinant phages were amplified with Escherichia coli strain LE392. The library was screened for plaques which hybridised to the 5% region of a birch NR cDNA clone [4]. Phage DNA from a hybridising clone was digested by SalI+EcoRI. A 2.1 kb
SalI/EcoRI fragment that hybridised to the NR cDNA was found to contain 1149 bp 5%upstream the translation start. Additional 499 bp of the NR promoter further upstream could be determined by primer walking.
2.3. Construction of NR promoter GUS gene fusions
The plasmid pSLJ4D4 was used to fuse NR promoter sequences with the GUS gene. pSLJ4D4 is a modified pUC19 with an insert that contains the 35S-CaMV promoter, the GUS gene and the polyadenylation signal of the octopine synthase gene (OCS) in fusion. Binary vectors derived from pGA482 were used to transform agrobacteria [10]. From the 2.1 kbSalI/EcoRI fragment obtained from recombinant phage, a 1.121 kb RsaI/Eco RI-subfragment was excised that represents a 5% flank-ing sequence of the birch NR gene spannflank-ing from +85 to −1036 relative to the transcription start site. This RsaI/EcoRI fragment was inserted into the plasmid pSLJ4D4 adjacent to the GUS gene thereby displacing the 35S-promoter. (In this fu-sion construct, 29 nucleotides of the NR leader sequence between the translation start site and position +85 are replaced by the first 51 nucle-otides of the GUS leader sequence containing a
SacI site that was used later on for the cloning of promoter deletion constructs in the pGA482 bi-nary vector). A fragment comprising the fused 1,121 bp birch sequence and the GUS encoding sequence was isolated from pSLJ4D4, inserted into the phagemid pBluescript KSII+ (Strata-gene, La Jolla, USA), and 5% deleted. Unidirec-tional deletion reactions in cloned inserts in pBluescript II KS(+) were performed with the
double-stranded Nested Deletion Kit (Pharmacia) based on a strategy described by Henikoff [11]. This resulted in NR promoter GUS fusions with end points at positions −820, −643, −535, − 408, −320 and −237 of the NR promoter se-quence. A longer strech of the NR promoter with the endpoint at −1535 could be cloned and fused to the GUS gene in pBluescript KSII+ after having created a NotI site by primer directed mutagenesis of the original recombinant Lambda phage DNA adjacent to the birch DNA insert.
From recombinant pBluescript KSII+, a se-quence spanning from position −183 of the NR promoter to GUS and OCS was isolated by a
isolated from the corresponding recombinant pBluscript KSII+ by a SacI+BamHI double digest and ligated into the recombinant pGA482 cleaved by SacI+BglII. The various NR pro-moter GUS gene fusion constructs are depicted in Fig. 1. A 35S-CaMV promoter GUS gene con-struct derived from pBI121 (Clontech, Palo Alto, USA) was also inserted into pGA482. Verification of constructs was carried out by restriction enzyme digests, DNA – DNA hybridisation and sequencing.
2.4. Transformation of N. plumbaginifolia, growth of transformants, and fluorimetric GUS assay
The pGA482-derived vectors were introduced into A. tumefaciens strain LBA4404 by the direct transformation method [10]. For selection of transformants, A. tumefacienscells were grown on a medium containing streptomycin, tetracycline, and kanamycin. Successful transformation was demonstrated by restriction analysis ofA. tumefa
-ciens DNA and Southern hybridisation with a radioactive labelled birch NR DNA fragment (po-sitions +85 to −183). The infection of leaf discs from N. plumbaginifolia with A. tumefaciens, the selection of transformed calli by use of cefotaxime and kanamycin, and the regeneration of transgenic plants were performed as described [12]. A PCR approach was employed to verify that regenerated kanamycin resistantN.plumbaginifolia plants con-tain a NR promoter GUS gene construct. A se-quence between nucleotide positions +199 and −197 relative to the transcription start site of the
fusion construct was amplified using 5% -TC-TACAGGACGTAACATAAG-3%as the antisense primer and 5% -AAGTGAAAAGCTTGACCGCA-3% as the sense primer. The PCR-products were identified by hybridisation with a DNA sample spanning from positions +105 to −184 of the fusion construct.
Transgenic plants were further cultivated on Murashige and Skoog (MS) medium containing 18.8 mM KNO3 and 20.6 mM NH4NO3 under a 16 h light/8 h dark regime at 24°C in a plant growth chamber. Light was generated by a cool-white fluorescent source with a light intensity at 150 mmol m−2 s−1.
Extraction of GUS from leaves and roots of primary transformants and fluorometric assays for GUS activity were performed according to Jeffer-son et al. [13] with methylumbelliferone (MU) as a standard. A detectable GUS activity was found in about 30% of primary transformed plants.
2.5. Statistical analysis
Gene expression in populations of first-genera-tion transgenic plants usually does not follow a normal distribution. The measure most suitable to describe the location of an unknown distribution probably is the median. An informative descrip-tion of the shape of such a distribudescrip-tion is the median absolute deviation or MAD, which is defined as the mean of the absolute differences between each observation and the median of all observations [14]. As a distribution-free statistical method we employed the nonparametric Mann – Whitney U-test that does not use the actual mea-surements, but instead the ranks of the measurements. This method was also used to test proposed hypotheses employing a multiplication constant [15]. In one set of experiments (see Sec-tion 3.3), the obtained data occur in pairs, and the Wilcoxon paired-sample test was applied [15]. Cal-culated values of the statistical significance level a
are given in Section 3.
3. Results
3.1. Comparison of 5% flanking sequences of NR
genes
From a birch genomic library we isolated a recombinant clone that contains a birch 1648 bp
Table 1
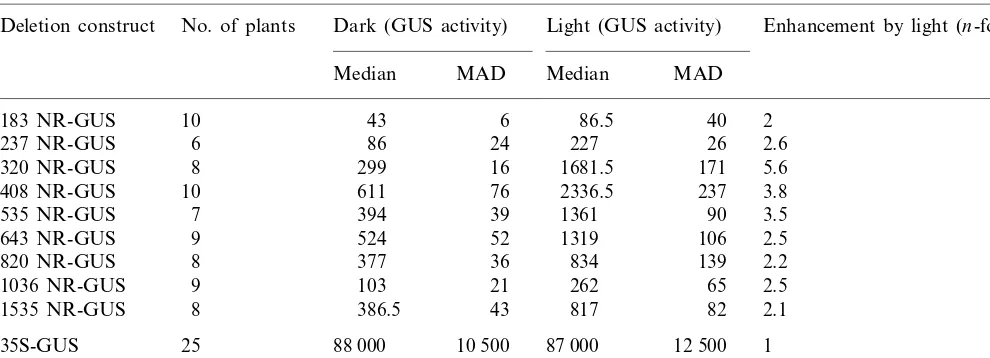
GUS activity in leaves ofN.plumbaginifoliaplants transformed with various birch NR promoter GUS fusion constructsa
Dark (GUS activity)
Deletion construct No. of plants Light (GUS activity) Enhancement by light (n-fold)b
Median MAD Median MAD
408 NR-GUS 611 76 2336.5 237 3.8
394 39 1361
820 NR-GUS 377 36 834 139 2.2
103 21 262 65 2.5
aGUS activity (pmol MU min−1mg−1protein) was measured in extracts from leaves harvested immediately after a 72 h dark
period (dark) and after 3 h of light (light).
ba50.05.
sequence 5% upstream the translation start of the NR gene (Fig. 2). The transcription start site (position +1) of the birch NR gene was found to be a T that is 113 bp upstream the translation start (data not shown). A TATA-motif (TATATATA) between positions −23 and −30 exactly meets the consensus sequence for plant TATA-boxes [16].
In order to reveal similarities between 5% flank-ing sequences of higher plant NR genes, an align-ment of eleven sequences is presented (Fig. 3). The alignment is restricted to 200 nucleotides upstream the translation start site of each sequence. No significant similarities could be determined further upstream. The consensus sequence of the TATA box is 5%-PyCTATAtAaPNcc-3%(capital letters and small letters, respectively: the nucleotide is present in 88 to 100% and 66 to 88% of all sequences). The 5% untranslated sequence of the transcripts of the NR genes from all dicotyledons contains an AG-rich and a UC-AG-rich region. In these regions, the AG-content is between 65 and 96% (68% in birch), and the UC-content is between 75 and 100% (76% in birch). The presence of an AG-rich and a UC-rich stretch would allow the formation of a stem – loop structure in the NR mRNA. Such a structure is known to increase the stability of various mRNAs [17,18] and, thereby, to be in-volved in translational regulation of gene expression.
3.2. NR promoter sequences necessary for light-dependent GUS acti6ation
For an analysis of light-regulated expression of NR promoter GUS gene fusions, primary trans-formants ofN. plumbaginifolia were transferred to darkness for a 72 h period to minimise GUS activity. Darkening plants for longer than 72 h reduced their vitality. The whole plants then were again illuminated with a light intensity at 150 mmol m−2 s−1 generated by a cool-white fluores-cence source. GUS activity was measured in ex-tracts obtained from roots and leaves immediately at the end of darkness and after 30, 60 and 90 min and 2, 3, 5, and 12 h in the light. Relative GUS activity after 12 h of illumination was set to 100%. At the end of 72 h darkness, 40% activity was detected, and more than 95% was obtained within 3 h of exposure to light. For further analyses, therefore, GUS activity was detected at the end of a 72 h dark period and after 3 h in light.
T
.
Strater
,
W
.
Hachtel
/
Plant
Science
150
(2000)
153
–
161
Fig. 3. Alignment of 5% flanking sequences upstream the translation start of higher plant NR genes. TATA boxes (italics, underlined), transcription start sites (bold,
underlined twice), regions rich in A+G (italics, bold), and T+C-rich stretches (underlined) are denoted. Abbreviations: BEAN 1 and 2,Phaseolus6ulgarisNR1 and NR2;
ARAB 1 and 2,Arabidopsis thalianaNR1 and NR2; TOB 1 and 2,N.tabacumNR1 and NR2; Petunia,Petunia hybrida; Tomato,Lycopersicum esculentum; Barley,Hordeum
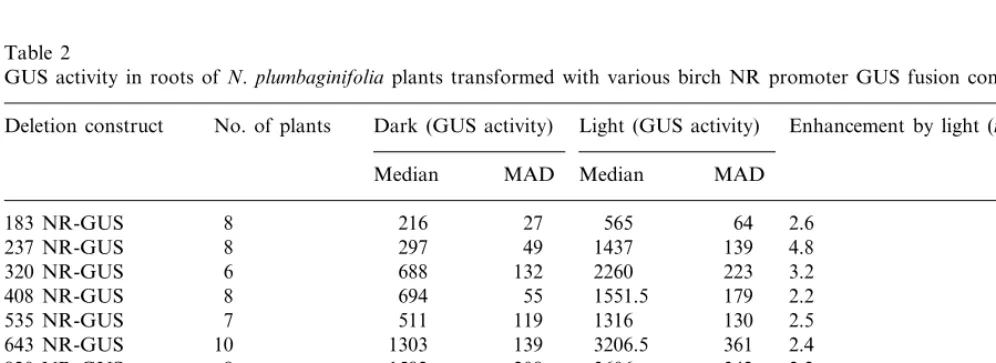
tween 643 and 1535 bp, GUS activity was higher than in roots containing shorter NR-promoters. Differences in GUS activity between roots and leaves can be summarised as follows: (1) the max-imum of GUS activity was higher in roots than in leaves, (2) the minimal promoter to reach highest GUS activity is significantly shorter in leaves than in roots, and (3) only in leaves but not in roots the −1036 promoter deletion con-struct does pass on a very low GUS gene expres-sion.
An enhancement of GUS activity by illumina-tion is conferred to both leaves and roots by all promoter lengths (Tables 1 and 2). Maximal stim-ulation is conferred by a promoter length of 237 bp in roots and by 320 bp in leaves. With these promoter lengths, a 4.8-fold enhancement by light in roots and a 5.6-fold enhancement in leaves is significant (a=0.05). In both leaves and roots,
inducibility is less pronounced not only with shorter but also in the presence of longer NR-promoter sequences.
3.3. Sequences necessary for nitrate-dependent GUS acti6ity
In order to study the influence of the nitrogen source on the expression of different 5% deleted NR promoter GUS gene constructs in transfor-mants of N. plumbaginifolia, two different ap-proaches were employed. In a first series of
experiments, plants were transferred from MS medium to a mixture of soil and sand and re-ceived a 10 mM ammonium succinate solution as the only nitrogen source for two months to ex-haust nitrate pools. Root and leaf material was then harvested and assayed for GUS activity. Subsequently, the plants were supplied with a 50 mM KNO3 solution for about 20 h, and har-vested again. A significant stimulation of GUS activity by nitrate (1.5 – 1.8-fold; a50.05) was observed in roots harbouring at least 643 bp of the birch NR promoter but not in those harbour-ing only 535 bp or less (data not shown). Nitrate did not stimulate GUS activity significantly in leaves of any kind of transformant in these exper-iments.
In a second approach, two cuttings of each transformant were made. One cutting was trans-ferred to solidified MS medium containing 10 mM KNO3 and 10 mM NH4NO3 whereas the other was placed on a medium with 10 mM NH4succinate as the sole nitrogen source. After
three weeks, GUS activity in root and leaf sam-ples was compared between the two plants of each pair of cuttings by calculating the ratio be-tween the two values. In Fig. 4 the mean of the ratios of correlated pairs is given. In these experi-ments, a significant response to nitrate was ob-tained in both roots and leaves of plants that were transformed with a NR promoter of at least 643 bp.
Table 2
GUS activity in roots of N.plumbaginifoliaplants transformed with various birch NR promoter GUS fusion constructsa
No. of plants
Deletion construct Dark (GUS activity) Light (GUS activity) Enhancement by light (n-fold)b
MAD
320 NR-GUS 688 132 2260 223 3.2
2.2
8 694 55 1551.5 179
408 NR-GUS
130 2.5
535 NR-GUS 7 511 119 1316
361 2.4
643 NR-GUS 10 1303 139 3206.5
2.2 342 3606
208
820 NR-GUS 8 1582
1036 NR-GUS 12 1230.5 133 2529.5 405 2
8 1981 293
1535 NR-GUS 3300 346 1.6
35S-GUS 25 61 500 1500 63 000 3750 1
WT 10 20.5 5 19.5 6 1
aGUS activity (pmol MU min−1mg−1protein) was measured in extracts from roots harvested immediately after a 72 h dark
period (dark) and after 3 h of light (light).
Fig. 4. Relative enhancement (n-fold) of GUS gene expression by nitrate as compared to ammonium in leaves (pale) and roots (dark) of matched pairs of transformed plants. Pairs were set up of two cuttings from each transformed plant, applying nitrate to one cutting and ammonium to the other cutting of each pair. Of each NR promoter GUS construct, four transformants were used to obtain four pairs of cuttings. The ratios between the absolute GUS activities measured in nitrate- and ammonium-grown cuttings of each pair were obtained, and n was calculated as the mean of the ratios obtained from the four pairs for each promoter length. With all fusion constructs containing a NR promoter ]643 bp, n=2 for roots andn=3 for leaves is significant at a level of a=0.05.
possibly is due to the presence of both activating factors in these promoter regions and antagonistic factors further upstream. The promoter region between −320 and −535 contains two GATA motifs (position −344 to −347 and −449 to −452). The recognition sequence GATA for NIT-2, a regulatory factor of nitrogen metabolism in fungi [19], putatively is involved in the transcrip-tional regulation of NR genes [20 – 22] and nitrite reductase genes [23]. Whether GATA motifs in the birch sequence between −320 and −535 are of importance for GUS inducibility is not known.
Deletion analysis of plant NR promoters has been difficult due to several problems. In some studies, only a very small number of transgenic plants was obtained in which the reporter gene was expressed and regulated by either light or nitrate [21,24]. Such low frequencies make the interpretation of results extremely difficult. The difficulties are further confounded by the observa-tion that the inducobserva-tion of host NR gene expression in tobacco has a negative effect on the expression of transgenes carrying NR sequences of related species [22,24]. A part of our results can best be compared to those reported by Jensen et al. [21] who analysed bean NR promoter mediated GUS activity in transgenic tobacco in the light. The comparison reveals both differences and similari-ties between birch and bean NR promoters. In contrast to our results, only small differences in GUS activity between leaves and roots are con-ferred by bean NR promoter fragments up to 859 bp, and an enhancing sequence in this region cannot be deduced. A leaf-specific negative cis -ele-ment located between −859 and −1223 is indi-cated by the data in the paper of Jensen et al. This is in parallel with the situation found in the birch promoter. Divergent from the birch promoter, in the bean promoter another negative cis-element down regulating expression in both leaf and root tissue seems to be present upstream −1223.
Some information regarding cis-acting se-quences necessary for nitrate dependent transcrip-tion of Arabidopsis NR genes is available. It has been demonstrated that 238 bp and 330 bp of the
Arabidopsis NR1 and NR2 promoters, respec-tively, are sufficient for induction by nitrate [2]. An Ac/gTCA motif was shown to be conserved between the 238 bp NR1 sequence and the 330 bp NR2 sequence and putatively is involved in nitrate induction [3]. The motif is present in the 5% flank-4. Discussion
Our data show distinct promoter sequences be-ing used differentially in leaves and roots. Among all NR promoter GUS constructs used, the 408 bp-promoter mediates the highest GUS activity in illuminated leaves of nitrate-grown transformants. The −1036 deletion mediates much weaker activ-ity than the −643 (and −820) and the −1535 deletions. This indicates the presence of a nega-tively acting element around −1036, and possibly the binding of a putative leaf specific repressor to this element. An abolition of this repression is observed in plants harbouring additional NR pro-moter DNA (1535 bp). To obtain ‘full’ GUS activity in roots, longer promoter sequences are required than in leaves. A strong increase in the GUS activity in roots was observed between pro-moter lengths of 535 bp and 643 bp. This indicates the presence of a cis-acting element in the −643 deletion that is absent in the −535 deletion, and possibly the binding of a putative root-specific enhancing factor to this element. The region be-tween −535 and −643 also is identified as being particularly important for nitrate-dependent GUS expression in both leaves and roots.
ing regions of nitrate inducible genes from a num-ber of species [3] including birch, however, at quite different positions in all these species. Whether ACTCA motifs in the birch NR promoter are functional has to be tested.
Acknowledgements
We wish to thank Dr H. Sommer (MPIZ; Cologne, Germany) and Dr A. Friemann for their help in establishing a genomic library, and Dr B. Davis (Leeds, UK) for providing plasmid pSLJ4D4. This work was supported by grant Ha 817/11-1 from the Deutsche Forschungs-gemeinschaft.
References
[1] N.M. Crawford, Nitrate: nutrient and signal for plant growth, Plant Cell 7 (1995) 859 – 868.
[2] Y. Lin, C.F. Hwang, J.B. Brown, C.L. Cheng, 5% proxi-mal regions of Arabidopsis nitrate reductase genes direct nitrate-induced transcription in transgenic tobacco, Plant Physiol. 106 (1994) 477 – 484.
[3] C.F. Hwang, Y. Lin, T. D‘Souza, C.L. Cheng, Sequences necessary for nitrate-dependent transcription of Ara-bidopsisnitrate reductase genes, Plant Physiol. 113 (1997) 853 – 862.
[4] A. Friemann, K. Brinkmann, W. Hachtel, Sequence of a cDNA encoding the bispecific NAD(P)H-nitrate reduc-tase from the tree Betula pendula and identification of conserved protein regions, Mol. Gen. Genet. 237 (1991) 97 – 105.
[5] A. Friemann, M. Lange, W. Hachtel, K. Brinkmann, Induction of nitrate assimilatory enzymes in the tree Betula pendula, Plant Physiol. 99 (1992) 837 – 842. [6] W.Y. Cheung, N. Hubert, B.S. Landry, A simple and
rapid DNA microextraction method for plant, animal and insect suitable for RAPD and other PCR analysis, PCR Methods Appl. 3 (1993) 69 – 70.
[7] S. de Vries, H. Hoge, T. Bisseling, Isolation of total and polysomal RNA from plant tissues, in: S.B. Gelvin, R.A. Schilperoort (Eds.), Plant Molecular Biology Manual, Dordrecht: Kluwer, 1988, pp. B6/1 – B6/13.
[8] S. Myohanen, J. Wahlfors, Automated fluorescent primer extension, Biotechniques 4 (1993) 16 – 17. [9] H.G. Murray, W.F. Thompson, Rapid isolation of high
molecular weight plant DNA, Nucleic Acids Res. 8 (1980) 4321 – 4325.
[10] G. An, Binary Ti vectors for plant transformation and promoter analysis, Methods Enzymol. 153 (1987) 292 – 305.
[11] S. Henikoff, Unidirectional digestion with exonuclease III in DNA sequencing, Methods Enzymol. 155 (1987) 156 – 165.
[12] T. Scho¨ndorf, W. Hachtel, The choice of reducing sub-strate is altered by replacement of an alanine by a proline in the FAD-domain of a bispecific NAD(P)H-nitrate reductase from birch, Plant Physiol. 108 (1995) 203 – 210. [13] R.A. Jefferson, T.A. Kavanagh, M.W. Bevan, GUS fu-sion: b-glucuronidase as a sensitive and versatile gene fusion marker in higher plants, EMBO J. 6 (1987) 3901 – 3907.
[14] J.-P. Nap, P. Keizer, R. Jansen, First-generation trans-genic plants and statistics, Plant Mol. Biol. Rep. 11 (1993) 156 – 164.
[15] J.H. Zar, Biostatistical Analysis, Prentice Hall, Engle-wood Cliffs, NJ, 1984.
[16] C.P. Joshi, An inspection of the domain between puta-tive TATA box and translation start site in 79 plant genes, Nucleic Acids Res. 15 (1987) 6643 – 6653. [17] S. Feng, E.C. Holland, HIV-1 tat trans-activation
re-quires the loop sequence within tar, Nature 334 (1988) 165 – 167.
[18] H. Vaucheret, J. Kronenberger, P. Rouze´, M. Caboche, Complete nucleotide sequence of the two homologous tobacco nitrate reductase genes, Plant Mol. Biol. 12 (1989) 597 – 600.
[19] Y.H. Fu, G.A. Marzluf, Nit-2, the major positive acting nitrogen regulatory gene ofNeurospora crassa, encodes a sequence-specific DNA-binding protein, Proc. Natl. Acad. Sci. USA 87 (1990) 5331 – 5335.
[20] G. Jarai, H.N. Truong, F. Daniel-Vedele, G.A. Marzluf, NIT2, the nitrogen regulatory protein of Neurospora crassa, binds upstream ofnia, the tomato nitrate reduc-tase gene, in vitro, Curr. Genet. 21 (1992) 37 – 41. [21] P.E. Jensen, T. Hoff, B.M. Stummann, K.W.
Hen-ningsen, Functional analysis of two bean nitrat reductase promotors in transgenic tobacco, Physiol. Plant. 96 (1969) 351 – 358.
[22] H. Vaucheret, M. Caboche, Induction of nitrate reduc-tase host gene expression has a negative effect on the expression of the two homologous tobacco nitrate reduc-tase genes, Plant Sci. 107 (1995) 95 – 104.
[23] R. Rastogi, N.J. Bate, S. Sivasankar, S.J. Rothstein, Footprinting of the spinach nitrite reductase gene pro-moter reveals the preservation of nitrate regulatory ele-ments between fungi and higher plants, Plant Mol. Biol. 34 (1997) 465 – 476.
[24] H. Vaucheret, A. Marion-Poll, C. Meyer, J.-D. Faure, E. Marin, M. Caboche, Interest in and limits to the utiliza-tion of reporter genes for the analysis of transcriputiliza-tional regulation of nitrate reductase, Mol. Gen. Genet. 235 (1992) 259 – 268.