www.elsevier.com / locate / bres
Research report
Estrogen receptors and aromatase activity in the hypothalamus of the
female frog, Rana esculenta. Fluctuations throughout the reproductive
cycle
a ,
*
b c a aGiulia Guerriero
, Charles E. Roselli , Marina Paolucci , Virgilio Botte , Gaetano Ciarcia
a
Department of Zoology, Federico II University, Via Mezzocannone, 8, 80134 Naples, Italy
b
Department of Physiology and Pharmacology, O.H.S.U., Portland, OR 97201, USA
c
Faculty of Science, University of Sannio, Benevento, Italy
Accepted 2 August 2000
Abstract
It is well known that certain actions of androgen are mediated through in situ aromatization to estrogen in neural target tissues. This study was undertaken to investigate androgen utilization in the hypothalamus of the female frog, Rana esculenta, through a quantification
3
of estrogen receptors and aromatase activity during the reproductive cycle. H-estradiol-binding molecules were present in both the
3 210
cytosol and the nuclear extract of the hypothalamus. These molecules bound specifically H-estradiol with high affinity (Kd 10 M) and low capacity (cytosol: 1.260.4 fmol / mg protein; nuclear extract: 7.960.6 fmol / mg protein). Aromatase activity was detected in the microsomal fraction of the hypothalamus using a sensitive in vitro radiometric assay. Both aromatase activity and nuclear estrogen receptor binding fluctuated in synchrony throughout the reproductive cycle. Western blot analysis of aromatase protein revealed one immunoreactive band with a molecular weight of approximately 56 kDa. In contrast to aromatase enzyme activity, the relative levels of aromatase protein changed little during the reproductive cycle suggesting that post-translational mechanisms may be involved in regulating estrogen synthesis in the frog brain. A possible role for estrogens in the modulation of the reproductive behavior in this species is suggested. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and antonomic regulation
Topic: Hypothalamic-pituitary-gonadal regulation
Keywords: Estrogen receptors; Aromatase; Hypothalamus; Frog
1. Introduction that estrogens, through in situ aromatization of circulating androgens, mediate certain androgen actions [6,10,39,51]. Sex steroids have potent effects on the central nervous In male mammals the preoptic area regulates copulatory system. In the adult vertebrate, androgens and estrogens behavior, concentrates estrogens, contains estrogen recep-control gonadotropin release and evoke the expression of tors, and synthesizes estrogens from circulating androgens sexual behavior [19,59]. Autoradiographic techniques [4,9,36,52,58].
show that nuclei in the preoptic area of the hypothalamus, In contrast to several vertebrate species of males, the limbic structures and mesencephalic nuclei close to the musk shrew (Sunus murinus) is the only well-studied optic tectum are responsible for the uptake of androgens species in which brain aromatization has been shown to be and estrogens in a variety of vertebrates. The critical role required for the expression of the female sexual behavior. of androgens in neuronal development and maintenance in Similar to males, aromatase is high in the preoptic area of males is supported by numerous studies. It is well known female musk shrews and implants of testosterone in this region reinstate sexual receptivity after ovariectomy [48,57]. Currently, little is known about the function of
*Corresponding author. Tel.:139-081-790-7069; fax: 1
39-081-790-aromatization in females of lower vertebrate species,
3326.
E-mail address: [email protected] (G. Guerriero). although circulating androgens are often higher in females
than in males [59]. Aromatase activity (AA) has been the anterior commissure. Hypothalamic regions were detected in the preoptic area, hypothalamus and amygdala pooled into three groups of about 20 animals each and of Rana catesbeiana [11], and aromatization seems to be stored in liquid nitrogen until use. Plasma was obtained involved in the regulation of male sexual behavior in Rana after centrifugation of the blood at 8003g for 10 min, at pipiens [7]. Autoradiography and immunocytochemical 48C and stored at2208C until use. Ovaries were dissected studies demonstrated, respectively, widespread distribution out and weighed.
of androgen and estrogen-concentrating nerve cells in
hypothalamic and limbic structures of Xenopus laevis 2.2. Preparation of microsomal pellet, cytosol and [30,38] and in the urodele Taricha granulosa [14]. How- nuclear extract
ever, a biochemical approach to characterize and quantify
sex steroid receptors was not utilized in any of these All operations were carried out at 48C. Analytical grade
vertebrate studies. chemicals were used. Minced tissues were weighed and
Schlinger and Callard [56] demonstrated in quail that homogenized with 1:4 (w / v) of TEMG (10 mM Tris–HCl, hypothalamic aromatase regulates testosterone-induced 1 mM EDTA, 1 mM 2-mercaptoethanol and 10% glycerol, aggressiveness by regulating the quantity of estradiol pH 7.8) containing 0.05 M NaCl (homogenization buffer). available for receptor binding. This was the first demon- The suspension was centrifuged at 8003g for 10 min. The stration of a quantitative relationship between aromatiza- recovered supernatant was centrifuged at 105,0003g for tion and estrogen receptor occupancy. It is not currently 60 min. The pellet of this centrifugation was employed for known whether this relationship exists in the brain of AA detection and is indicated as microsomal pellet, the amphibians. In Rana esculenta, a distinctive feature of supernatant constituted the cytosol. The 8003g pellet female reproductive endocrinology is the relatively high (crude nuclear pellet) was suspended and washed three level of circulating androgens during the reproductive times with homogenization buffer. Smears of the last pellet cycle [42,44]. Although some hypotheses have been were inspected by a phase-contrast microscope to assess advanced to explain the role of androgens in the female the presence and integrity of nuclei. Thereafter, the pellet frog [16], the role of aromatization has not been addressed. was suspended in 1:4 (w / v) TEMG containing 0.7 M KCl Thus, the present study was undertaken in order to test the (extraction buffer). The suspension was left 1 h in an ice hypothesis that testosterone acts through conversion to bath, with continuous stirring. It was then centrifuged at estradiol and subsequent binding to nuclear estrogen 105,0003g for 60 min. The supernatant constituted the receptors. For this reason, we investigated both the pres- nuclear extract.
ence and amount of both AA and estradiol binding
3
molecules in the hypothalamus of the female frog, Rana 2.3. H-estradiol binding assays esculenta, during the reproductive cycle.
3
[2,4,6,7] H-estradiol (90 / 110 Ci / mmol) was purchased from Amersham (Buckinghamshire, UK). For Scatchard
3
2. Materials and methods analysis [55] increasing amounts (0.3–5.0 nM) of H-estradiol were incubated with aliquots (200ml) of cytosol
2.1. Animals and nuclear extract in the presence or absence of 100-fold
excess of unlabeled 17b-estradiol (Sigma, St Louis, MO) Adult female frogs of Rana esculenta were collected to determine total and non-specific binding, respectively. from the surrounding of Naples during the following Incubations were carried out for 16 h at 48C. Bound and periods of the reproductive cycle: prebreeding (February to free steroids were separated by adding a suspension of March); breeding (from April to June); recovery, the charcoal-dextran (0.5–0.05% w / v) (Pharmacia, Piscata-period when vitellogenesis resumes in the ovaries (from way, NJ). The mixture was vortexed and kept in ice bath October to January). About 60 adult females were captured for 5 min [41]. It was then centrifuged at 8003g for 10 for each of the above reported periods. Animal mainte- min. The supernatant was transferred to vials containing nance and all surgical and experimental procedures were 4.5 ml Maxifluor scintillation fluid (Packard, Milan, Italy). conducted in accordance with the European Communities Radioactivity was measured in a Packard spectrometer Council Directive of 1986, (86 / 809 / EEC). Soon after (Packard 1600) at 45% counting efficiency. Data of
capture, the animals were anesthetized with MS 222 specific binding were plotted according to Scatchard
(Sigma, St Louis, MO). Animals were bled with a heparin- graphical procedure. For steroid specificity experiments, coated glass capillary inserted into the heart. Successively aliquots (200 ml) of cytosol and nuclear extract were
3
pro-cedure were carried out as reported before. Because of the acterized, was a polyclonal antibody raised in rabbit, 3
low levels of H-estradiol binding molecules in the brain, directed against human placenta aromatase, and purified by Scatchard analyses were used for single binding assay [35]. ammonium sulfate fractionation and affinity chromatog-Values were extrapolated from Scatchard analysis per- raphy [24]. About 63mg of total protein was used for each formed on three different pools of animals captured during lane. Samples were electrophoresed on a SDS–PAGE (8% the main periods of the reproductive cycle. Values of total acrylamide) according to Laemmli [31], and electrotrans-receptors were obtained from the intercept with the X-axis, ferred onto a 0.45mm nitrocellulose membrane (Schleicher normalized for the total protein content and expressed as and Schuell, Keene, USA) with a transblot cell apparatus
fmol / mg protein. (Bio-Rad). Membranes were blocked overnight by
incuba-tion in 10% non-fat milk powder suspended in 20 mM Tris
2.4. Measurement of AA (pH 7.6) containing 137 mM NaCl and 0.1% Tween-20
(TBS). Membranes were then washed three times with To measure AA, the microsomal pellet (the pellet of the TBS and incubated for 60 min in TBS containing Harada’s first ultracentrifugation at 105,0003g) was sonicated in aromatase antibody (1 mg / ml) (diluted at 1:5000). Parallel 1:30 (w / v) phosphate buffers (10 mM KPO , 100 mM4 strips were incubated in normal rabbit IgG. After three KCl and 1 mM EDTA, pH 7.4). The incubation mixture, washes in TBS, membranes were stained in horseradish which contained NADPH-generating system and 0.3 mM peroxidase-labelled goat antirabbit IgG. Proteins were
3
[1b- H] androstenedione (NEN Research Products, DuP- detected using enhanced chemiluminescence (ECL Amer-ont Co., Boston, MA; SA, 25.4 Ci / mmol) in 100 ml of sham, Life Science, Arlington Heights, IL). Human phosphate buffer, was pre-incubated at 378C for 30 min to placenta was used as a positive control for the presence of generate sufficient NADPH for the aromatase reaction. aromatase. The negative control included incubation with Aliquots (100ml) of hypothalamic microsome samples and normal rabbit IgG. Quantitative analysis of the autoradiog-human placental microsomes that were used as positive raphic bands of interest was performed using Multi-controls, were then added and the incubation was carried Analyst software (Bio-Rad).
out for 1 h at 258C. These incubation conditions were
adapted from a previous study in frogs by Callard et al. 2.6. Androgens and 17b-estradiol measurement in the 3
[48], and were within the linear range for H O production2 plasma (data not shown). The reaction was stopped with 400 ml
10% trichloroacetic acid containing 20 mg charcoal / ml. The steroid content in plasma samples was assayed by 3
The H O generated was purified on Dowex minicolumns2 RIA methods, adapted to Rana esculenta plasma [18]. The (Bio-Rad Laboratories, Richmond, CA). Tubes containing intraassay and interassay coefficient of variation were 7%
3
incubation buffer and a known amount of H O were2 and 13%, for androgens and 5% and 10% for 17b
-es-carried through the entire procedure to correct for re- tradiol, respectively. Testosterone antibodies reacted also coveries (mean6S.E.M., 95.863.6%; n59). The tritium with dihydrotestosterone (about 80%), therefore the term was counted at about 30% efficiency for sufficient time to androgens will be used throughout the paper. Dr Bolelli
3
give 5% counting error. Details of the H O assay used in2 (CNR, Physiopathology of Reproduction Service, Uni-our laboratory have been published previously [50]. To versity of Bologna, Italy) provided androgen and 17b
-3
validate the H O assay for use in the frog brain, aliquots2 estradiol antibodies. of hypothalamic microsomes were incubated with
increas-3
ing amounts of [1b- H] androstenedione (e.g. 6.2–200 2.7. Measurement of proteins 3
nM) and the amount of H O generated was measured and2
corrected for counts in buffer blanks. The data obtained Proteins were determined by the method of Lowry et al. were plotted as a saturation plot and the maximal velocity [33], using BSA as standards.
(Vmax) and Michaelis constant (Km) were derived by
non-linear regression analysis. In addition, incubations 2.8. Statistical methods containing serial dilutions of substrate (from 12.5 to 200
nM) were carried out in the presence of inhibitor 1,4,6- Data were analyzed with one-way analysis of variance androstatriene-3,17 dione (ATD) at concentrations of 30 (ANOVA). Duncan’s multiple range tests were applied to and 300 nM, with or without the substrate itself. determine which means differed significantly.
2.5. Western blotting
3. Results
All immunoblotting procedures were carried out at room
3
temperature. Dr Harada (Fujita Health University, 3.1. H-estradiol binding activity assay Toyoake, Aichi, Japan) kindly furnished anti-aromatase
3
3
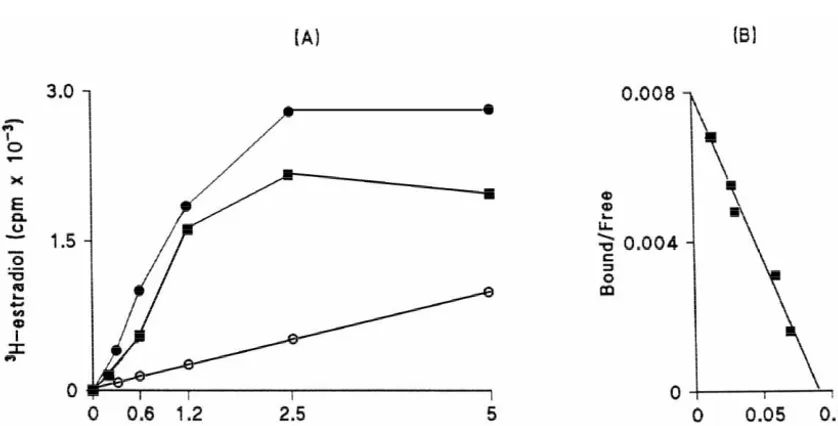
Fig. 1. Saturation (A) and Scatchard analysis (B) of H-estradiol binding in the cytosol of the female frog (Rana esculenta) hypothalamus. Only the specific binding is shown in the Scatchard plot. These results are representative of three different experiments carried out during each period of the reproductive cycle (pre-breeding, breeding and recovery). Kd values did not show any significant variation.j, specific binding;d, total binding;s, non-specific binding.
and nuclear extract of the female frog hypothalamus. for 17b-estradiol. In the nuclear extract 17b-estradiol was Saturation was reached around a value of 2.5 nM of the only competitor. In the cytosol, 17b-estradiol was the 3
H-estradiol when the total protein content was kept at 2 best competitor, followed by DES, which competed,
210
mg / ml. The average Kd value was 7.561.1310 M and although at a lesser extent. T, P and C did not compete
210
6.160.9310 M in the cytosol and nuclear extract, effectively in either the cytosol or in the nuclear extract 3
respectively (Figs. 1 and 2). Kd values did not show any (Fig. 3). H-estradiol binding specificity experiments were significant variation throughout the reproductive cycle performed once for each period of the year (prebreeding,
3
(data not shown). H-estradiol binding moiety was specific breeding, and recovery) and gave similar results.
3
3
Fig. 3. Specificity of H-estradiol binding in the cytosol (open bar) and nuclear extract (black bar) of the female frog (Rana esculenta) hypo-thalamus. The percent binding is calculated as the specific binding in the presence of a 1,000-fold excess of competitor (B) divided by the specific binding in the absence of competitor (B )03100. The data represent the
3
means of duplicate determinations. H-estradiol binding specificity experi-ments were performed once for each period of the reproductive cycle and
Fig. 5. Competitive inhibition of AA in the microsomes of the female gave similar results.
frog (Rana esculenta) hypothalmus by 1,4,6-androstatriene-3,17-dione (ATD) at 30 and 300 nM. These results are representative of three different experiments carried out during each period of the reproductive cycle (prebreeding, breeding, recovery). Substrate concentration (1b
-3.2. Aromatase activity assay 3
H)androstenedione5300 nM.
3
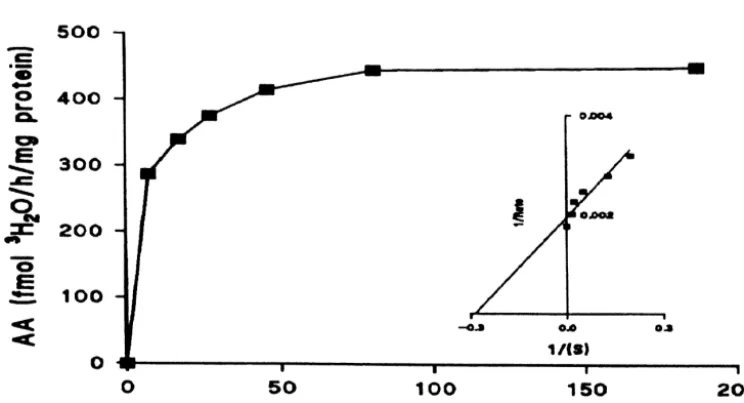
The validity of the H O assay for use with the female2 frog hypothalamus was tested by saturation analysis and
competitive inhibition with ATD. Fig. 4 shows that 3.3. Western blotting
aromatase activity in female frog hypothalamic
micro-somes exhibited typical Michaelis–Menten kinetics. The In Fig. 6 the immunoblotting of aromatase in the female
apparent Km was 4.2 nM and the Vmax was 471 fmol frog hypothalamus of Rana esculenta is reported. The
3
H O / h / mg protein. When microsomes were incubated in2 antibody cross-reacted with a single band with an apparent two different concentrations of ATD, activity (Vmax) was mol.wt of 56 kDa both in the hypothalamus of Rana decreased but the apparent Km was unaffected as would be esculenta in the three periods of the reproductive cycle expected by competitive inhibition with 30 and 300 nM of (lanes 1, 2, 3) and in the human placenta (lane 4), used as
ATD (Fig. 5). a positive control. No immunoreactive bands were detected
Fig. 4. Effect of substrate concentration on AA in the female frog (Rana esculenta) hypothalamus. Duplicate aliquots of microsomes were incubated for 1
3
h at 258C in 10 mM potassium phosphate buffer containing [1b- H] androstenedione. The reaction was terminated by the addition of 10% cold
3
Fig. 6. Western blot analysis of Rana esculenta hypothalamus and human placenta (as positive control). Female frogs captured in prebreeding (lines 1; 5), breeding (lines 2; 6), recovery (lines 3; 7) periods; human placenta (4; 8). Samples were transferred onto nitrocellulose and incubated with either the polyclonal antibody against aromatase (lines 1, 2, 3, 4) or rabbit IgG as a control (lines 5; 6; 7; 8). The position of marker proteins is indicated on the left. Western blots were performed on four different pools of animals with consistent results. Insert, quantitative analysis of band optical densities (1, prebreeding; 2, breeding; 3, recovery periods).
in samples when normal rabbit IgG was used in place of the specific antibody (lanes 5, 6, 7, 8). To determine if the level of aromatase protein expression changed over the reproductive cycle, quantitative analysis of sample relative optical density was performed (Fig. 6, insert). The results indicated an increase in expression between prebreeding period (band 1) and the breeding and recovery periods (bands 2 and 3), ranging from 1.2 to 1.28, respectively. No significant differences were measured between aromatase expression in the breeding (band 2) and recovery (band 3) periods.
3
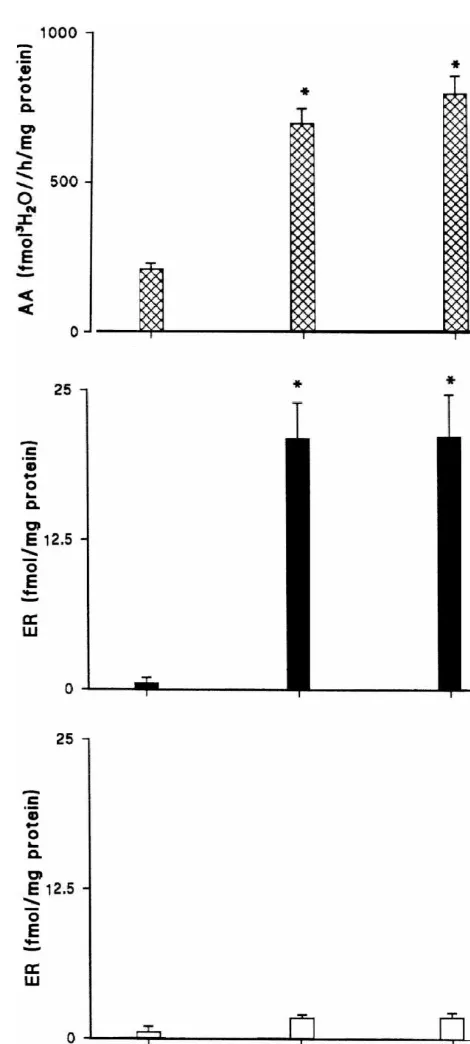
3.4. Aromatase and H-estradiol binding activity and fluctuations throughout the reproductive cycle
3
Both aromatase and H-estradiol binding activity in the nuclear extract of the hypothalamus of the female of Rana esculenta were low during the prebreeding period and increased during the breeding and recovery periods (P,
3
0.05). H-estradiol binding activity in the cytosol was very
3
Fig. 7. Aromatase activity (A) and H-estradiol binding levels in the
low at any time of the reproductive cycle and did not show
cytosol (B) and nuclear (C) extracts of the female frog (Rana esculenta)
any significant variation (Fig. 7).
hypothalamus during each period of the reproductive cycle (prebreeding, breeding, recovery). Three groups of pooled hypothalamic regions were analyzed for each period of the reproductive cycle. Asterisks indicate
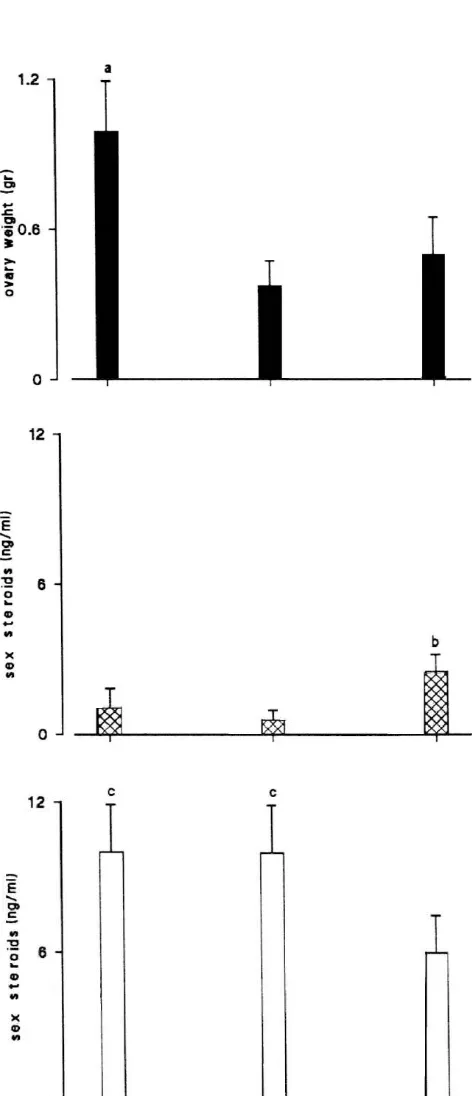
3.5. Plasma steroid levels and ovarian weight
significant difference from the prebreeding period (P,0.05). Values are
fluctuations
means6S.D.
The annual pattern of plasma steroids and ovarian weight variations throughout the reproductive cycle of
during the recovery period, due to vitellogenesis resump-tion.
4. Discussion
In this study, we characterized and quantified an es-trogen binding molecule and aromatase activity (AA) in the hypothalamus of the female frog, Rana esculenta. Estrogen binding in the hypothalamus displayed high affinity for estradiol and exhibited a finite number of binding sites; it showed biochemical characteristics (high affinity, low capacity, specificity) in agreement with estrogen receptors in the brains of other lower vertebrates [28,34,58], as well as the liver of Rana esculenta [40].
AA exhibited high substrate affinity and maximal ve-locity with typical Michaelis–Menten kinetics. The AA levels in the frog hypothalamus were higher than those reported in the rat [47,52,53], but lower than in quail [2] and goldfish [43]. Aromatase is considered an ancient enzyme system and this view is reinforced by its almost ubiquitous presence in vertebrates [12,51]. However, aromatase localization and AA are highly variable both in different groups of vertebrates and in the same species during different periods of the reproductive cycle, making it difficult to draw a consistent pattern.
Western blot analysis of frog hypothalamus microsomes reveals a major protein band identical in molecular size and labeling intensity to the 56 kDa protein previously identified as aromatase in the brain [8,20,54] and in non-nervous tissue [32,37] of other vertebrates. The expression of aromatase protein changed slightly during the three periods of the reproductive cycle. However, the relative change in aromatase protein expression calculated from Fig. 6 was small (1.2- and 1.28-fold increase between prebreeding and breeding and recovery periods, respective-ly) in comparison to the relative change in aromatase enzymatic activity during the same periods (3.0 and 3.3-fold increase between prebreeding and breeding and recovery periods, respectively). Therefore, we suggest that AA may be regulated mostly through post-translational modifications that alter enzyme activity rather than by changes in absolute levels of the protein. Studies are in progress to further test this idea.
Our results confirm and extend the previous autoradiog-raphic study of estrogen receptors in Xenopus [38] as well as an earlier demonstration of AA in Rana catesbeiana [11]. The presence of both high levels of AA and estrogen receptors, within the female frog hypothalamus, suggests that in situ aromatization in brain is an integral component
Fig. 8. Fluctuation in ovary weight (A), 17b-estradiol (B) and androgens
(C) plasma levels in Rana esculenta during each period of the reproduc- of androgen action in females of this species. AA has been
tive cycle (prebreeding, breeding, recovery). n55 determinations for each reported to exist in the brain of the other vertebrate period of the reproductive cycle. Letters over bars indicate significantly species, including birds and mammals [15,27,29,51,62]. different value (P,0.05): (a) ovary weight prebreeding vs. breeding and
However, only for the shrew, has a specific role for
recovery periods; (b) 17b-estradiol recovery vs. prebreeding and breeding
aromatization been demonstrated in the physiology of the
periods; (c) androgens prebreeding and breeding vs. recovery periods.
Levels of hypothalamic estrogen receptors and AA throughout the reproductive cycle [45] and vitellogenin fluctuated in synchrony throughout the reproductive cycle accumulation into the oocytes is reflected in the increased in females. They were both low during the pre-breeding ovarian weight during the recovery period (present data). period and high during the breeding and recovery periods. The high levels of AA and specific estrogen binding during Although the most parsimonious explanation of these data the recovery period of Rana esculenta indirectly sustain would be that a functional relationship may exist between the importance of a feedback mechanism played by the levels of AA and the levels of estrogen receptor estrogens at the hypothalamus–pituitary level, since the occupation such as that which was first demonstrated by rate of vitellogenin intake from oocytes requires and is Schlinger and Callard in quail [56], it is not possible to correlated with circulating gonadotropin levels [25]. draw a firm conclusion until further experimentation has In the female of Rana esculenta the major circulating been performed. However, in the female Rana esculenta, steroids are androgens, and their concentrations in the circulating androgen substrate did not show the same cycle plasma are greater than that of the 17b-estradiol at any of fluctuation as metabolism and so these data do not offer period of the reproductive cycle [22,42]. We made no a clear understanding of the role, if any, of T and its attempts to separate testosterone (T) from dihydrotestos-estrogenic-metabolites in the control of female reproduc- terone (DHT). However, an indication of the relative tion, calling, receptivity or, aggression. In addition, the amount of T and DHT could be provided by a study present results raise the possibility that factors acting carried out in a related species, the bullfrog Rana cates-independently or in conjunction with sex steroids are beiana, where DHT represents approximately 25% of the T involved in the regulation of AA in amphibians. In [11]. In Rana esculenta androgens do not always parallel mammals and birds, aromatase is controlled by a synergis- AA fluctuations in the hypothalamus, being high during tic action of androgen and estrogen, with androgen playing the prebreeding and breeding periods. This asynchronous the major role in mammals [49] and estrogens playing the trend of androgens and AA lead us to speculate that major role in birds [3]. In cultured turtle brain, both cAMP although aromatase is present during the recovery period, and a phosphodiesterase inhibitor (3-isobutyl-1-methyl little estrogen is produced in situ due to the lower levels of xanthine) stimulate AA, whereas androstenedione, testo- circulating androgens. The presence of a specific androgen sterone, and dihydrotestosterone inhibit it [5]. Recent receptor in hypothalamus of Rana esculenta could provide studies in birds suggest that neurotransmitters such as an explanation for the observed high levels of circulating dopamine can regulate AA trans-synaptically through androgens in the female of this species [23]. Eventually, if second messenger systems [1]. That AA in amphibians not all circulating androgens are aromatized to estrogens, may also be modulated through alternate mechanisms then it is plausible to hypothesize the existence of a
should be given further consideration in future ex- mechanism through which androgens act on their own.
perimentation. Androgen receptor have been detected in the liver of the
Since in all vertebrates the hypothalamus controls female of Rana esculenta and their levels fluctuate gonadotropin secretion, we hypothesize that locally formed throughout the reproductive cycle is parallel those of estrogens are involved in the modulation of reproductive circulating androgens [17].
control of central neuronal interactions and functions, J. Steroid
Acknowledgements
Biochem. Mol. Biol. 40 (1991) 123–132.
[20] D. Gelinas, G.V. Callard, Immunolocalization of aromatase and
This work was supported by a grant from M.U.R.S.T. androgen receptor neurons in the goldfish brain, Gen. Comp.
(GC) and a grant from NSF IBN 9817037 (CER) Endocrinol. 106 (1997) 155–168.
[21] D. Gelinas, G.A. Pitoc, G.V. Callard, Isolation of a goldfish brain cytochrome P450 aromatase cDNA: mRNA expression during the seasonal cycle and after steroid treatment, Mol. Cell. Endocrinol.
References 138 (1998) 81–93.
[22] G. Guerriero, C.E. Roselli, J.V.A. Choate, M. Paolucci, G. Ciarcia, V. Botte, Hypothalamus aromatase activity, hypothalamic testosterone [1] J. Balthazart, G.F. Ball, New insights into the regulation and
and estradiol binding molecules, and plasma testosterone levels in function of brain estrogen synthase (aromatase), Trends Neurosci. 21
the female frog Rana esculenta,, in: 18th Conference of European (1998) 243–249.
Comparative Endocrinologists. Rouen, France, 1996. [2] J. Balthazart, M. Schumacher, L. Evrard, Sex differences and steroid
[23] G. Guerriero, M. Paolucci, V. Botte, G. Ciarcia, Immunolocalization control of testosterone metabolizing enzyme activity in the quail
of aromatase- and androgen receptor-positive neurons in the frog brain, J. Neuroendocrinol. 2 (1990) 675–683.
brain, in: 19th Conference of European Comparative Endocrinolog-[3] J. Balthazart, R. Stoop, A. Foidart, N. Harada, Synergistic control by ists. 1–5 September. Nijmegen, Netherlands, 1998.
androgens and estrogens of aromatase in the quail brain, NeuroRe- [24] N. Harada, Novel properties of human placental aromatase as port 5 (1994) 1729–1732. cytochrome P-450: purification and characterization of a unique [4] T.J. Brown, R.B. Hochberg, J.E. Zielinski, N.J. MacLusky, Regional form of aromatase, J. Biochem. 103 (1988) 106–113.
sex differences in cell nuclear estrogen-binding capacity in the rat [25] S. Ho, Endocrinology of vitellogenesis, in: D.O. Norris, R.E. Jones hypothalamus and preoptic area, Endocrinology 123 (1988) 1761– (Eds.), Hormones and Reproduction in Fishes, Amphibians and
1770. Reptiles, Plenum Press, New York, 1987, pp. 145–159.
[5] G.V. Callard, Aromatization is cyclic AMP-dependent in cultured [26] J.B. Hutchison, How does the environment influence the behavioural brain cells, Brain Res. 204 (1981) 461–464. action of hormones? in: The Development and Integration of [6] G.V. Callard, Androgen and estrogen actions in the vertebrate brain, Behaviour Essay in Honour of Robert Hinde, P. Bateson, Cambridge
Amer. Zool. 23 (1983) 607–620. University Press, UK, 1991, pp. 149–170.
[7] G.V. Callard, Aromatization in brain and pituitary: an evolutionary [27] R.E. Hutchison, A.W. Wozniak, J.B. Hutchison, Regulation of perspective, in: F. Celotti, et al. (Eds.), Metabolism of Hormonal female brain aromatase activity during the reproductive cycle of the Steroids in the Neuroendocrine Structures. Raven Press, New York, dove, J. Endocrinol. 134 (1992) 385–396.
1984, pp. 79–102. [28] N. Jenkins, J.P. Joss, J.M. Dodd, Biochemical and autoradiographic [8] G.V. Callard, M. Drygas, D. Gelinas, Molecular and cellular physi- studies on the estradiol concentrating cells in the diencephalon and ology of aromatase in the brain and retina, J. Steroid Biochem. pituitary gland of the female dogfish, Gen. Comp. Endocrinol. 40
Molec. Biol. 44 (1993) 541–547. (1980) 211–219.
[9] G.V. Callard, P. Mak, D.J. Solomon, Effects of short days on [29] M. Karolczak, E. Kuppers, C. Beyer, Developmental expression and aromatization and accumulation of nuclear estrogen receptors in the regulation of aromatase and 5-alpha reductase type 1 mRNA in the hamster brain, Biol. Reprod. 35 (1986) 282–291. male and female mouse hypothalamus, J. Neuroendocrinol. 10 [10] G.V. Callard, Z. Petro, K.J. Ryan, Conversion of testosterone to (1998) 267–274.
estrogen and other steroids in the vertebrate brain, Amer. Zool. 18 [30] D.B. Kelley, J.I. Morrell, D.W. Pfaff, Autoradiographic localization (1978) 511–523. of hormone-concentrating cells in the brain of an amphibian [11] G.V. Callard, Z. Petro, K.J. Ryan, Androgen metabolism in the brain Xenopus laevis. I. Testosterone, J. Comp. Neurol. 164 (1975)
and non-neural tissues of the bullfrog Rana catesbeiana, Gen. 47–59.
Comp. Endocrinol. 34 (1978) 18–25. [31] U.K. Laemmli, Cleavage of structural proteins during the assembly [12] G.V. Callard, Z. Petro, K.J. Ryan, Phylogenetic distribution of of bacteriophage T4, Nature 227 (1970) 680–685.
aromatase and other testosterone-converting enzymes in the central [32] E.D. Lephart, K.G. Peterson, J. Noble, F.W. George, M.J. McPhaul, nervous system, Endocrinology 103 (1978) 2283–2288. The structure of cDNA clones encoding the aromatase P-450 [13] G. Chieffi, R. Pierantoni, Regulation of ovarian steroidogenesis, in: isolated from a rat Leydig cell tumor line demonstrates differential D.O. Norris, R.E. Jones (Eds.), Hormones and Reproduction in processing of aromatase mRNA in rat ovary and a neoplastic cell Fishes, Amphibians and Reptiles, Plenum Press, New York, 1987, line, Mol. Cell Endocrinol. 70 (1990) 31–40.
pp. 117–137. [33] O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, Protein [14] G.A. Davis, F.L. Moore, Neuroanatomical distribution of androgen measurement with the folin phenol reagent, J. Biol. Chem. 193
and estrogen receptor-immunoreactive cells in the brain of the male (1951) 265–275.
roughskin newt, J. Comp. Neurol. 372 (1996) 294–308. [34] P. Mak, S.M. Ho, I.P. Callard, Estrogen receptor in the turtle brain, [15] T.L. Dellovade, N. Harada, E.F. Rissman, Distribution and steroid Brain Res. 231 (1982) 63–74.
dependence of aromatase enzyme immunoreactivity in limbic nuclei [35] M.Y. McGinnis, P.G. Davis, M.J. Meaney, M. Singer, B.S. McEwen, of the female musk shrew brain, Brain Res. 634 (1994) 141–149. In vitro measurement of cytosol and cell nuclear androgen receptors [16] G. Delrio, M. d’Istria, L. Iela, G. Chieffi, The possible significance in the male rat brain and pituitary, Brain Res. 275 (1983) 75–82.
of testosterone in the female green frog, Rana esculenta, Boll. Zool. [36] R. Meisel, B. Sachs, The physiology of male sexual behavior, in: E. 46 (1979) 1–9. Knobil, J.D. Neill (Eds.), The Physiology of Reproduction, Raven [17] M.M. Di Fiore, L. Assisi, V. Botte, Aromatase and testosterone Press, New York, 1994, pp. 1395–1485.
receptor in the liver of the female green frog, Rana esculenta, Life [37] C.R. Mendelson, E.E. Wright, C.T. Evans, J.C. Porter, E.R. Simp-Sci. 62 (1998) 1949–1958. son, Preparation and characterization of polyclonal and monoclonal [18] M. d’Istria, G. Delrio, V. Botte, G. Chieffi, Radioimmunoassay of antibodies against human aromatase cytochrome P-450 and their use testosterone, 17b-estradiol and oestrone in male and female plasma in its purification, Arch. Biochem. Biophys. 243 (1985) 480–491. of Rana esculenta during sexual cycle, Steroids Lipids 5 (1974) [38] J.I. Morrell, D.B. Kelley, D.W. Pfaff, Autoradiography localization
42–48. of hormone-concentrating cells in the brain of an amphibian
[39] F. Naftolin, K.J. Ryan, I.J. Davies, V.V. Reddy, F. Flores, Z. Petro, [50] C.E. Roselli, W.E. Ellinwood, J.A. Resko, Regulation of brain R.J. White, Y. Takaoka, L. Wolin, The formation of estrogens by aromatase activity in rats, Endocrinology 114 (1984) 192–199. central neuroendocrine tissue, Recent Prog. Horm. Res. 31 (1975) [51] C.E. Roselli, L.E. Horton, J.A. Resko, Distribution and regulation of
295–298. aromatase activity in the rat hypothalamus and limbic system,
[40] M. Paolucci, V. Botte, Hepatic estradiol receptor in the female lizard Endocrinology 117 (1985) 2471–2476.
Podarcis s. sicula. Changes during the breeding period related to [52] C.E. Roselli, J.A. Resko, Androgens regulate brain aromatase plasma estradiol and vitellogenin levels, Gen. Comp. Endocrinol. 6 activity in adult rats through T receptor mechanism, Endocrinology
(1987) 181–185. 114 (1984) 2183–2189.
[41] M. Paolucci, V. Botte, Estradiol-binding molecules in the hepat- [53] C.E. Roselli, J.A. Resko, The distribution and regulation of aromat-ocytes of the female water frog Rana esculenta, and plasma estradiol ase activity in the central nervous system, Steroids 50 (1987) and vitellogenin during the reproductive cycle, Gen. Comp. Endo- 496–508.
crin. 70 (1988) 466–476. [54] M.K. Sanghera, E.R. Simpson, M.J. McPhaul, G. Kozlowski, A.J. [42] M. Paolucci, V. Esposito, M.M. Di Fiore, V. Botte, Effects of short Conley, E.D. Lephart, Immunocytochemical distribution of aromat-postcapture confinement on plasma reproductive hormone and ase cytochrome P450 in the rat brain using peptide-generated corticosterone profiles in Rana esculenta during the sexual cycle, polyclonal antibodies, Endocrinology 129 (1991) 2834–2844. Boll. Zool. 57 (1990) 253–259. [55] G. Scatchard, The attractions of proteins for small molecules and [43] M. Pasmanik, G.V. Callard, Changes in brain aromatase and 5a- ions, Ann. N.Y. Acad. Sci. 51 (1949) 660–672.
reductase activities correlate significantly with seasonal reproductive [56] B.A. Schlinger, G.V. Callard, Aggressive behavior in birds: an cycles in goldfish (Carassius auratus), Endocrinology 122 (1988) experimental model for studies of brain-steroid interactions, Comp.
1349–1356. Biochem. Physiol. A 97A (1990) 307–316.
[44] R. Pierantoni, L. Iela, G. Delrio, R.K. Rastogi, Seasonal plasma sex [57] U.R. Sharma, E.F. Rissman, Testosterone implants in specific neural steroid levels in the female Rana esculenta, Gen. Comp. Endocrinol. sites activate female sexual behavior, J. Neuroendocrinol. 6 (1994)
53 (1984) 126–134. 423–432.
[45] A.M. Polzonetti Magni, R. Curini, O. Carnevali, C. Novara, M. [58] K. Sinckak, C.E. Roselli, L.G. Clemens, Levels of serum steroids, Zerani, A. Gobetti, Ovarian development and sex steroid hormones aromatase activity and estrogen receptors in preoptic area, hypo-during the reproductive cycle of Rana esculenta complex, Zool. Sci. thalamus and amygdala of B6D2F1 male house mice that differ in 7 (1990) 265–271. the display of copulatory behavior after castration, Behav. Neurosci. [46] R.K. Rastogi, J.A. King, M.M. Di Fiore, B. D’Aniello, C. Pinelli, 110 (1996) 593–602.
Sex and reproductive status related brain content of mammalian and [59] N.L. Staub, M. De Beer, The role of androgens in female verte-chicken-II GnRHs in Rana esculenta, J. Neuroendocrinol. 9 (1996) brates, Gen. Comp. Endocrinol. 108 (1997) 1–24.
519–522. [60] K.A. Sullivan, J.W. Witkin, M. Ferin, A.J. Silvermann, Gonado-[47] J.A. Resko, C.E. Roselli, Brain steroid synthesis and metabolism, tropin-releasing hormone neurons in the rhesus macaque are not Neuroprotocols 1 (1992) 27–34. immunoreactive for estrogen receptor, Brain Res. 685 (1995) 198– [48] E.F. Rissman, N. Harada, C.E. Roselli, Effect of vorozole, an 200.
aromatase enzyme inhibitor, on sexual behavior, aromatase activity [61] R.A. Wallace, Vitellogenesis and oocytes growth in non-mammalian and neural immunoreactivity, J. Neuroendocrinol. 9 (1996) 199– vertebrates, in: L.W. Bowder (Ed.), Developmental Biology, Plenum
210. Publishing Corporation, New York, 1985, pp. 127–177.
[49] C.E. Roselli, Synergistic induction of aromatase activity in the rat [62] N.M. Yamada, S. Hirata, J. Kato, Distribution and postnatal changes brain by estradiol and 5a-dihydrotestosterone, Neuroendocrinology of aromatase mRNA in the female rat brain, J. Steroid Biochem.