www.elsevier.com / locate / bres
Short communication
Tonic inhibition of food intake during inactive phase is reversed by
the injection of the melanocortin receptor antagonist into the
paraventricular nucleus of the hypothalamus and central amygdala of
the rat
a ,
*
b¨
Ants Kask
, Helgi B. Schioth
a
Department of Pharmacology, Faculty of Medicine, University of Tartu, Ravila 19, Tartu 51014, Estonia
b
Department of Neuroscience, Uppsala University, Uppsala, Sweden
Accepted 19 September 2000
Abstract
Melanocortins inhibit food intake and melanocortin 4 receptor (MC R) antagonists stimulate feeding behaviour. These effects may4
occur due to stimulation or blockade of MC receptors in the hypothalamus. To test the validity of this hypothesis, a cyclic peptide, the4 MC R selective antagonist HS014 (20, 100 and 500 pmol), or vehicle, was injected unilaterally into the paraventricular nucleus of the4
hypothalamus (PVN). As MC receptors are expressed also in extrahypothalamic sites involved in the regulation of feeding behaviour, HS014 was injected bilaterally into the vicinity of the central nucleus of the amygdala (CA) and the nucleus accumbens region (Acc). All doses of HS014 induced a dose-dependent increase in food intake when injected into the PVN. Intra-amygdalar injections of HS014 (50 and 250 pmol / side) also stimulated food intake, whereas a 10-pmol dose was inactive. Local microinjections of HS014 into the Acc failed to stimulate feeding. These data suggest that endogenous melanocortin receptor agonists exert a tonic inhibitory influence on food consumption by stimulating MC receptors in the hypothalamus and amygdala.4 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Peptides: anatomy and physiology
Keywords: Feeding; Melanocortin receptor; MC receptor antagonist, HS014; Amygdala; Nucleus accumbens; Paraventricular nucleus; Hypothalamus; Rat4
Energy consumption and expenditure in mammals are coupled to changes in activity of POMC [39] and altered controlled by the co-ordinated action of several neuro- POMC-expression in the arcuate nucleus of the hypo-peptides in the central nervous system. Pro-opiomelanocor- thalamus is seen in various physiological and pathological tin (POMC) is cleaved by prohormone convertases PC1 conditions such as lactation [35], infection [8] and obesity [21] and PC2 [28] to a number of smaller biologically [18]. Although the effects of melanocortin peptides on active neuropeptides, commonly referred to as melanocor- feeding were described in the 1980s [29], the concept that tins. One of them, alpha-melanocyte stimulating hormone POMC is an endogenous regulator of feeding was estab-(a-MSH), initially known for its effects on skin pigmenta- lished only recently [5]. The obesity in yellow obese tion in amphibians, induces hypophagia [1,14,29] and (Ay /Ay) mice was explained with overproduction and increases thermogenesis in rats [31].b-MSH also inhibits ectopic expression of Agouti peptide that normally reg-food intake, whereas g1-MSH and g2-MSH, the preferen- ulates pigment synthesis in melanocytes. It was suggested tial melanocortin 3 receptor (MC R) agonists in the CNS,3 that Agouti competes witha-melanocyte stimulating hor-do not [1,14]. Circadian patterns of food intake seem to be mone binding at melanocortin subtypes. The Agouti pep-tide is a potent antagonist of MC R and MC R and3 4 targeted disruption of MC R function results in the pheno-4
*Corresponding author. Present address: Douglas Hospital Research
type seen in obese Agouti mice [12]. Therefore, it is likely ´
Center, 6875, Boul. LaSalle, Verdun, Quebec, Canada H4H 1R3.
E-mail address: [email protected] (A. Kask). that signalling at MC R is important for regulating both4
A. Kask, H.B. Schioth / Brain Research 887 (2000) 460 –464 461
short-term and long-term food intake. This proposal has into the lumen of each cannula to help maintain patency. gained support from studies where MC R antagonists, such4 Rats recuperated for at least 7 days after surgery and they as SHU9119 [11] and HS014 [34], have been shown to were frequently handled and weighed during the recovery increase feeding [7,16,17,32,37]. MC R may also have an4 period to habituate them to partial restraint during i.c.v. endogenous antagonist, the C-terminal fragment of Agouti- injections and testing procedure. All feeding experiments related protein (Agrp) that has been shown to antagonise were performed not earlier than 7 days after the surgery. MC receptors and increase food intake in rats [32]. On the day of the experiment, rats were then injected with
11 14 18
Interestingly enough, MC R antagonists not only interfere4 vehicle (aCSF) or cyclic peptide (AcCys ,D-Nal , Cys ,
22
with satiety mechanisms, but they alone elicit robust Asp-NH )-2 b-MSH11 – 22, a melanocortin MC R antagonist4 feeding response during light phase, at a time when (HS014, 20, 100 and 500 pmol in 0.5 ml of aCSF). For nocturnal animals such as rats and mice eat very little. This bilateral administration, the amount of drug given per each suggests that melanocortins exert tonic inhibitory influence site of injection was two time lower, so that the total on neural pathways that facilitate food intake and endogen- amount of HS014 given per rat was always the same. The ous MC R antagonists either disinhibit neurotransmitter4 injections were carried out over 1 min using fine stainless systems that normally suppress ingestive behaviour or they steel injector connected to the 10 ml SGE syringe and amplify other orexigenic signals such as neuropeptide Y infusion pump (World Precision Instruments, Sarasota, [26]. While the orexigenic effects of MC R antagonists4 USA). The movement of a small air bubble (less than 1 have been found in several laboratories, the neural sites mm in length within polyethylene tubing) confirmed the mediating these responses are largely unknown. MC R and3 flow of the fluid. The needle was left in place for 30 s to MC R have been found in several brain regions including4 prevent backflow through the needle track and the cannula the hypothalamus, limbic structures and hindbrain, and was closed with stylet. Following the injection, animals MC R seems to be most abundant MC receptor subtype in3 were returned to their home cages, containing known these areas [22]. Recently it was shown that the effects of amount (30–40 g) of the pellets in food holders. Three synthetic analogue of a-MSH, MTII and non-selective days after first experiment, rats were re-tested in counter-MCR antagonist SHU9119 modulate feeding behaviour balanced order so than none of the rats received the same after intrahypothalamic application [38]. The results of the treatment twice. All experiments were carried out between studies where fourth and lateral ventricle injections of 11:00 and 17:00 h. Food intake was measured by weighing MTII and SHU9119 were used, led Grill et al. [10] to remaining pellets and the spillage using Mettler balance to propose that caudal brainstem might be involved in the nearest 0.01 g. Verification of injection sites was carried melanocortic regulation of feeding. However, MC R has4 out using standard methods [15]. Namely, after the last been found also in more rostral areas of the brain that experiment, all rats were overdosed with chloral hydrate participate in the regulation of ingestive behaviour and (600 mg / kg, i.p.) and 0.3 ml of Fast Green dye was energy homeostasis [24]. The purpose of this study was to infused to mark the injection site. The brains were compare the effects of the MC R selective antagonist4 removed and immersed in 10% formaldehyde for at least 1 HS014 on food intake after unilateral application into the week. Injection sites were examined from 15-mm thick paraventricular nucleus of the hypothalamus and bilateral hematoxylin–eosin stained coronal sections under low injections into the amygdala and nucleus accumbens. magnification microscope by an observer unaware to the Sixty male Wistar / Kuo rats (National Laboratory Ani- behavioural results. Data from animals with improperly mal Center, Kuopio, Finland) weighing 350–390 g at the placed cannulae were not included in data analysis. Cyclic time of surgery, were housed individually in wire-bot- MSH analogue HS014 was provided by Melacure Thera-tomed cages (45337319 cm) with free access to food peutics AB, (Uppsala, Sweden). HS014 was dissolved in (Diet R35, Lactamin AB, Vadstena, Sweden) and water in a distilled water and stored in aliquots at 2208C. Final temperature controlled room at 21628C in a room with a dilutions were made with aCSF. All results are expressed 12-h light / dark cycle (lights on at 08:00 h). Stainless steel as mean6S.E.M. The cumulative food intake data were cannulae (O.D. 0.5 mm) were implanted 1 or 2 mm analysed by analysis of variance (ANOVA) for repeated (amygdala) above the target site under general chloral measures and the amount of food consumed by specific hydrate anaesthesia (350 mg / kg / 10 ml i.p.). The final time points was analysed by factorial ANOVA. When coordinates, determined were as follows: (1) the paraven- ANOVA yielded with positive results, multiple compari-tricular nucleus of the hypothalamus (PVN) — A: 20.4; sons between treatment groups were carried out using L: 20.4; V: 27.2 with tooth bar at 13.0; (2) central Scheffe’s post-hoc test. Experimental procedures were nucleus of the amygdala (CA) — A: 22.3; L: 64.2; V: carried out in accordance with guidelines of the European
28.1 and (3) the nucleus accumbens (Acc) — A:11.4; L: Community, local laws and policies, and were approved by
61.6; V:25.5 in skull flat position. For the Acc, cannulas the Ethics Committee of Animal Experiments at the were not aimed specifically at the core or shell part and in University of Tartu.
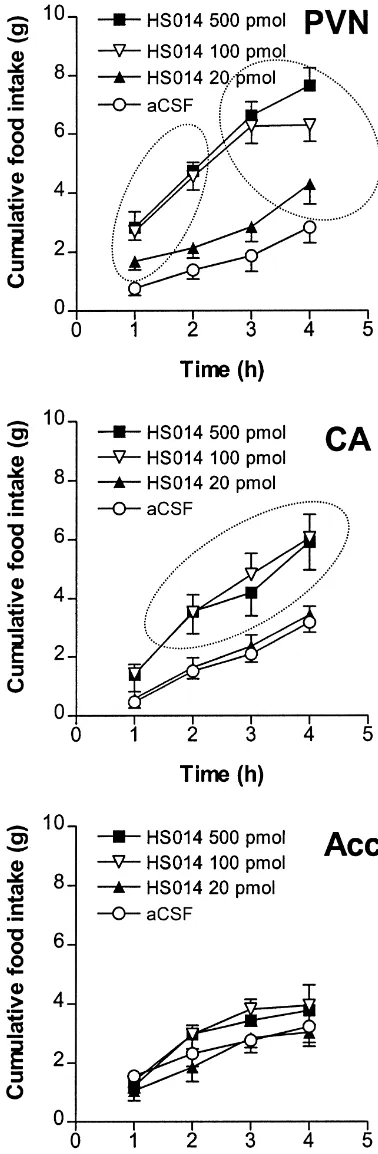
effects were, respectively: F(3,76)518.84, P,0.0001; F(3,228)5136.06, P,0.0001; F(9,228)55.97, P,0.0001. Food intake differed between the groups at 1, 2, 3 and 4 h — F(4,39)511.63; 17.55; 17.11 and 16.57, respectively, P,0.0001. Individual comparisons with Scheffe’s test indicated that maximal effect of HS014 was observed already at 100 pmol dose. The effects of 100 and 500 pmol of HS014 in the PVN were similar. HS014 stimulated feeding at 20 pmol, but less efficiently than at the two higher doses. Bilateral application of HS014 into the central nucleus of the amygdala resulted in dose-dependent stimulation of feeding (Fig. 1). Repeated measures ANOVA for the drug, time and interaction effects was, respectively: F(3,29)56.18, P,0.005; F(3,87)589.95, P,0.0001; F(9,87)52.01, P,0.05. Factorial ANOVA carried out on 1, 2, 3 and 4 h cumulative food intake data indicated that, at 1 h after treatment, rats treated with vehicle or different doses of HS014 ate the same amount of food. At subsequent measurements, food intake was significantly higher in rats treated with 100 and 500 pmol dose of HS014 as compared to vehicle-treated rats (Fig. 1). When HS014 was microinjected bilaterally into the nu-cleus accumbens, no significant effects on food intake was seen: time and treatment interaction F(9,66)50.78, NS (Fig. 1).
Our results confirm the critical role of MC R in the4 regulation of food intake. These results complement the study of Giraudo et al. [9] showing that intra-hypothalamic administration of MC R antagonist SHU9119 increased4 feeding. Furthermore, our data demonstrate that prophagic effect of the same magnitude is seen after microinjection of MC R antagonist HS014 into the vicinity of central4 nucleus of the amygdala. There is accumulating evidence that melanocortins regulate feeding via MC R. The first4 and most convincing evidence is the development of obesity and tolerance to anorectic effects of MTII in MC R knockout mouse [12,25]. Secondly, MC R antago-4 4 nists potently stimulate food intake [2,7,9,10,16,17,32,37], and, finally, MC R selective agonists3 g1-MSH and g2 -MSH do not inhibit food intake [1,14]. POMC-synthe-sising neurons are predominantly located in the arcuate nucleus of the hypothalamus [39]. These neurones also contain several other neurotransmitters, including mono-amines, serotonin and several neuropeptides [13].a -MSH-containing neurones project to a number of brain areas including the amygdala and the nucleus accumbens [6], brain regions that are known to be involved in the regulation of food intake.
Lindblom et al. [23] have recently shown that MC R in4
Fig. 1. A robust increase in food intake in freely-feeding male rats in the
the Acc are downregulated in genetically obese OLETF middle phase of the light after intracerebral injection of the MC R4
antagonist HS014. Vehicle or HS014 (20, 100, 500 pmol, n56–9 in each rats. Although there are few studies that link POMC in the group) was injected unilaterally into the vicinity of the paraventricular Acc to addictive behaviours such as innate preference for nucleus (PVN), bilaterally (10, 50 and 250 pmol / side) into the region of
alcohol [30], the effects of MCR agonists on food intake central amygdala (CA) or into the nucleus accumbens (Acc). Food intake
have not been studied after microinjection into the Acc was measured each hour up to 4 h following injection. Doses in figure
region. With these findings in mind, one could expect that refer to total amount of HS014 given per rat. Encircled (???) data values
refer to the results that are significantly different from controls (P,0.05, microinjection of the MC R antagonists into this brain4
A. Kask, H.B. Schioth / Brain Research 887 (2000) 460 –464 463
region could also modulate food intake, but this was not amygdala. Notably, amygdala also has a high density of the case. Yet, this does not rule out the possibility that melanocortin receptors [23]. Thus, it may be concluded MC R in the Acc could be involved in the regulation of4 that both hypothalamic and amygdalar MC Rs exert tonic4 feeding behaviour under these conditions. inhibitory tone on neural pathways that facilitate feeding
The hypothalamus is a complex structure with multiple behaviour.
neurotransmitters controlling a variety of functions. The Several classical neurotransmitters and neuropeptides pathway from the arcuate nucleus to PVN is one of the inhibit food intake when given i.c.v. but there is not always projections regulating feeding behaviour [13]. PVN is enough evidence to suggest that these agents inhibit sensitive to a number of orexigenic hormones such as NPY feeding under physiological conditions. A stronger case is [13], galanin [22], melanin-concentrating hormone [33] made when antagonist, preventing the action of endogen-and orexins [36]. Until now it was not known whether ous neurotransmitter, causes a gain of function by stimulat-MCR agonists inhibit feeding when microinjected into ing feeding behaviour. In this study we show that intra-extrahypothalamic areas regulating feeding behaviour. PVN and intra-amygdalar injection of the MC R HS0144 Intracerebroventricularly injected MC agonists have been potently stimulates food intake. Our data suggest that shown to induce c-fos expression in several brain areas activation of MC R by endogenous MCR agonists in the4 including the PVN, the arcuate nucleus [4] and the PVN and amygdala inhibits food intake. The blockade of amygdala [2]. It was also shown that MTII and SHU9119, MC R in these regions either by exogenously applied4 potent MCR agonist and antagonist respectively, have very synthetic MC R antagonist or Agouti-related protein4 potent effects on food intake following intra-PVN in- (Agrp), endogenous MCR antagonist, reverses this tonic jections [9]. Our data with HS014, a cyclic peptide that has inhibition and leads to stimulation of food intake. more than 300-fold selectivity for rat MC R vs. rat MC R4 3
[23], confirm this, adding further support to the hypothesis that the ARC–PVN pathway is involved in integrating a
Acknowledgements
variety of signals regulating feeding behaviour [13]. We also show that the MC R antagonist microinjected4
This study was supported by research grant from into the central nucleus of amygdala increases food intake.
Melacure Therapeutics AB to the University of Tartu Feeding response induced by highest dose of HS014 in the
¨
(VARFR 09598). Helgi B. Schioth was supported by amygdala was somewhat weaker that orexia induced by
¨ ¨
SSMF (Svenska Sallskapet for Medicinsk Forskning) and intra-PVN HS014. Moreover, in the PVN HS014, in a dose
¨
the Swedish Brain Foundation (Hjarnfonden). We wish to as low as 20 pmol, stimulated feeding whereas in the
thank Maia Orav and Eve Proovel for skilled technical amygdala HS014 was inactive. A more delayed onset and
assistance and Anna Skottner for useful comments on the lower magnitude of feeding response to intra-CA HS014
manuscript. would be suggestive of drug diffusion into the PVN
(calculated distance between injection sites is ¯4 mm). However administration of HS014 into the Acc (PVN–Acc distance¯3 mm) failed to increase food intake confirming
References
site specificity of the effect. MTII administered i.c.v. increases c-fos expression in the central nucleus of the
[1] C.R. Abbott, M. Rossi, M.-S. Kim, S.H. AlAhmed, G.M. Taylor, M. amygdala [2] and bed nucleus of stria terminalis [3],
Ghatei, D.M. Smith, S.R. Bloom, Investigation of the melanocyte suggesting that these brain areas may be involved in stimulating hormones on food intake: Lack of evidence to support a melanocortin-induced inhibition of food intake [27]. C-fos role for the melanocortin-3-receptor, Brain Res. 869 (2000) 203–
210. in these brain regions is also increased by stress [4] but the
[2] C. Benoit, M.W. Schwartz, J.L. Lachey, M.M. Hagan, P.A. Rushing, stimulation of food feeding in our study should not be
K.A. Blake, K.A. Yagaloff, G. Kurylko, L. Franco, W. Danhoo, R.J. interpreted as anxiolytic effects because rats were tested in
Seeley, A novel selective melanocortin-4 receptor agonist reduces a home cage and were habituated to the testing procedure. food intake in rats and mice without producing aversive conse-Although most of the studies of ingestive behaviour have quences, J. Neurosci. 20 (2000) 3442–3448.
[3] K.S. Brown, R.M. Gentry, N.E. Rowland, Central injection in rats of focused on the hypothalamus, the role of other limbic
alpha-melanocyte-stimulating hormone analog: effects on food structures in ingestive behaviour has been known for a
intake and brain Fos, Regul. Pept. 78 (1998) 89–94. long time. After lesioning of the amygdala, hyperphagia
[4] S. Campeau, W.A. Falls, W.E. Cullinan, D.L. Helmreich, M. Davis, and obesity is observed in rats [19], both in females and S.J. Watson, Elicitation and reduction of fear: behavioural and males [20]. King et al. [20] proposed the existence of an neuroendocrine indices and brain induction of the immediate-early
gene c-fos, Neuroscience 78 (1997) 1087–1104. amygdala–PVN pathway that is involved in the regulation
[5] R.D. Cone, The central melanocortin system and its role in energy of feeding behaviour. Since the majority of the brain
homeostasis, Ann. Endocrinol. (Paris) 60 (1999) 3–9.
a-MSH seems to originate from the arcuate nucleus and
[7] W. Fan, B.A. Boston, R.A. Kesterson, V.J. Hruby, R.D. Cone, Role involved in food intake and reward mechanisms in obese (OLETF) of melanocortinergic neurons in feeding and the Agouti obesity rats, Brain Res. 852 (2000) 180–185.
syndrome, Nature 385 (1997) 165–168. [24] J. Lindblom, H.B. Schioth, A. Larsson, J.E. Wikberg, L. Bergstrom,¨ [8] D. Gayle, S.E. Ilyin, C.R. Plata-Salaman, Feeding status and Autoradiographic discrimination of melanocortin receptors indicates bacterial LPS-induced cytokine and neuropeptide gene expression in that the MC3 subtype dominates in the medial rat brain, Brain Res. hypothalamus, Am. J. Physiol. 277 (1999) 1188–1195. 810 (1998) 161–171.
[9] S.Q. Giraudo, C.J. Billington, A.S. Levine, Feeding effects of [25] D.J. Marsh, G. Hollopeter, D. Huszar, R. Laufer, K.A. Yagaloff, S.L. hypothalamic injection of melanocortin 4 receptor ligands, Brain Fisher, P. Burn, R.D. Palmiter, Response of melanocortin-4 receptor-Res. 809 (1998) 302–306. deficient mice to anorectic and orexigenic peptides, Nat. Genet. 21 [10] H.J. Grill, A.B. Ginsberg, R.J. Seeley, J.M. Kaplan, Brainstem (1999) 119–122.
application of melanocortin receptor ligands produces long-lasting [26] D.J. Marsh, G.I. Miura, K.A. Yagaloff, M.W. Schwartz, G.S. Barsh, effects on feeding and body weight, J. Neurosci. 18 (1998) 10128– R.D. Palmiter, Effects of neuropeptide Y deficiency on hypothalamic 10135. agouti-related protein expression and responsiveness to melanocortin [11] V.J. Hruby, D. Lu, S.D. Sharma, A.L. Castrucci, R.A. Kesterson, analogues, Brain Res. 848 (1999) 66–77.
F.A. al-Obeidi, M.E. Hadley, R.D. Cone, Cyclic lactam alpha- [27] B. Murphy, C.N. Nunes, J.J. Ronan, C.M. Harper, M.J. Beall, M. melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7, Lys10] Hanaway, A.M. Fairhurst, L.H. Van der Ploeg, D.E. MacIntyre, T.N. alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aro- Mellin, Melanocortin mediated inhibition of feeding behavior in matic amino acids at position 7 show high antagonist potency and rats, Neuropeptides 32 (1998) 491–497.
selectivity at specific melanocortin receptors, J. Med. Chem. 38 [28] L. Paquet, A. Zhou, E.Y. Chang, R.E. Mains, Peptide biosynthetic (1995) 3454–3461. processing: distinguishing prohormone convertases PC1 and PC2, [12] D. Huszar, C.A. Lynch, V. Fairchild-Huntress, J.H. Dunmore, Q. Mol. Cell. Endocrinol. 120 (1996) 161–168.
Fang, L.R. Berkemeier, W. Gu, R.A. Kesterson, B.A. Boston, R.D.
[29] R. Poggioli, A.-V. Vergoni, A. Bertolini, ACTH-(1-24) and alpha-Cone, F.J. Smith, L.A. Campfield, P. Burn, F. Lee, Targeted
MSH antagonize feeding behavior stimulated by kappa opiate disruption of the melanocortin-4 receptor results in obesity in mice,
agonists, Peptides 7 (1986) 843–848. Cell 88 (1997) 131–141.
[30] D.D. Rasmussen, C.A. Bryant, B.M. Boldt, E.A. Colasurdo, N. [13] S.P. Kalra, M.G. Dube, S. Pu, B. Xu, T.L. Horvath, P.S. Kalra,
Levin, C.W. Wilkinson, Acute alcohol effects on opiomelanocor-Interacting appetite regulating pathways in the hypothalamic
regula-tinergic regulation, Alcohol. Clin. Exp. Res. 22 (1998) 789–801. tion of body weight, Endocr. Rev. 20 (1999) 68–100.
[31] G.E. Resch, W.R. Millington, Glycyl-L-glutamine antagonizes
alpha-¨ ¨
[14] A. Kask, L. Rago, J.E. Wikberg, H.B. Schioth, Differential effects of
MSH-elicited thermogenesis, Peptides 14 (1993) 971–975. melanocortin peptides on ingestive behaviour in rats: evidence
[32] M. Rossi, M.S. Kim, D.G. Morgan, C.J. Small, C.M. Edwards, D. against the involvement of MC receptor in the regulation of food3
Sunter, S. Abusnana, A.P. Goldstone, S.H. Russell, S.A. Stanley, intake, Neurosci. Lett. 283 (2000) 1–4.
D.M. Smith, K. Yagaloff, M.A. Ghatei, S.R. Bloom, A C-terminal ¨
[15] A. Kask, L. Rago, J. Harro, Anxiogenic-like effect of the NPY Y1
fragment of Agouti-related protein increases feeding and antagon-receptor antagonist BIBP3226 administered into the dorsal
izes the effect of alpha-melanocyte stimulating hormone in vivo, periaqueductal gray matter in rats, Regul. Pept. 75–76 (1998)
Endocrinology 139 (1998) 4428–4431. 255–262.
[33] M. Rossi, S.A. Beak, S.J. Choi, C.J. Small, D.G. Morgan, M.A.
¨ ¨
[16] A. Kask, L. Rago, F. Mutulis, R. Pahkla, J.E. Wikberg, H.B.
Ghatei, D.M. Smith, S.R. Bloom, Investigation of the feeding effects ¨
Schioth, Selective antagonist for the melanocortin 4 receptor
of melanin concentrating hormone on food intake: action indepen-(HS014) increases food intake in free-feeding rats, Biochem.
dent of galanin and the melanocortin receptors, Brain Res. 846 Biophys. Res. Commun. 245 (1998) 90–93.
(1999) 164–170.
¨ ¨ ¨
[17] A. Kask, R. Pahkla, A. Irs, L. Rago, J.E. Wikberg, H.B. Schioth,
¨
[34] H.B. Schioth, F. Mutulis, R. Muceniece, P. Prusis, J.E. Wikberg, Long-term administration of MC4 receptor antagonist HS014 causes
Discovery of novel melanocortin 4 receptor selective MSH ana-hyperphagia and obesity in rats, NeuroReport 10 (1999) 707–711.
logues, Br. J. Pharmacol. 124 (1998) 75–82. [18] E.-M. Kim, E. O’Hare, M.C. Grace, C.C. Welch, C.J. Billington,
[35] M.S. Smith, Lactation alters neuropeptide-Y and proopiomelanocor-A.S. Levine, ARC POMC mRNA and PVN a-MSH are lower in
tin gene expression in the arcuate nucleus of the rat, Endocrinology obese relative to lean Zucker rats, Brain Res. 862 (2000) 11–16.
133 (1993) 1258–1265. [19] B.M. King, K.N. Rossiter, J.T. Cook, H.M. Sam, Amygdaloid
[36] D.C. Sweet, A.S. Levine, C.J. Billington, C.M. Kotz, Feeding lesion-induced obesity in rats in absence of finickiness, Physiol.
response to central orexins, Brain Res. 821 (1999) 535–538. Behav. 62 (1997) 935–938.
[37] A.-V. Vergoni, A. Bertolini, F. Mutulis, J.E. Wikberg, H.B. Schioth, [20] B.M. King, B.L. Rollins, S.G. Stines, S.A. Cassis, H.B. McGuire,
Differential influence of a selective melanocortin MC receptor
M.L. Lagarde, Sex differences in body weight gains following 4
antagonist (HS014) on melanocortin-induced behavioral effects in amygdaloid lesions in rats, Am. J. Physiol. 277 (1999) 975–980.
rats, Eur. J. Pharmacol. 362 (1998) 95–101. [21] J. Korner, J. Chun, D. Harter, D. Axel, Isolation and functional
[38] T.E. Thiele, G. van Dijk, K. Yagaloff, S.L. Fisher, M. Schwartz, P. expression of a mammalian prohormone processing enzyme, murine
Burn, R.J. Seeley, Central infusion of melanocortin agonist MTII in prohormone convertase 1, Proc. Natl. Acad. Sci. USA 88 (1991)
rats: assessment of c-Fos expression and taste aversion, Am. J. 6834–6838.
Physiol. 43 (1998) R248–R254. [22] S.E. Kyrkouli, B.G. Stanley, R.D. Seirafi, S.F. Leibowitz,
Stimula-tion of feeding by galanin: anatomical localizaStimula-tion and behavioral [39] B. Xu, P.S. Kalra, W.G. Farmerie, S.P. Kalra, Daily changes in specificity of this peptide’s effects in the brain, Peptides 11 (1990) hypothalamic gene expression of neuropeptide Y, galanin,
995–1001. proopiomelanocortin, and adipocyte leptin gene expression and
¨
[23] J. Lindblom, H.B. Schioth, H. Watanobe, T. Suda, J.E. Wikberg, L. secretion: effects of food restriction, Endocrinology 140 (1999) ¨