www.elsevier.com / locate / bres
Research report
Cellular dynamics of corneal wound re-epithelialization in the rat
III. Mitotic activity
a ,
*
b,c a aIan S. Zagon
, Joseph W. Sassani
, Torre B. Ruth , Patricia J. McLaughlin
a
Department of Neuroscience and Anatomy, The Milton S. Hershey Medical Center, The Pennsylvania State University, College of Medicine, 500 University Drive, Hershey, PA 17033, USA
b
Department of Ophthalmology and Pathology, The Milton S. Hershey Medical Center, The Pennsylvania State University, College of Medicine, 500 University Drive, Hershey, PA 17033, USA
c
The Pennsylvania Lions Vision and Research Center, The Milton S. Hershey Medical Center, The Pennsylvania State University,
College of Medicine, 500 University Drive, Hershey, PA 17033, USA Accepted 15 August 2000
Abstract
The number and distribution of mitotic epithelial cells in the ocular surface during homeostasis and in response to abrasion of the mammalian cornea were determined. Normal rats and those receiving a 3 mm diameter centrally located epithelial defect, received an intraperitoneal injection of colchicine 6 h prior to sacrifice. Mitosis in the basal epithelium during homeostasis was comparable in magnitude across the ocular surface epithelium, with the exception of a few mitotic figures in the midline. Thirty percent of the mitotic figures were in the basal layer (layer 1), and 70% were in layer 2; the cells in layer 2 were often noted to retain connection to the basal lamina by cytoplasmic stalks. Mitosis was rarely noted in the regenerating epithelium. However, summation of M phase cells in both the basal and suprabasal epithelium adjacent to the wound showed increases of 3- and 5-fold at 30 and 36 h after abrasion, respectively, from levels at homeostasis and the time of injury. In striking contrast to homeostatic epithelium, 80% of the mitotic cells were located in layer 1 of the corneal epithelium, with normal distribution observed by 72 h. Mitosis in the limbus and conjunctiva was increased 3-fold at 30 h and 24 h, respectively, from values at homeostasis and the time of debridement. These results, using rigorous statistical analysis and precise topographic assessment, showed that mitosis is not impeded — but rather often accelerated — following denuding of the corneal epithelium and that the spatial distribution of mitotic cells is correlated with wounding. The data revealed that re-epithelialization of the corneal epithelium is not dependent on mitosis in the regenerating epithelium, but rather in the adjacent unwounded epithelium of the cornea, with most cells being located in the basal layer until re-epithelialization is completed. Mitotic cells in the limbus and conjunctiva may be related to replenishment of ocular surface epithelial cells used in the repair process rather than directly supplying the abraded surface. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Regeneration
Keywords: Cornea; Mitosis; Colchicine; Epithelium; Wound healing; Limbus; Conjunctiva
1. Introduction Thoft and Friend [26] proposed the X, Y, Z hypothesis as the organizing principle of corneal epithelial maintenance, The ocular surface epithelium is in a constant state of with X representing the proliferation of basal epithelial physiological renewal, with a dependence upon a balance cells, Y the contribution to the cell mass by centripetal between the generation of cells and the loss of cells. In movement of peripheral cells, and Z the epithelial cell loss humans and rats, for example, the corneal epithelium has from the surface. Thus, the kinetics of the corneal epi-been estimated to become renewed every 7 days [2,12]. thelium can be expressed as X1Y5Z, with cell loss balancing cell replacement. This steady state of cell renewal is crucial to the functions of the ocular surface
*Corresponding author. Tel.: 11-717-531-6409; fax: 1
1-717-531-epithelium, which serves as a protective barrier between
5003.
E-mail address: [email protected] (I.S. Zagon). the external and intraocular environments, and for
tion of fluid transport for normal stromal hydration and ocular surface. Colchicine was used to induce a mitotic
corneal transparency [9,19,35]. arrest in order to identify cells in the M phase of the cell
It is understandable that injury to the cornea must be cycle. We expected to resolve the following queries: (a) repaired rapidly in order to re-establish function. Three Where does mitosis take place in ocular surface epithelium phases of epithelial healing have been described: (a) during homeostasis? (b) Is there a relationship between the epithelial cell migration and wound closure, (b) re-estab- location of mitosis and DNA synthesis during homeosta-lishment of cell number by cellular replication and dif- sis? (c) Does the covering of the wounded surface depend ferentiation, and (c) reassembly of adhesion structures upon mitosis of the cells populating the re-epithelialized [1,4–8,10,11,14,15,21,23,25,27,28,35]. Unfortunately, dis- area? (d) Is wound closure contingent upon mitosis of agreement exists regarding the time course of these events adjacent cells in the undamaged corneal epithelium? (e) Do following epithelial injury. Some studies have reported that cells of the limbus, cited as progenitors of the corneal the early stages of epithelial wound closure predominately epithelial cells [20,22,24] respond to injury of the central rely on cell migration rather than cell replication, with region of the cornea by alterations in mitosis? (f) How do mitosis inhibited up to 3 to 4 days after injury depending cells of the conjunctiva, which have the capability of upon wound size [1,6,7,21]. Nevertheless, other workers covering the corneal epithelium [29–31], react to an insult have suggested that DNA synthesis and mitosis do occur in in the central region of the cornea with respect to mitotic the regenerating epithelium and / or the adjacent corneal activity? (g) Which cell layers, basal and / or suprabasal, epithelium within 24 h following injury [5,10,13,14,23, undergo mitosis? (h) What is the relationship of mitotic 27,28]. Finally, it has been reported that little change in behavior and DNA synthesis during repair of abrasions of cell division from homeostatic levels in the corneal the corneal epithelium? The information obtained in these epithelium is observed after wounding [25]. There are experiments concerned with mitosis was integrated with many factors that contribute to this lack of consensus about that from previous studies on DNA synthesis in order to the process of wound healing of the corneal surface. Some reveal, in a more comprehensive fashion, the role of cell of these include: differences in species used (e.g., rat, proliferation in wound healing.
mouse, rabbit), lack of specification of the regions ex-amined (e.g., corneal epithelium, limbus, conjunctiva) or
layers (i.e., basal, suprabasal) or plane of section investi- 2. Materials and methods gated, subjective impressions rather than statistically valid
statements, methodology that may not be optimal and 2.1. Animals specific (e.g., scintillation counting of the corneal surface),
differences in wounding techniques (e.g., n-heptanol, Adult (250–300 g) male Sprague–Dawley rats (Charles mechanical), type of wound (superficial, full thickness), River Labs, Wilmington, MA) were utilized in this study. and / or damage to the integrity of the basal lamina with Animals were housed in an environment of 2160.58C with
respect to injury. a relative humidity of 50610%. The room had a complete
To address these concerns, we have designed a series of exchange of air 15–18 times per hour and a 12 h light– experiments that incorporate statistical analysis in a rigor- dark cycle with no twilight. Water and Purina 5010 Rodent ously defined model that examined a mechanical dis- Chow were continuously available. The rats were accli-turbance of the epithelial surface that left the basal lamina mated to the animal facilities for at least 1 week prior to intact. In two earlier reports [33,34] we tagged cells with study.
radioactive thymidine prior to abrasion [33] and at discrete All investigations conformed to the Association for points after debridement [34] and inquired about DNA Research in Vision and Ophthalmology Resolution on the synthesis during repair. Some of the principles formulated Use of Animals in Research, the regulations of the from these earlier studies include: (a) DNA synthesis and National Institutes of Health, and the guidelines of the centripetal migration of cells observed in homeostasis is Department of Comparative Medicine of The Pennsylvania retained after injury to the corneal epithelium, (b) cell State University College of Medicine.
replication occurs simultaneously with centripetal migra- Animals were examined pre-operatively to exclude tion during re-epithelialization, (c) vertical migration is not subjects with ocular surface disease.
repressed by injury, (d) the regenerating surface of
epi-thelium is not dependent on DNA synthesis for repopula- 2.2. Wound healing tion, (e) re-epithelialization relies on the activity of cells in
the adjacent unwounded area, and (f) the repair of injuries The procedures for wounding followed Zagon and to the central corneal epithelium does not rely upon the colleagues [33,34]. In brief, rats were anesthetized by
limbus or conjunctiva. intraperitoneal injections of ketamine (10 mg / kg) and
was demarcated only in the right cornea with a disposable epithelial cells of this area. The limbus was approximately dermatology skin punch (Acu-Punch, Acuderm, Inc., Ft. 2.3 mm from the edge of the wound. The conjunctiva was Lauderdale, FL). The encircled corneal epithelium was analyzed over four 0.16 mm grid lengths beginning 1 mm removed with a [15 Bard-Parker scalpel blade. To from the limbo-corneal junction on each of the superior
facilitate accurate measurements of the wounded areas, and inferior aspects of the eye; goblet cells distinguished special efforts were made to produce abrasions with round this bulbar region of the conjunctiva.
and smooth perimeters. Following surgery, 50 ml of
Polytrim (Allergan Pharmaceuticals, Irvine, CA) was ap- 2.5. Thin section analysis plied to the injured eye [33,34].
Animals were examined post-operatively to exclude In addition to evaluating paraffin embedded sections as ocular surface disease, and removed from the study if to the location of mitotic figures in colchicine preparations,
inflammation or infection occurred. some unwounded animals were injected with colchicine
and 6 h later, their eyes were fixed in 2% glutaraldehyde,
2.3. Colchicine treatment 2.5% paraformaldehyde, 3% sucrose, and 0.025% CaCl in
2 0.1 M sodium cacodylate buffer at 48C for 18 h, postfixed Based on preliminary findings in which colchicine (10 in OsO for 2 h, and embedded in Epon 812. One-micron
4
mg / kg; Sigma, Indianapolis, IN) was injected intraperito- sections of the corneal epithelium were placed on glass neally into 2 animals / time point at 1, 2, 4, 6, and 24 h and slides and stained with toluidine blue. Images were cap-examined for mitotic activity, 6 h and 24 h were found to tured with a three-chip Sony CCD camera (model DKC-be optimal for recording a mitotic index; the 6 h time point 5000) mounted on an Olympus BH-2 light microscope. following colchicine injection was chosen for subsequent
studies. At each time point, 4 animals were anesthetized
2.6. Data presentation and decapitated, and the eyes proptosed and enucleated.
Eyes were fixed in 10% neutral buffered formalin for 24 h,
All studies were conducted in a masked fashion, and the and embedded in paraffin. Six-micron sections that
in-same individual performed the surgery and the cell count-cluded the entire corneal surface, limbus, and conjunctiva
ing. Data are presented as the mean mitotic index (percent) were collected and stained with hematoxylin and eosin.
for every two grid areas (5segment). In some cases, the In some cases, surgery was performed and rats given
average MI for a region (e.g., regenerating surface) was colchicine for 6 h. All surgeries were performed at 0800–
provided. Thus, there are 2 segments (54 grids) each for 1000 h. Animals also were collected at 12, 18, 24, 30, 36,
the inferior and superior aspects of the limbus and conjun-48, 72, and 120 h following surgery.
ctiva, and 25 segments (550 grids) across the corneal surface. No differences in the MIs of the superior and 2.4. Mitotic indexes
inferior poles in any region of the ocular surface epi-thelium at any time point could be detected, and data were The number of cells with mitotic figures (i.e., prophase,
combined. All data within a region across time were metaphase, anaphase or telophase) in the basal and
sup-subjected to ANOVA, with subsequent comparisons made rabasal epithelial layers of the superior and inferior cornea,
using the Newman–Keuls test. limbus, and conjunctiva were counted from 2 non-serial
sections per eye; 4 corneas were assessed at each time point. Only cells in the deepest aspect of the basal
epithelium were considered basal cells. The suprabasal 3. Results layer extended from layer 2 (just superficial to the basal
3.1. The number and distribution of mitotic epithelial or conjunctiva. The number of cells undergoing mitosis in cells in the uninjured cornea an approximately 0.64 mm extent constituting the center of
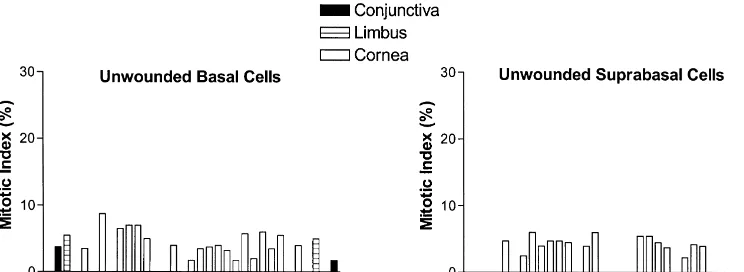
the cornea had a mean MI of 0.8%. 3.1.1. Basal cells
The range of the MI of basal cells in the unwounded 3.1.2. Suprabasal cells
cornea was 0 to 8.7%, and for the four grids of limbus and The index of mitotic suprabasal cells in the corneal conjunctiva the MI ranged from 0 to 5.5% and 0 to 3.7%, epithelium of uninjured animals was 2.660.5%, and for respectively (Figs. 1A,C, 2). It should be noted that in the limbus and conjunctiva the MI was less than 0.5% some specimens there were no mitotic cells in one or more (Figs. 1B,D, 2). The region of 0.64 mm extent constituting of the 0.32 mm segments of the peripheral cornea, limbus, the center of the cornea rarely contained mitotic figures. As mentioned earlier, the mitotic cells in the suprabasal layer, were predominately located in layer 2.
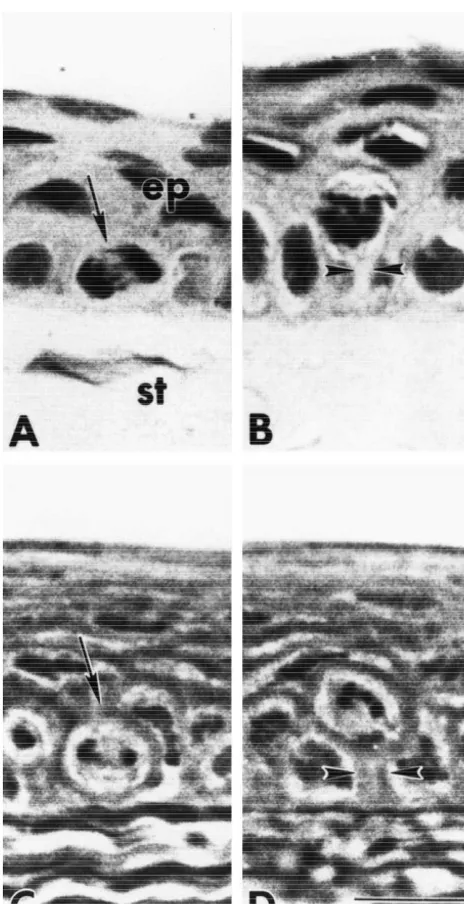
Study of 1mm plastic thick sections revealed that some of the mitotic cells in layer 2 were noted to have a ‘stalk’ of cytoplasm that connected to the basement membrane (Fig. 1B,D). Evaluation of 31 cells with mitotic figures in layer 2 from 20 thin sections showed that 16% had stalks compared to 84% of the cells without visible extensions to the basement membrane. However, the number of cells with stalks may be an underestimation because the plane of section may have obscured such connections.
3.1.3. Distribution of mitotic cells
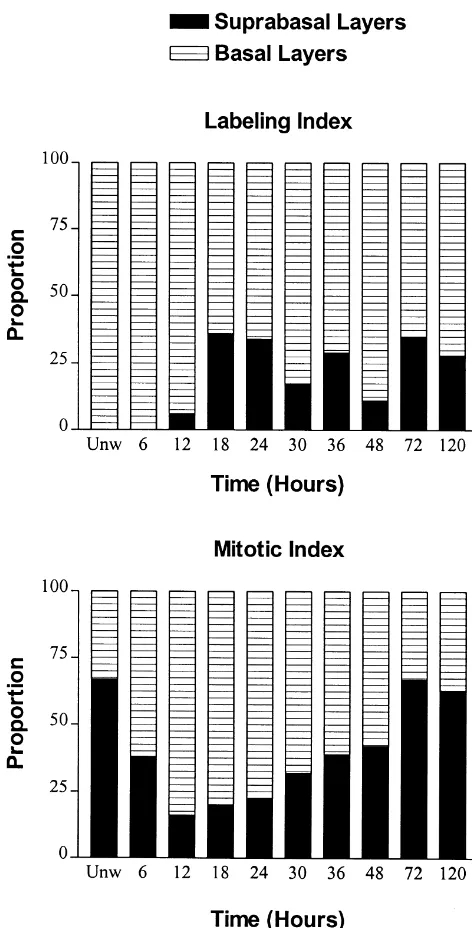
The number of mitotic cells in the basal and suprabasal layers of the ocular surface epithelium of the uninjured eye was calculated. In the epithelium of the cornea, limbus, and conjunctiva, each region contained mitotic figures in a ratio of approximately 30% in the basal layer to 70% in layer 2 (Fig. 3).
3.2. The number and distribution of mitotic epithelial cells after injury
3.2.1. Wounded region of the cornea
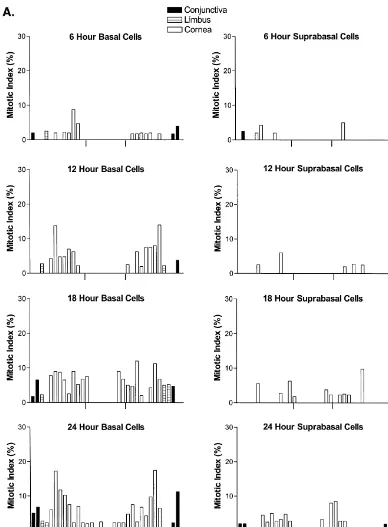
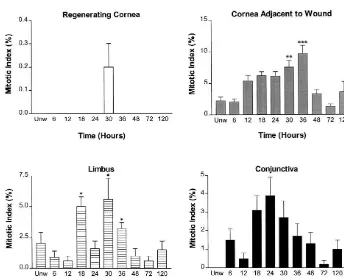
Mitotic cells were not recorded in the originally abraded area in the first 12 h following injury (Fig. 4). From 18 h until the conclusion of the 5-day examination period, little or no mitotic activity was observed in the basal epithelial cells populating the regenerating region of the cornea; however, at 18 h some segments at the margins of the wound did have an MI that reached 6–8%.
No mitotic activity was noted in the suprabasal layer for the first 12 h post-injury (Fig. 4). From 18 h after abrasion until termination of the experiment (i.e., 5 days), mitosis was infrequent in these suprabasal cells. At the margins of the wound, some segments observed at 18 h and 24 h had an MI that occasionally reached 5–8%.
3.2.2. Cornea peripheral to the wounded region
Within 6 h following injury, the MI of the basal cells of
Fig. 1. Photomicrographs of the corneal epithelium in uninjured animals the corneal epithelium extending from the margins of the
demonstrating mitotic cells in the basal (arrow) (A) and suprabasal (B) wound to the limbus was comparable to specimens ob-layers of paraffin sections, and the basal (C) and suprabasal ob-layers (D) of tained from uninjured animals (Fig. 4), although there was 1mm plastic sections. All animals were injected with colchicine 6 h prior
a general impression of a subnormal MI in many segments.
to termination. Note the stalk-like cytoplasmic processes (arrowheads)
A significant elevation in the MI was noted at 18 h
extending from mitotic cells in layer 2 to the basal lamina (B, D).
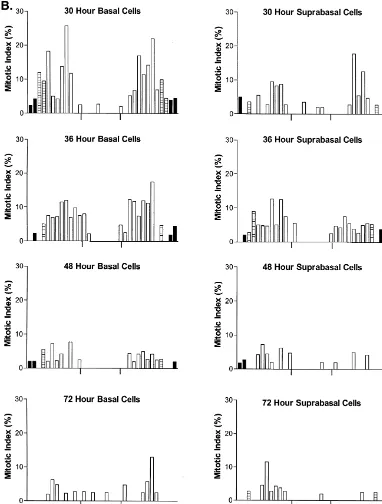
Fig. 2. Histogram of the mean mitotic index of basal and suprabasal epithelial cells occupying the ocular surface of unwounded rats; standard errors are not included on the graphs because they were minimal. Epithelial cells located in the inferior and superior aspects of the cornea are denoted to the left and right sides, respectively, of each figure. Animals received colchicine 6 h prior to termination at the designated times.
(P,0.001), with up to 5-fold more mitotic figures ob- MI of approximately 4% at 36 h following denuding of the served at 30 h than at 6 h following abrasion. The MI at 30 corneal epithelium; this value differed significantly (P, h and 36 h in the cornea peripheral to the wounded region 0.05) from those recorded in uninjured rats.
differed significantly (P,0.001) from preparations of Summation of mitotic cells in both the basal and
uninjured animals. suprabasal layers revealed significant (P,0.05) elevations
With regard to the suprabasal region of the corneal in mitotic activity at 18, 30, and 36 h relative to both epithelium adjacent to the wounded region, the MIs were homeostatic levels and in abraded specimens at 6, 12, 24, comparable to uninjured specimens for the first 36 h (Fig. 48, 72, and 120 h (Fig. 5).
4). At 36 h the MI was increased significantly (P,0.001)
by 7-fold from the 6 h level of injured corneas, and 2-fold 3.2.4. Conjunctiva
over the unwounded preparations. The MIs at 48, 72, and The MI of the basal epithelium of the conjunctiva was 120 h were similar to both the 6 h indexes of injured not changed from homeostatic levels after injury to the
corneas and unwounded specimens. cornea except at 24 h when a 3-fold MI was noted; this
Summation of mitotic cells in both the basal and difference was statistically significant (P,0.05) (Fig. 4). suprabasal layers of the corneal epithelium adjacent to the The difference in MI of the conjunctiva at 24 h in injured abraded area (Fig. 5) showed that at 30 h and 36 h there animals was statistically significant from the 12, 48, and 72 were 3-fold (P,0.01) and 5-fold (P,0.001) increases, h time points (P,0.05).
respectively, from unwounded and wounded subjects. In the suprabasal epithelial cells of the conjunctiva from Examination of the proportion of mitotic cells in the animals with an abrasion of the corneal epithelium, mitotic basal and suprabasal layers in the corneal epithelium activity was negligible at all time points studied (Fig. 4) as adjacent to the abraded region indicated that from 12 h to well as from homeostatic levels.
24 h post-injury approximately 81% of the cells undergo- Summation of mitotic cells in both the basal and ing mitosis were in layer 1 whereas 19% of the mitotic suprabasal layers in the conjunctiva revealed no significant figures were in layer 2 (Fig. 3). Relative to the proportion difference between wounded and unwounded specimens, of mitotic cells in layers 1 and 2 during homeostasis, there or across the time points of wounded cornea preparations was a change of 2.5-fold within the first day following (Fig. 5).
injury. During the second day following abrasion, layer 1 had 62% of the mitotic figures and layer 2 had 38%. At 72
h, the proportion of mitotic cells in layers 1 and 2 of 4. Discussion corneal epithelial cells in the area adjacent to wounding
was similar to homeostatic levels. A major objective of this study was to define the number
and location of mitotic cells in the homeostatic corneal
3.2.3. Limbus epithelial surface, and integrate this knowledge with
Buschke and colleagues [3] and Haskjold et al. [16], but in disagreement with Kaufman and coworkers [18] who reported mitosis to be more diminished in the central cornea than in the periphery. These differences as to the distribution of mitotic figures in the corneal epithelium may have been due to the use of mitotic arresting agents in the present case and with Buschke et al. [3] and Haskjold et al. [16], but not in the studies of Kaufman et al. [18]. The accumulation of mitotic figures over 4 to 6 h using drugs such as colchicine may have allowed for a finer discrimination as to the location of mitotic cells. An exception to observations of mitotic activity in ocular surface epithelium occurred in the midline of the corneal epithelium, where few basal or suprabasal mitotic cells were detected in our experiments. Buschke et al. [3] also noted a similar paucity of mitoses in the central zone of the corneal epithelium. The integration of this data with information in previous studies on rat [33,34] and human [17] showing the infrequency of DNA synthesizing cells in the midline of the cornea, suggests the special nature of this region as to cell replication. Presumably, basal epi-thelial cells bordering the middle of the cornea and moving centripetally towards the center undergo S, G , and M and2 are no longer proliferatively active (i.e., G ) as they reach0 the midline where they are at the termination point of centripetal migration and poised to desquamate.
Using the present data, one can extrapolate the number of epithelial cells undergoing mitosis daily in the ocular surface. Thus, if there is approximately a 3% mitotic index in the corneal epithelium for the 6 h of colchicine treatment (data similar to that presented by Bertalanffy and Lau [2]), and the duration of mitosis has been estimated to be 70 min [25], in a 24 h period about 12% of the cells have been in the M phase. Therefore, approximately every 8 days there is a renewal of epithelial cells in the rodent cornea, an estimation close to the 7 days for the human cornea [12] and the rat cornea [2] reported by others. With respect to the magnitude of cell replication in the entire corneal epithelium during homeostasis, Buschke and
co-Fig. 3. The proportion of cells undergoing DNA synthesis (labeling
workers [3] have estimated that there are 2 000 000
index) or mitosis (mitotic index) that were recorded in the basal and
epithelial cells in the rat cornea so that every hour
suprabasal layers from corneal epithelium. Data were collected from the
region peripheral to the wound at the designated time points, as well as approximately 10 000 cells undergo mitosis as computed from unwounded specimens. from our study, a calculation that is similar to the
Fig. 4. (A–C) Histogram of the mean mitotic index of basal and suprabasal epithelial cells occupying the ocular surface of the rat following a 3 mm abrasion of the central region of corneal epithelium (denoted by tick marks); animals received colchicine 6 h prior to sacrifice. Standard errors are not included on the graphs because they were minimal. Epithelial cells located in the inferior and superior aspects of the cornea are denoted to the left and right sides, respectively, of each figure.
Fig. 4. (continued )
layer 1 (see Fig. 5). Therefore, there is a compartmen- injury. The results of our study show that in the regenerat-talization in phases of the cell cycle, with cells in S phase ing region there is little mitotic activity in either the basal situated in layer 1, and then a critical decision must be or suprabasal layers. The corneal epithelium extending made to either complete the M phase in layer 2 whereby from the wound margin to the limbus was not altered in the cells are postmitotic (a pathway taken by roughly mitotic activity in response to injury within the first 18 h, two-thirds of the dividing cells) or complete the M phase indicating that abrasion to the corneal epithelium does not in layer 1 in order to be available for another round of cell perturb ongoing cell replicative processes. However, the replication (a pathway taken by about one-third of the number of mitotic cells was notably increased from 18 h to
dividing cells). 36 h after wounding in the basal layer, and at one time
Fig. 4. (continued )
changed to 80% in layer 1 and 20% in layer 2 within 12 h that mitosis is neither important to the limbus nor conjun-of debridement and slowly returned to that conjun-of normal ctiva in the immediate covering of the debrided surface, conditions from 24 h to 72 h. This increase in mitotic cells but rather increases by the time at least 1 to 2 epithelial and the change in location to the basal layer of the corneal layers have been replaced over the injured area. Therefore, epithelium adjacent to the wound at a time when wound the limbus and to a lesser degree, the conjunctiva, may be closure (i.e., 24 h) and the establishment of layers 2 and 3 responsible for replenishing cells that have migrated into of the regenerating area occurred, suggest a need for the wounded area after injury.
centripetally migrating cells in order to complete re-epi- Integration of our previous data regarding DNA syn-thelialization. Thus, replacement of cells in the abraded thesis following wounding [33,34] with the information on region is dependent on the epithelium adjacent to the mitotic behavior during corneal repair is revealing (Fig. 3). injury and not from the repopulating area, and involves a The regenerating region of the corneal epithelium has little change in the tempo of cell replication and the anatomical DNA synthesis or mitosis, suggesting that cells repopulat-location of the generative cells. Finally, our studies show ing the damaged surface are not reliant upon cell
replica-Fig. 5. Histogram of the average mitotic activity in both basal and suprabasal cells in different regions of the ocular surface in animals receiving a 3 mm abrasion of the central region of the corneal epithelium. Data are presented as means6S.E. Significantly different from homeostatic levels at P,0.05 (*),
tion. Our data also show that cells undergoing DNA between the spatial distribution of mitotic cells and the synthesis in the regions adjacent to the wound migrate into temporal course of wound repair.
the denuded surface. However, the completion of cell Now that we have more clearly elucidated the cell generative processes must be at the periphery of the wound proliferative processes in homeostasis and wound healing because little mitosis was observed in the regenerative of the corneal epithelium, future studies will be designed to region after injury. Wounding of the corneal surface did not perturb these processes utilizing growth factors in order to immediately disrupt DNA synthesis in the uninjured examine the mechanism regulating the biology of cell region. However, from 12 h onwards to day 5, these basal behavior in the maintenance and repair of the corneal epithelial cells demonstrated extraordinary activity in epithelium.
respect to DNA synthesis. Moreover, these cells complete the cell division process because a correlative increase in
mitosis can be observed. A major change in cell behavior Acknowledgements in response to wounding is the change in location of S and
M phase cells. The proportion of cells in S phase in layer 2 This work was supported by NIH grant EY10300. The increases dramatically from 12 h to day 5 (the termination authors thank Mary Haldeman, Jennifer Lehman, Jamie of experiments), presumably as an accommodation for the Fry, and Jim Wiley for technical assistance.
increased DNA synthesis needed to replace the cells eliminated by debridement. The ratio of mitotic cells in
layers 1 and 2 also changes notably so that more of these References cells undergoing mitosis are in the basal layer, presumably
reflecting the need for more centripetally migrating cells to [1] L.B. Arey, W.M. Covode, The method of repair in epithelial wounds of the cornea, Anat. Rec. 86 (1943) 75–86.
repopulate the wounded region. There also appears to be a
[2] F.D. Bertalanffy, C. Lau, Mitotic rate and renewal time of the
difference in the temporal recovery of the distribution
corneal epithelium in the rat, Arch. Ophthalmol. 68 (1962) 144–
pattern of mitotic and DNA synthesizing cells with respect 148.
to wound healing. Finally, mechanical injury to the central [3] W. Buschke, J.S. Friedenwald, W. Fleischmann, Studies on the
corneal epithelium does not appear to have a major impact mitotic activity of the corneal epithelium. Methods. The effects of colchicine, ether, cocaine and ephedrin, Bull. Johns Hopkins
Hospi-on DNA synthesis or mitosis in the limbus or cHospi-onjunctiva,
tal 73 (1943) 143–168.
particularly within the time frame examined. Whether
[4] K.Y. Chan, D.L. Patton, Y.T. Cosgrove, Time-lapse
videomicro-long-term perturbations in these regions of the ocular scopic study of in vitro wound closure in rabbit corneal cells, Invest. surface epithelium occur after re-epithelialization of the Ophthalmol. Vis. Sci. 30 (1989) 2488–2498.
wound needs clarification. [5] E.H. Chung, A.E.K. Hutcheon, N.C. Joyce, J.D. Zieske,
Synchroni-zation of the G1 / S transition in response to corneal debridement,
The present study which defines the number and
loca-Invest. Ophthalmol. Vis. Sci. 40 (1999) 1952–1958.
tion of mitosis in the epithelium of the ocular surface adds
[6] J.S. Friedenwald, W. Buschke, Mitotic and wound-healing activities
to the base of information provided by our previous studies of the corneal epithelium, Arch. Ophthalmol. 32 (1944) 410–413. [32–34] of DNA synthesis in homeostasis and wound [7] J.S. Friedenwald, W. Buschke, The influence of some experimental
healing. Principles elucidated with regard to mitotic be- variables on the epithelial movements in the healing of corneal wounds, J. Cell. Comp. Physiol. 23 (1944) 95–107.
havior and homeostasis include: (i) mitosis occurs in the
[8] I.K. Gipson, S. Spurr-Michaud, A. Tisdale, M. Keough, Reassembly
basal epithelium of the cornea, limbus, and conjunctiva in
of the anchoring structures of the corneal epithelium during wound
approximately equal rates, (ii) mitosis in the suprabasal repair in the rabbit, Invest. Ophthalmol. Vis. Sci. 30 (1989) 425– layers is of unequal magnitude in the regions of the ocular 434.
surface epithelium, with the corneal epithelium containing [9] I.K. Gipson, S.P. Sugrue, Cell biology of the corneal epithelium, in: D.M. Albert, F.A. Jakobiec (Eds.), Principles and Practice of
approximately 5-fold more mitotic figures than in the
Ophthalmology. Basic Sciences, Saunders, Philadelphia, PA, 1994,
limbus or conjunctiva, (iii) the midline of the cornea
pp. 3–16.
contains a region that is of low abundance in mitotic [10] R.S. Grillo, B.A. Knowles, D.F. Cooperstein, An autoradiographic figures, (iv) mitosis is more than twice as abundant in evaluation of the regeneration of corneal epithelium in Bufo
epithelial layer 2 than in the basal layer, and (v) mitotic marinus, J. Exp. Zool. 203 (1978) 201–206.
[11] C. Hanna, Proliferation and migration of epithelial cells during
cells in layer 2 often retain connection to the basal lamina.
corneal wound repair in the rabbit and the rat, Am. J. Ophthalmol.
With respect to mitosis and wound healing of the corneal
61 (1966) 55–63.
epithelium: (i) mitosis continues after injury, (ii) the [12] C. Hanna, D.S. Bicknell, J.E. O’Brien, Cell turnover in the adult regenerating region does not depend upon mitosis for cell human eye, Arch. Ophthalmol. 61 (1961) 111–114.
repopulation, (iii) basal epithelial cells in the region [13] J. Hara, An autoradiographic study of regeneration of the corneal epithelium, Folia Ophthalmol. Jpn. 13 (1962) 443–466.
adjacent to the wound margin of the wound are largely
[14] E. Haskjold, R. Bjerknes, S.B. Refsum, Cell kinetics during healing
responsible for the production of cells contributing to
of corneal epithelial wounds, Acta Ophthalmol. 67 (1989) 174–180.
healing, (iv) re-epithelialization is not dependent on [15] E. Haskjold, S.B. Refsum, R. Bjerknes, Cell renewal of the rat mitosis in the suprabasal layers, (v) cell proliferation is corneal epithelium, Acta Ophthalmol. 66 (1988) 533–537.
mitotic rate of the rat corneal epithelium. Cell divisions and [27] H.W. Thompson, J.S. Malter, T.L. Steinemann, R.W. Beuerman, migration are analyzed by a mathematical model, Virchows Arch. B Flow cytometry measurements of the DNA content of corneal Cell Pathol. 58 (1989) 123–127. epithelial cells during wound healing, Invest. Ophthalmol. Vis. Sci. [17] N.C. Joyce, B. Meklir, S.J. Joyce, J.D. Zieske, Cell cycle protein 32 (1991) 433–436.
expression and proliferative status in human corneal cells, Invest. [28] M. Yamada, Y. Mashima, Changes in proliferation and differentia-Ophthalmol. Vis. Sci. 37 (1996) 645–655. tion of basal cells during wound healing of rabbit corneal epithelial [18] B. Kaufman, H. Gay, A. Hollaender, Distribution of mitoses in the abrasions, J. Jap. Ophthalmol. Soc. 99 (1995) 10–16.
corneal epithelium of the rabbit and the rat, Anat. Rec. 90 (1944) [29] Z.-G. Wei, G. Cotsarelis, T.-T. Sun, R.M. Lavker, Label-retaining 161–178. cells are preferentially located in fornical epithelium: Implications [19] H.E. Kaufman, B.A. Barron, M.B. McDonald, S.T. Waltman, The on conjunctival epithelial homeostasis, Invest. Ophthalmol. Vis. Sci.
Cornea, Livingstone, New York, 1988. 36 (1995) 236–246.
[20] S. Kinoshita, T.C. Kiorpes, J. Friend, R.A. Thoft, Limbal epithelium [30] Z.-G. Wei, T.-T. Sun, R.M. Lavker, Rabbit conjunctival and corneal in ocular surface wound healing, Invest. Ophthalmol. Vis. Sci. 23 epithelial cells belong to two separate lineages, Invest. Ophthalmol.
(1982) 73–80. Vis. Sci. 37 (1996) 523–533.
[21] T. Kuwabara, D.G. Perkins, D.G. Cogan, Sliding of the epithelium [31] Z.-G. Wei, R.-L. Wu, R.M. Lavker, T.-T. Sun, In vitro growth and in experimental corneal wounds, Invest. Ophthalmol. 15 (1976) differentiation of rabbit bulbar, fornix, and palpebral conjunctival
4–14. epithelia, Invest. Ophthalmol. Vis. Sci. 34 (1993) 1814–1828.
[22] R.M. Lavker, G. Dong, S.Z. Cheng, K. Kudoh, G. Cotsarelis, T.-T. [32] I.S. Zagon, J.W. Sassani, E.R. Kane, P.J. McLaughlin, Homeostasis Sun, Relative proliferative rates of limbal and corneal epithelia, of ocular surface epithelium in the rat is regulated by opioid growth Invest. Ophthalmol. Vis. Sci. 21 (1991) 1864–1875. factor, Brain Res. 759 (1997) 92–102.
[23] K.U. Sandvig, E. Haaskjold, S.B. Refsum, Time dependency in the [33] I.S. Zagon, J.W. Sassani, P.J. McLaughlin, Cellular dynamics of regenerative response to injury of the rat corneal epithelium, corneal wound re-epithelialization in the rat. I. Fate of ocular surface Chronobiol. Internat. 11 (1994) 173–179. epithelial cells synthesizing DNA prior to wounding, Brain Res. 822 [24] A. Schermer, S. Galvin, T.-T. Sun, Differentiation-related expres- (1999) 149–163.
sion of a major 64 K corneal keratin in vivo and in culture suggests [34] I.S. Zagon, J.W. Sassani, P.J. McLaughlin, Cellular dynamics of limbal location of corneal epithelial stem cells, J. Cell Biol. 103 corneal wound re-epithelialization in the rat. II. DNA synthesis of (1989) 49–62. the of the ocular surface epithelium following wounding, Brain Res. [25] G.K. Smelser, V. Ozanics, Effect of chemotherapeutic agents on cell 839 (1999) 243–252.
division and healing of corneal burns and abrasions in the rat, Am. J. [35] J.D. Zieske, I.K. Gipson, Agents that affect corneal wound healing: Ophthalmol. 27 (1944) 1063–1072. Modulation of structure and function, in: D.M. Albert, F.A. Jakobiec [26] R.A. Thoft, J. Friend, The X, Y, Z hypothesis of corneal epithelia (Eds.), Principles and Practice of Ophthalmology. Basic Sciences,