www.elsevier.comrlocaterapplanim
A comparison of cell-mediated immune responses
in rhesus macaques housed singly, in pairs, or in
groups
Steven J. Schapiro
), Pramod N. Nehete, Jaine E. Perlman,
K. Jagannadha Sastry
Department of Veterinary Sciences, The UniÕersity of Texas M. D. Anderson Cancer Center, Rt. 2, Box 151-B1, Bastrop, TX 78602, USA
Accepted 5 January 2000
Abstract
A variety of psychosocial factors have been shown to influence immunological responses in laboratory primates. The present investigation examined the effects of social housing condition on
Ž .
cell-mediated immune responses, comparing rhesus macaques Macaca mulatta in three housing
Ž .
conditions single, pair, and group . Subjects included 12 adults of both sexes in each housing
Ž . Ž .
condition Ns36 . Multiple blood samples 0, 4, 8, and 12 months were collected for
immunological analyses, including lymphocyte subsets, lymphocyte proliferation to pathogens and
nonspecific mitogens, natural killer cell activity, and cytokine production. CD4qto CD8qratios
differed significantly across housing conditions and singly caged subjects had significantly lower
q q Ž .
CD4 rCD8 after the 4-month timepoint than did socially housed pair and group subjects.
CD4qto CD8qratios were positively correlated within subjects, suggesting a trait-like aspect to
this parameter. Lymphocyte proliferation responses to all four gastrointestinal pathogens differed
Ž .
across housing conditions at least at the 0.08 level , as did proliferation responses to StaphA, and
Ž .
the production of cytokines IFN-g, IL-2, and IL-10 . Proliferation responses of singly caged
monkeys did not differ from socially housed monkeys and the highest levels of both IFN-gand
IL-10 were produced by group housed subjects. The data demonstrate that social housing condition affects immune responses. While not unidirectional, these effects generally suggest enhanced immune responses for socially housed animals. Since rhesus monkeys live socially in nature, and the immune responses of singly housed animals differed from those housed socially,
)Corresponding author. Tel.:q1-512-321-3991; fax:q1-512-332-5208.
Ž .
E-mail address: [email protected] S.J. Schapiro .
0168-1591r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
there is considerable motivation and justification for suggesting that the use of singly housed rhesus macaques may complicate interpretations of normal immunological responses. This may have important implications for the management, treatment, and selection of primate subjects for
immunological studies.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Primate housing; Immune response; Macaca mulatta
1. Introduction
Housing captive primates socially, either in pairs or in groups, usually results in Ž
behavioral benefits for the animals so housed Crockett et al., 1994; Reinhardt, 1994;
. Ž
Schapiro et al., 1996a,b . While this is not always the case Coe, 1991; Ruppenthal et .
al., 1991; Crockett et al., 1994; Clarke et al., 1995 , if group or pair mates are properly Ž
chosen and attention is paid to other relevant psychosocial variables Reinhardt, 1989, 1994; Reinhardt et al., 1988, 1995; Crockett et al., 1994; Schapiro and Bloomsmith,
.
1994; Schapiro et al., 1996a , social housing can be an extremely effective enrichment strategy, providing numerous opportunities for the expression of species-typical
behav-Ž .
iors Bramblett, 1989; Mason, 1991; Novak and Suomi, 1991 . Behavioral measures provide important information about subjects’ psychological well-being in these types of studies, but it is also important to attempt to correlate behavioral measures with physiological measures, to further illustrate the relevance of the variables being manipu-lated and assessed.
A number of investigators have examined the effects that psychosocial variables can Ž
have on immunological responses in captive macaques Boccia et al., 1992; Cohen et al., 1992; Gust et al., 1992; Coe, 1993; Terao et al., 1995; Capitanio et al., 1996, 1998a;
.
Clarke et al., 1996; Schapiro et al., 1998 . A large proportion of these efforts have examined the effects of manipulations to the social environments of the subjects, with
Ž
many of these analyzing the effects of mother–infant separations Coe et al., 1988, .
1989; Laudenslager et al., 1990; Terao et al., 1995 . One normally observes diminished Ž
immune function in response to stressful social manipulations Cohen et al., 1992;
. Ž
Gordon et al., 1992; Coe, 1993; Lubach et al., 1995 . As described previously Schapiro
. Ž .
et al., 1998 , immune responses typically measured include: 1 lymphocyte subset
q qŽ
levels, primarily CD4 and CD8 Gust et al., 1991; Gordon et al., 1992; Lubach et al., . Ž .
1995; Capitanio et al., 1998a; Lilly et al., 1999 ; 2 lymphocyte proliferation responses ŽKaplan et al., 1991; Boccia et al., 1992; Cohen et al., 1992; Lubach et al., 1995 ; and. Ž .3 natural killer cell NK activity Boccia et al., 1992; Lubach et al., 1995; Terao et al.,Ž . Ž
. 1995 .
Since nonhuman primates are currently used in a variety of immunological studies ŽVillinger et al., 1993; Benveniste et al., 1996; Capitanio et al., 1998b; Gold et al., 1998;
.
Nehete et al., 1998; Marcario et al., 1999; Sarkar et al., 1999 , we feel that it is crucial to quantify the effects that behavioral management strategies, such as single housing during experiments, have on the immunological responses of captive primates. Work by
Ž .
Ž
singly caged rhesus macaques were allowed to spend regular periods 100 minrsession, .
3–5 sessionsrweek interacting in stable social groups, while the remaining subjects
Ž .
spent regular periods same duration and frequency as above interacting in social groups whose membership was constantly altered by the experimenters. Capitanio et al. Ž1998b found that the macaques that had been in stable social groups spent significantly. more time in affiliative interactions and had significantly longer survival times after
Ž .
infection with simian immunodeficiency virus SIV than macaques that had been in Ž
unstable social groups. They concluded that psychosocial factors the social opportuni-ties and relationships provided by interacting with a stable set of partners, specifically
.
the amount of time spent affiliating , could influence a critical immunological factor, Ž .
survival time after infection with a deadly pathogen SIV . This finding highlights the relevance of psychosocial factors and their effects on laboratory primates in biomedical research.
Nonhuman primate subjects for biomedical research are usually chosen in a fairly nonsystematic fashion. Typically, available andror inexpensive animals are chosen first ŽKaplan et al., 1991; Benveniste et al., 1996 , with little regard for other factors. Prior to. the beginning of an experiment, monkeys are removed from their ‘‘normal’’ housing and placed in the experimental condition. A period of acclimation to allow the subjects to adapt to their new surroundings is usually provided. The acclimation period can vary
Ž .
in duration from several weeks to several months Capitanio et al., 1996 , but is rarely rigidly controlled or explicitly stated. There are few empirical data that demonstrate that an acclimation period of a specific length is necessary for subjects to return to baseline
Ž .
functioning. In fact, recent work by Lilly et al. 1999 shows that acclimation is unlikely to have occurred, even 28 weeks after removal from the ‘‘normal’’ housing condition. Clearly, an enhanced understanding of the time course of acclimation processes would be germane to biomedical research with laboratory primates.
Ž .
We were specifically interested in determining 1 whether there were any differences in the cell-mediated immune responses of adult rhesus monkeys that were housed singly,
Ž .
in pairs, or in small groups and 2 whether these responses changed over time. Clearly, housing adult rhesus monkeys individually is a fairly stressful psychosocial manipula-tion; one that may be comparable to the more commonly studied mother–infant separation or reintroduction to groups of conspecifics. We measured lymphocyte sub-sets, mitogen- and pathogen-induced lymphocyte proliferation, NK activity, and cy-tokine production on multiple occasions over a 12-month period. Measuring lymphocyte proliferation responses to gastrointestinal pathogens is a novel approach with potential ecological relevance.
2. Animals, materials, and methods
2.1. Subjects and housing
Ž .
We studied 36 adult rhesus monkeys Macaca mulatta of both sexes, that had been Ž .
Ž .
colony Schapiro et al., 1994 . All subjects were at least 5.5 years of age at the time of sampling, and all had been socially housed for a minimum of 4 years of their lives. All but two of the subjects were socially housed for the 2 years prior to the study.
Ž .
The 12 monkeys in the group housed condition five males and seven females were living in outdoor buildings in stable social groups comprised of one male, four to seven females, and their most recent offspring at the time the study began. No alterations were made to their social or physical settings.
Ž .
Eight of the twelve pair housed subjects five males and seven females were also living in similar social groups prior to the study. On the day the study began, these animals were removed from their groups, a blood sample was obtained, and they were reformed into pairs with a single familiar groupmate. The other four pair housed subjects were already living in pairs when the study began. No alterations were made to their social or physical settings. Three pairs were housed in runs in outdoor buildings and three pairs were housed in double cages connected by a tunnel in indoor rooms.
Ž .
Ten of the twelve singly housed subjects five males and seven females were living in social groups prior to the study. On the day the study began, these animals were removed from their social groups, a blood sample was obtained, and they were then
Ž 2 2
placed in an appropriately sized either 0.40 m or 0.57 m , dependent on the subject’s
. Ž .
weight and enriched toys, perch, feeding devices single cage. The other two singly housed subjects were singly housed prior to the beginning of the study and no alterations were made to their social setting, although one of these monkeys was moved from one room to another, a change in physical setting. All but one of the singly caged subjects were housed in cages on racks in indoor rooms; the lone exception was a male housed in an outdoor run because he was too large for any of our cages.
2.2. Blood collection and cell preparation
All monkeys were initially sampled in February, 1996, the end of the 1995–1996 breeding season in our colony. Monkeys that had undergone a change in social andror
Ž
physical settings at the initiation of the study 11 singly housed subjects and 8 pair .
housed subjects were sampled immediately prior to the change, 2 days after the change, and again 1, 4, 8, and 12 months after the change. Monkeys that had not changed social
Ž .
or physical settings 1 singly housed, 4 pair housed, and all 12 group housed subjects were sampled on four occasions; once at the beginning of the study, and again 4, 8, and 12 months into the study.
Ten milliliters of peripheral blood were obtained from the femoral vein using an EDTA-coated syringe and were then transferred to an EDTA sterile vacutainer tube ŽBecton-Dickinson between 08:30 and 10:45. All monkeys were restrained using light.
Ž . Ž .
ketamine anesthesia 10 mgrkg . Peripheral blood mononuclear cells PBMC were
Ž .
separated using Histopaque-1077 Sigma, St. Louis, MO density gradient centrifuga-tion. PBMC present at the plasma–Histopaque interface were harvested. These cells
Ž
2.3. Immunological assays
2.3.1. T-lymphocyte subsets
PBMC were assayed for the presence of T-cell surface antigens using a whole-blood Ž .
staining procedure and a FACScan flow cytometer Coulter . A series of flourescein-ŽFITC and phycoerythrin- PE conjugated monoclonal antibody reagents previously. Ž . characterized to react with subsets of PBMC of rhesus macaques were utilized to determine the frequency of each subset. The monoclonal reagents included
PE-con-q Ž . q Ž
jugated CD4 -Leu-3a Becton Dickinson and FITC-conjugated CD8 -Leu-2a Becton .
Dickinson . A total of 10,000 cells per sample were analyzed for the frequency of FITCq and PEqcells. Calculation of the absolute numbers of cell subpopulations were
based on a complete blood cell count using a Baker System 9000 hematology instrument and a differential done from Wright–Giemsa-stained blood smears. Results are reported as absolute number of cellsrmilliliter.
2.3.2. Lymphocyte proliferation assays
Ž 6 .
A 0.1-ml aliquot of the cell suspension 1=10 cellsrml was dispensed into each well of a U-bottom, 96-well microtiter plate and incubated in triplicate with
concentra-Ž . Ž .
tions of concanavalin A ConA; 1:100 , phytohemagglutinin PHA; 1:50 , pokeweed
Ž . Ž .
mitogen PWM; 1:250 , lipopolysaccharide LPS; 1:100 , and staphylococcal
entero-Ž .
toxin A StaphA; 1:1000 .
Ž .
In addition, we tested two concentrations each 1:5 and 1:10 of four different common gastrointestinal pathogens of rhesus monkeys: Escherichia coli, Salmonella
typhimurium, Shigella flexneri, and Campylobacter jejuni. These organisms can cause
Ž
severe diarrhea in captive macaques Holmberg et al., 1982; Hird et al., 1984; Reinhardt, .
1993; Wolfensohn, 1998 .
All cultures were incubated for 4 days at 378C in a humidified 5% CO atmosphere;2
w3 x Ž
and during the final 16–18 h, 1mCi of H thymidine 6.7 Cirmmol; ICN Biomedicals, .
Costa Mesa, CA was added. Cells were then harvested onto filter strips for estimation
w3 x Ž
of H thymidine incorporation using a Skatron cell harvester Skatron Instruments,
. Ž .
Sterling, VA . The specific radioactivity DCPM, counts per minute of cells treated with various additions were calculated in each case by subtracting the values obtained with cells cultured in medium alone.
2.3.3. Natural killer cell assay
NK activity against51Cr-labeled K562 target cells was measured. 5=106 K562 cells Ž
were incubated for 2 h at 378C, with 100 mCi of sodium chromate New England
. 4
Nuclear, Boston, MA . Cells were washed twice and resuspended at 5=10 rml in medium containing 10% heat-inactivated fetal calf serum. 5=105 PBMC per well were
Ž .
added to 96-well U-bottom microtiter plates Falcon , and cocultured with the K562 Ž .
cells in triplicate at effector:target ratios E:T of 100:1, 50:1, 25:1, and 12.5:1. The cells were then incubated for 4 h at 378C in a humid atmosphere containing 5% CO . After2 incubation, the plate was centrifuged at 250 g for 5 min, 100 ml of supernatant were
w
Ž .
removed from each well and added to Titertube Micro Tubes Bio Rad , and the tubes Ž .
determined by measuring the release of the radiolabel into the culture supernatant. The percentage of specific51Cr release was calculated as:
100=
Ž
experimental releaseyspontaneous release. Ž
r maximum releaseyspontaneous release .
.
Maximum release was determined from supernatants of cells that were lysed by adding 5% Triton X-100. Spontaneous release was determined from target cells incubated without added effector cells.
2.3.4. Cytokine assay
Ž 6. Ž .
PBMC 1=10 were incubated with 20ml of mitogen PHA; 1:10 and complete
Ž .
medium total volume of 200ml per well in a 96-well U-bottom plate for 48 h at 378C. One hundred microliters of supernatant were then removed from each well and frozen at
y708C in another 96-well U-bottom plate. At the time of assay, the plate was thawed and assayed for cytokines IFN-g, IL-2, IL-4, and IL-10 using Cytoscreen immunoassay
Ž .
kits Biosource International , according to the manual instructions. It has been shown that the human test kits for these cytokines are acceptable for use with rhesus monkey
Ž .
cells Villinger et al., 1993 . The minimal detection limits for these assays were 4 pgrml for IFN-g; 8.7 pgrml for IL-2; 2 pgrml for IL-4; and 5 pgrml for IL-10.
2.4. Analyses
Ž .
Data were analyzed using analysis of variance ANOVA techniques with housing
Ž . Ž
condition single, pair, or group as a between-subjects factor and sample timepoint 0, .
4, 8, and 12 months as a within-subjects factor. Planned comparisons were used to determine if singly caged subjects differed significantly from socially housed subjects Žpair and group housed combined . Separate one-way ANOVAs were used to determine. if there were housing effects at baseline or at the 12-month timepoint. A few subjects were not assayed for all dependent variables at all timepoints, as reflected in the degrees of freedom in certain analyses.
Since no systematic differences in immune responses existed between pair housed subjects that had been previously paired and those that had been group housed, or between pair housed subjects living in outdoor runs and those living in indoor double cages, for purposes of analysis, the pair housed data were treated as one data set. Similarly, since singly housed subjects that had been previously singly housed and those that had been group housed did not differ systematically, the single housed data were also treated as one data set in the analysis.
3. Results
3.1. T-lymphocyte subsets
The ratio of CD4q to CD8q lymphocytes differed significantly across the three
w Ž . x q
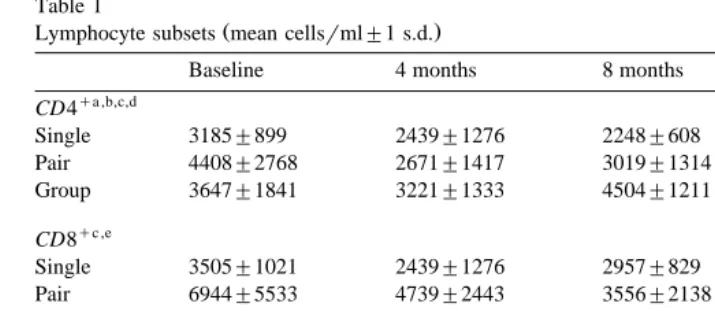
Table 1
Ž .
Lymphocyte subsets mean cellsrml"1 s.d.
Baseline 4 months 8 months 12 months
qa ,b,c,d
CD4
Single 3185"899 2439"1276 2248"608 2131"701 Pair 4408"2768 2671"1417 3019"1314 2692"1373 Group 3647"1841 3221"1333 4504"1211 4078"1958
qc ,e
CD8
Single 3505"1021 2439"1276 2957"829 3358"1165 Pair 6944"5533 4739"2443 3556"2138 3814"2585 Group 3366"1851 2642"991 3974"1361 4069"1816
q qa,d,f
PF0.05 for comparison across housing conditions.
b
PF0.05 for comparison across timepoints.
c
PF0.05 for housing condition=timepoint interaction.
d
P-0.05 for comparison at 12 month timepoint only.
e
PF0.05 for comparison at baseline timepoint only.
f Ž .
PF0.05 for planned comparison of single housedÕs. socially housed pair and group at 8 months and
12 months.
w Ž . x q
lymphocytes F 2,29 s7.42, PF0.01; see Table 1 . The absolute number of CD8 lymphocytes did not differ. Importantly, across housing conditions, there were no significant differences in CD4qrCD8q or in CD4q numbers at the baseline timepoint,
but significant differences across housing conditions existed at the 12-month timepoint
q qw Ž . x q w Ž .
for both CD4 rCD8 F 2,29 s5.38, PF0.01 and CD4 numbers F 2,30 s5.43,
x q
PF0.01 . CD8 numbers differed significantly across the three housing conditions at
w Ž . x
baseline F 2,33 s4.21, PF0.05 , but not at 12 months. Planned comparisons re-vealed that singly caged subjects had significantly lower CD4qrCD8qthan did socially
Ž . w Ž . x
housed in pairs and groups subjects at the 8-month F 1,30 s9.11, PF0.01 and the
w Ž . x
12-month F 1,29 s6.04, PF0.05 timepoints only.
q w Ž .
Only CD4 numbers differed significantly across all timepoints F 3,84 s2.75,
x PF0.05 .
Five of six pairs of CD4q to CD8q ratios were significantly positively correlated
Ž
across timepoints for all subjects combined significant Pearson’s correlation coeffi-cients ranged from 0.48 to 0.72, P ’s allF0.009, the Bonferroni alpha level, see Table
.
2 . Only the correlation between the baseline sample and the 4-month sample was not significant at the corrected alpha level.
3.2. Lymphocyte proliferation assays
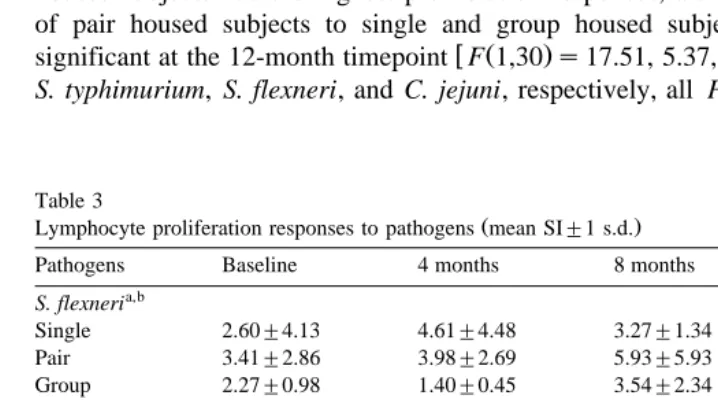
Proliferation responses to S. flexneri differed significantly across housing conditions
Table 2
Pearson’s correlation matrix for CD4q rCD8q
Baseline 4 months 8 months 12 months
Baseline –
PF0.08 all approached significance. There were no significant differences in prolifera-tion responses to any of the pathogens across housing condiprolifera-tions at the baseline
w Ž . x w Ž .
timepoint, but responses to S. flexneri F 2,30 s3.32, PF0.05 , E. coli F 2,30 s
x w Ž . x
11.59, PF0.001 , and S. typhimurium F 2,30 s3.97, PF0.05 all differed signifi-cantly across housing conditions at the 12-month timepoint. For all four pathogens, pair housed subjects had the highest proliferation responses, although planned comparisons of pair housed subjects to single and group housed subjects combined, were only
w Ž .
significant at the 12-month timepoint F 1,30 s17.51, 5.37, 4.67, and 4.13 for E. coli,
x S. typhimurium, S. flexneri, and C. jejuni, respectively, all P ’sF0.05 .
Table 3
Ž .
Lymphocyte proliferation responses to pathogens mean SI"1 s.d.
Pathogens Baseline 4 months 8 months 12 months
a,b
Single 6.95"12.5 1.81"1.33 20.07"0.67 3.24"1.45 Pair 10.2"12.1 1.77"0.92 2.41"1.06 4.26"2.45
PF0.05 for comparison across housing conditions.
b
PF0.05 for comparison at 12 month timepoint only.
c
PF0.08 for comparison across housing conditions.
d
There were significant differences in proliferation responses to three of the four
w Ž .
pathogens across all timepoints F 3,90 s4.40, 7.91, and 5.98 for E. coli, S.
ty-x
phimurium, and C. jejuni, respectively, all P ’sF0.01 . There were no significant housing=timepoint interactions.
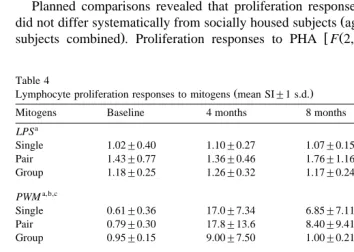
w Ž . Subjects differed by housing condition in their proliferation response to LPS F 2,30
x w Ž . x w Ž .
s5.11, PF0.05 , to PWM F 2,30 s3.37, PF0.05 , and to StaphA F 2,30 s3.27,
x Ž .
PF0.05, see Table 4 . In our immunological laboratory, a Stimulus Index SI value of Ž
at least 2.0 is required for a proliferation response to be considered positive Nehete et .
al., 1998 . All SIs less than 2.0 are considered indicative of no proliferation response, are referred to as negative, and are treated as biologically identical. Therefore, although the difference between LPS SI values across housing conditions was statistically significant, it was not biologically meaningful, since SI values to LPS never averaged greater than 2.0.
Planned comparisons revealed that proliferation responses of singly caged subjects Ž
did not differ systematically from socially housed subjects again, pair and group housed
. w Ž . x
subjects combined . Proliferation responses to PHA F 2,33 s4.85, PF0.05 and
Table 4
Ž .
Lymphocyte proliferation responses to mitogens mean SI"1 s.d.
Mitogens Baseline 4 months 8 months 12 months
a
PF0.05 for comparison across housing conditions.
b
PF0.05 for comparison across timepoints.
c
PF0.05 for comparison at baseline timepoint only.
d
PF0.05 for housing condition=timepoint interaction.
e
w Ž . x
PWM F 2,33 s4.35, PF0.05 differed statistically across housing conditions at the baseline timepoint, but mean SI values for PWM for all housing conditions were less than 1.0 at baseline and were therefore also considered negative. At the 12-month
w Ž . timepoint, PHA responses still differed across housing conditions F 2,30 s4.71,
x
PF0.05 and StaphA responses, which did not differ at baseline, now differed
Ž . x
F 2,30 s11.74, PF0.001 .
There were significant differences in proliferation responses to four of the five
w Ž .
mitogens across the four timepoints F 3,90 s23.76, 13.14, 24.29, and 11.80 for PHA,
x w Ž .
ConA, PWM, and StaphA, respectively, all P ’sF0.001 . Both PHA F 6,90 s4.80,
x w Ž . x
PF0.001 and StaphA F 6,90 s4.07, PF0.001 also evidenced significant housing =timepoint interactions.
3.2.1. Natural killer cell assays
Ž .
There was an important trend in NK activity at an effector to target E:T ratio of
w Ž . x
100:1 across the three housing conditions F 2,30 s2.86, PF0.07, see Table 5 .
w Ž . x
Timepoint also affected NK activity F 3,90 s9.07, PF0.001 , but there was no significant housing by timepoint interaction effect. At the baseline timepoint, NK
w Ž . x
activity differed significantly across housing conditions F 2,33 s6.62, PF0.01 , with group housed animals showing the lowest NK activity.
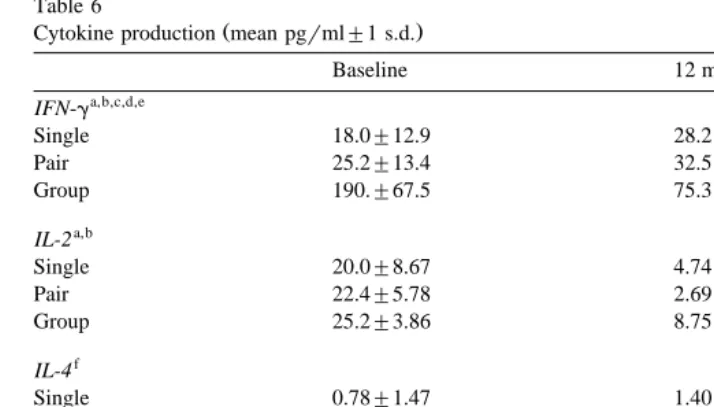
3.2.2. Cytokine assays
Cytokine samples were only assayed from the baseline and 12-month timepoints. There were significant differences across housing conditions in production of IFN-g
wF 2,30Ž .s64.49, PF0.001 , IL-2 F 2,30x w Ž .s3.72, PF0.05 , and IL-10 F 2,30x w Ž .s x
26.57, PF0.001 . Significant differences in production of IFN-g and IL-10 across
w Ž .
housing conditions existed at the baseline timepoint F 2,33 s69.22 and 49.87,
x
respectively, both P ’sF0.001 . Planned comparisons of singly caged subjects to socially housed subjects revealed that singly caged subjects produced significantly lower
w Ž . Ž .
levels of IFN-g at both timepoints F 1,30 s32.37, PF0.001 and F 1,30 s9.47,
x
PF0.01 for baseline and 12-months, respectively and of IL-10 at the baseline
w Ž . x
timepoint only F 1,30 s18.89, PF0.001 .
There were significant differences across timepoints for these same three cytokines
wIFN-g: F 1,30Ž .s17.58; IL-2: F 1,30Ž .s131.77; and IL-10: F 1,30Ž .s105.46, all
Table 5
Ž .
Natural killer cell responses mean % lysis"1 s.d.
a,b,c
E:Ts100:1 Baseline 4 months 8 months 12 months
Single 20.1"11.1 22.8"9.09 22.5"5.99 15.3"8.76 Pair 19.0"7.65 24.6"9.54 18.6"9.96 17.2"9.20 Group 8.88"5.24 22.2"9.67 13.5"9.08 13.3"8.29
a
PF0.07 for comparison across housing conditions.
b
PF0.05 for comparison across timepoints.
c
Table 6
Ž .
Cytokine production mean pgrml"1 s.d.
Baseline 12 months
a,b,c,d,e
IFN-g
Single 18.0"12.9 28.2"16.7
Pair 25.2"13.4 32.5"19.0
Group 190."67.5 75.3"29.0
a,b
IL-2
Single 20.0"8.67 4.74"6.86
Pair 22.4"5.78 2.69"4.82
Group 25.2"3.86 8.75"5.37
f
IL-4
Single 0.78"1.47 1.40"2.37
Pair 0.42"0.97 1.59"2.11
Group 0.23"0.79 2.24"2.13
a,b,c,d
IL-10
Single 20.5"5.68 10.7"6.02
Pair 19.6"5.25 12.1"6.43
Group 40.5"6.40 10.0"3.72
a
PF0.05 for comparison across housing conditions.
b
PF0.05 for comparison across timepoints.
c
PF0.05 for housing condition=timepoint interaction.
d
PF0.05 for planned comparison at baseline timepoint only.
e
PF0.05 for planned comparison at 12 month timepoint only.
f
Results are reported, but the values are below the accurate detection limits of the test kit.
x w Ž . x
P ’sF0.001 and significant interactions for IFN-g F 2,30 s31.89, PF0.001 and
w Ž . x Ž .
IL-10 F 2,30 s22.11, PF0.001 Table 6 .
4. Discussion
Numerous statistically significant differences in cell-mediated immune responses were identified across the three housing conditions, supporting our initial hypothesis that housing condition can affect a variety of in vitro assays of cell-mediated immune responses. Specifically, the ratios of CD4q to CD8q lymphocytes were significantly
to experimental housing conditions may be slow to develop, taking considerably longer than previously suspected.
In general, our lymphocyte subset data are in agreement with previous reports in which lower CD4qrCD8q were observed for subjects under conditions that involved
w
stressful manipulations to their psychosocial environments mother–infant separations ŽCoe et al., 1988, 1989; Laudenslager et al., 1990; Terao et al., 1995 , enforced social.
Ž .
instability Kaplan et al., 1991; Cohen et al., 1992; Line et al., 1996 , and separation Ž
from andror reunion with their social group Gust et al., 1991, 1992, 1993; Gordon et
. q q
al., 1992 . Additionally, the ratio of CD4 to CD8 lymphocytes has been identified as a potentially ‘‘trait-like’’ characteristic of laboratory monkeys, varying consistently across a variety of situations and timepoints, and thus may be a useful measure of
Ž .
biobehavioral organization Capitanio et al., 1998a; Lilly et al., 1999 . Our data support these findings; CD4qrCD8q for individual monkeys were significantly positively
Ž . correlated across housing conditions and timepoints. Like Capitanio et al. 1998a , but
Ž .
unlike Lilly et al. 1999 , we found no consistency across situations or timepoints for CD4q counts.
Differences observed in pathogen-specific lymphocyte proliferation responses were of great interest to us because they represent a specific response to an infectious agent and may be more representative of in vivo immune function than responses to nonspecific
Ž .
mitogens LPS, PWM, etc. . We performed T-cell proliferation assays to S. flexneri, C.
jejuni, S. typhimurium, and E. coli; pathogens that can cause severe diarrhea and a
Ž
variety of related health problems in captive macaques Holmberg et al., 1982; Hird et .
al., 1984; Reinhardt, 1993; Wolfensohn, 1998 . These organisms were chosen for specific reasons. First, they are common, but treatable, in many captive primate colonies, thereby representing a more explicit ‘‘ecologically’’ and immunologically relevant challenge than PHA, ConA, or some of the other commonly used mitogens. Also, while debilitating and potentially life threatening, infection with these organisms is considerably less devastating than infection with experimental strains of SIV, many of
Ž
which have been chosen for their virulence characteristics Benveniste et al., 1996; Joag .
et al., 1998 . This again enhances the ecological and immunological suitability of the gastrointestinal pathogens as a set of appropriate in vitro experimental challenges. Unlike SIV, most monkeys are likely to have been exposed to and survived infectious episodes of Shigella, Campylobacter, Salmonella, andror E. coli. Thus, given their severe, but not deadly disease course, these organisms could serve as potential surviv-able in vivo challenges as well.
Ž .
In general, higher Stimulation Index SI values on lymphocyte proliferation assays are indicative of better immune response. In most comparisons, the highest SI values for all four gastrointestinal pathogens were observed among pair housed subjects, although the difference between pair housed, and single and group housed subjects combined, was only significant at the 12-month timepoint. Since in vitro proliferation to a given antigen is a measure of a subject’s specific immunological memory, these data suggest that pair housed monkeys may be better equipped to deal with such infectious agents. This supposition is supported to some extent by previous findings from our laboratory in which pair housed monkeys had significantly less diarrhea that required treatment, than
Ž .
among the first data from nonexperimentally infected primates that may address the Ž
relationship between responses on in vitro immunological assays proliferation re-.
sponses and in vivo measures of health. Definition of this relationship is one of the key Ž
challenges currently facing the field of primate psychoneuroimmunology Lubach et al., .
1995; Laudenslager et al., 1996 .
It is our contention that strong social relationships, particularly the affiliative interac-tions that characterize our pair housed monkeys, may diminish the likelihood of severe infection with potentially diarrhea-inducing agents. The findings of Capitanio et al. Ž1998b are extremely relevant to this contention. Half of their subjects spent regular. periods interacting in stable groups, while the other half of the subjects spent regular periods in unstable groups. Monkeys in stable groups affiliated more, and more
Ž
importantly, survived significantly longer an increase of over 40% in median survival
. Ž .
times , after SIV infection than monkeys in unstable groups Capitanio et al., 1998b . If stable social groups and high levels of affiliative behavior can increase a monkey’s ability to survive after SIV infection, then it is relatively unsurprising that affiliative interactions may also lessen the severity of the effects of infection with gastrointestinal pathogens. Just as allowing SIV-infected subjects the opportunity to regularly interact in stable social groups may enhance experimental validity for SIV studies, social housing may enhance validity for other types of biomedical studies. Thus, from applied management and research perspectives, pair housing may be an extremely effective strategy to increase levels of social affiliation while simultaneously minimizing diarrhea-related problems.
While we did find significant differences in proliferation responses to StaphA across Ž
housing conditions the LPS and PWM results were statistically, but not biologically .
significant , our proliferation data, in general, are not in agreement with previous reports. Whereas many have found diminished proliferation responses under conditions
Ž
that involved stressful manipulations to subjects’ psychosocial environments Kaplan et .
al., 1991; Boccia et al., 1992; Cohen et al., 1992; Lubach et al., 1995 , we have repeatedly found no decrements in proliferation responses among animals that were
Ž
experiencing psychosocial environments that should have been more stressful Schapiro .
et al., 1998 . This includes marginally higher proliferation responses in unenriched than in enriched rhesus monkeys, significantly higher responses in middle-ranking than in highest-ranking female rhesus, and the present finding of marginally higher responses in singly housed monkeys. As mentioned previously, we have chosen to emphasize pathogen-specific immune memory responses because we suspect that proliferation responses to actual disease-inducing agents are more sensitive to subtle changes in psychosocial conditions than are responses to nonspecific mitogens, and thus, are better indicators of an animal’s capacity to immunologically respond to such ‘‘ecologically relevant’’ changes.
Our natural killer cell activity data do not agree with previous reports. Diminished NK activity usually prevails under conditions that involve stressful manipulations to
Ž
subjects’ psychosocial environments Boccia et al., 1992; Lubach et al., 1995; Terao et .
Alternatively, some singly caged subjects may have been more comfortable living alone. However, this is counterintuitive and is not supported by the observed decreases in CD4qrCD8q. At the moment, we have no good explanation for either the lower NK activity in group housed monkeys or the relatively low levels of NK activity in our monkeys overall.
Ž
It has been suggested that variability in disease progression in HIV and SIV; Clerici .
and Shearer, 1993; Benveniste et al., 1996; Capitanio et al., 1998b is associated with
Ž . Ž .
shifts in T helper cell TH1 and T suppressor cell TH2 responses. The timing of acceleration of disease progression in SIV and HIV appears to correlate with a shift
Ž .
from TH1 to TH2 responses Benveniste et al., 1996 . A primary motivation for us to collect and analyze cytokine production data was the prospect that these measures would help us address this shift in T cell responses, and thereby clarify the relationship between functional immune assays and animal health. Production of IL-2 and IFN-g Žamong other cytokines is typically associated with TH1 responses and production of.
Ž .
IL-4 and IL-10 among other cytokines is typically associated with TH2 responses. Ž
Although our data reveal no evidence of a TH1 to TH2 shift one really would not be .
expected, given that no disease challenge was involved , we still found significant differences across housing conditions. Cytokine production is an important step in the immunological response to challenge, and the fact that social housing condition can affect the production of these signaling compounds is additional evidence establishing the relationship between psychosocial factors and immune responses.
During this longitudinal study, repeated immunological samples were collected at baseline and at timepoints 4, 8, and 12 months into the study. A number of the immunological responses we measured differed significantly across timepoints, includ-ing E. coli, Salmonella, Campylobacter, PHA, ConA, PWM, and StaphA proliferation responses; NK activity; and IL-2, IL-10, and IFN-g production. While timepoint related effects are mildly interesting, the existence of differences across housing conditions at late timepoints, particularly at 12 months, would be of even greater significance. This would be especially true for measures on which there were no housing condition effects at baseline, implying that acclimation to new housing conditions is a process that takes a considerable period of time for laboratory macaques. By acclimation, we are essentially referring to a return to baseline response levels. In fact, our data reveal that for 6 of 10 measures on which groups did not differ at baseline, they did differ at 12 months. Interestingly, for three of four measures on which they differed at baseline, significant differences no longer existed at 12 months. Such results suggest that after a year of
Ž .
single housing, monkeys are a not identical to group housed or pair housed monkeys, Ž .
and to a lesser extent, they are b not identical to what they were when they were Ž .
socially normally housed.
Ž . differ significantly at the start of the study 0.95, 0.94, and 1.13, respectively , when
Ž .
most animals 30 of 36 were actually group housed. Twelve months later, when some monkeys had been singly or pair housed for a year, CD4qrCD8q for group housed
Ž .
animals had not changed 1.15 , but the ratios for pair housed monkeys had decreased to 0.83, and those for singly caged subjects had fallen more precipitously to 0.68. The StaphA data demonstrate a similar effect, with group housed animals maintaining
Ž . Ž
consistent responses at baseline and 12 months 35.8 and 35.4 , while both single 61.3
. Ž .
to 110 and pair housed monkeys 57.4 to 120 showed dramatic increases in prolifera-Ž .
tion responses. Lilly et al. 1999 have also found no apparent acclimation on a variety of immunological measures up to 28 weeks after a change in housing condition.
Housing captive macaques in different social conditions results in significant differ-ences in many aspects of their immune profiles. Thus, when studying macaque immune responses, one must be aware of the effects that housing condition, as one aspect of the psychosocial environment, has on the animals, independent of the experimental manipu-lation. There are similar influences which also should be accounted for, including
Ž
rearing and housing history Laudenslager et al., 1985, 1990; Coe et al., 1989, 1992;
. Ž
Lilly et al., 1999 , dominance rank Boccia et al., 1992; Clarke et al., 1996; Schapiro et
. Ž
al., 1998 , social instability Kaplan et al., 1991; Cohen et al., 1992; Line et al., 1996;
. Ž
Capitanio et al., 1998b , pre-sampling exposure to technician activity Capitanio et al.,
. Ž .
1996 , and even enrichment history Schapiro et al., 1998 . Therefore, there is consider-able impetus to effectively manage and even to attempt to optimize the psychosocial environment encountered by nonhuman primate subjects in biomedical research. One way to eliminate undesirable effects of uncontrolled psychosocial influences would be to apply findings such as those cited above to establish specific criteria for the selection, treatment, and management of primate subjects in the research laboratory.
We have demonstrated that immunological data gathered from singly housed adult rhesus monkeys are not identical to data gathered from pair housed or group housed adult rhesus monkeys. This generalization applies even after a year spent in the target housing condition, suggesting that long acclimation periods may be necessary for
Ž .
monkeys to immunologically adapt to single caging if they ever do; Lilly et al., 1999 . While we were hoping that our data would clearly identify one type of housing condition that was superior on all immunological indices, our results do not allow such an interpretation at this time. Measuring a large number of immunological responses is advantageous when attempting to get a complete picture of an animal’s immunological functioning, but may be disadvantageous when attempting to interpret the large number of results.
5. Conclusions
responses and the suitability of particular housing conditions. Future research efforts in this area should include prospective studies that focus on controlled manipulations of
Ž .
psychosocial factors particularly levels of affiliative behavior and the relationship Ž between in vitro measures of immune response and in vivo measures of health i.e.,
. responses to challenge with common primate pathogens like C. jejuni and S. flexneri .
Acknowledgements
Thanks to S. Buchl and the animal care staff of the primate section for assistance during blood sampling, to R. Cocke for help with immunological interpretations, and to M. Hook-Costigan and R. Stavisky for suggestions on an earlier draft of this manuscript. Animals are maintained in facilities approved by the Association for Assessment and Accreditation for Laboratory Animal Care International and in accordance with current United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health regulations and standards. Financial support for parts of this project came from the National Institutes of HealthrNCRR grants U42-RR05080 and R01-RR05092, NASA Cooperative Agreement NCC9-36, and Texas Higher Educa-tion Coordinating BoardrATP Award 15-014.
References
Benveniste, O., Vaslin, B., Le Grand, R., Cheret, A., Matheux, F., Theodoro, F., Cranage, M.P., Dormont, D.,
Ž . Ž .
1996. Comparative interleukin IL -2rinterferon IFN -gand IL-4rIL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SIVmac251 virus. Proc. Natl. Acad. Sci. USA 93, 3658–3663.
Boccia, M.L., Laudenslager, M.L., Broussard, C.L., Hijazi, A.S., 1992. Immune responses following competi-tive water tests in two species of macaques. Brain Behav. Immun. 6, 201–213.
Ž .
Bramblett, C., 1989. Mental well-being in anthropoids. In: Segal, E.F. Ed. , Housing, Care and Psychological Wellbeing of Captive and Laboratory Primates. Noyes Publications, New Jersey, pp. 1–11.
Capitanio, J.P., Mendoza, S.P., Lerche, N.W., 1998a. Individual differences in peripheral blood immunological
Ž .
and hormonal measures in adult male rhesus macaques Macaca mulatta : evidence for temporal and
situational consistency. Am. J. Primatol. 44, 29–41.
Capitanio, J.P., Mendoza, S.P., Lerche, N.W., Mason, W.A., 1998b. Social stress results in altered glucocorti-coid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc. Natl. Acad. Sci. USA 95, 4714–4719.
Capitanio, J.P., Mendoza, S.P., McChesney, M., 1996. Influences of blood sampling procedures on basal
Ž
hypothalamic–pituitary–adrenal hormone levels and leukocyte values in rhesus macaques Macaca .
mulatta . J. Med. Primatol. 25, 26–33.
Clarke, A.S., Czekala, N.M., Lindburg, D.G., 1995. Behavioral and adrenocortical responses of male cynomolgus and lion-tailed macaques to social stimulation and group formation. Primates 36, 41–56. Clarke, M.R., Harrison, R.M., Didier, E.S., 1996. Behavioral, immunological, and hormonal responses
Ž .
associated with social change in rhesus monkeys Macaca mulatta . Am. J. Primatol. 39, 223–233. Clerici, M., Shearer, G.H., 1993. A TH1 to TH2 switch is a critical step in the etiology of HIV infection.
Immunol. Today 14, 107–111.
Ž .
Coe, C.L., 1993. Psychosocial factors and immunity in nonhuman primates: a review. Psychosom. Med. 55, 298–308.
Coe, C.L., Ershler, W.B., Champoux, M., Olson, J., 1992. Psychosocial factors and immune senescence in the aged primate. Ann. NY Acad. Sci. 650, 276–282.
Coe, C.L., Lubach, G.R., Ershler, W.B., Klopp, R.G., 1989. Influence of early rearing on lymphocyte proliferation responses in juvenile rhesus monkeys. Brain Behav. Immun. 3, 47–60.
Coe, C.L., Rosenberg, L.T., Levine, S., 1988. Effect of maternal separation on the complement system and antibody responses in infant primates. Int. J. Neurosci. 40, 289–302.
Cohen, S., Kaplan, J.R., Cunnick, J.E., Manuck, S.B., Rabin, B.S., 1992. Chronic social stress, affiliation, and cellular immune response in nonhuman primates. Psychol. Sci. 3, 301–304.
Crockett, C.M., Bowers, C.L., Bowden, D.M., Sackett, G.P., 1994. Sex differences in compatibility of pair-housed adult longtailed macaques. Am. J. Primatol. 32, 73–94.
Gold, L.H., Fox, H.S., Henriksen, S.J., Buchmeier, M.J., Weed, M.R., Taffe, M.A., Huitron-Resendiz, S., Horn, T.F.W., Bloom, F.E., 1998. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J. Med. Primatol. 27, 104–112.
Gordon, T.P., Gust, D.A., Wilson, M.E., Ahmed-Ansari, A., Brodie, A.R., McClure, H.M., 1992. Social separation and reunion affects immune system in juvenile rhesus monkeys. Physiol. Behav. 51, 467–472. Gust, D.A., Gordon, T.P., Hambright, M.K., 1993. Response to removal from and return to a social group in
adult male rhesus monkeys. Physiol. Behav. 53, 599–602.
Gust, D.A., Gordon, T.P., Wilson, M.E., Ahmed-Ansari, A., Brodie, A.R., McClure, H.M., 1991. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav. Immun. 5, 296–307.
Gust, D.A., Gordon, T.P., Wilson, M.E., Ahmed-Ansari, A., McClure, H.M., 1992. Removal from natal social group to peer housing affects cortisol levels and absolute numbers of T cell subsets in juvenile rhesus monkeys. Brain Behav. Immun. 6, 189–199.
Hird, D.W., Anderson, J.H., Bielitzki, J.B., 1984. Diarrhea in nonhuman primates: a survey of primate colonies for incidence rates and clinical opinion. Lab. Anim. Sci. 34, 465–470.
Holmberg, C.A., Leningere, R., Wheeldon, E., Slater, D., Henrickson, R., Anderson, J., 1982. Clinicopatho-logical studies of gastrointestinal disease in macaques. Vet. Pathol., Suppl. 7, 163–170.
Joag, S.V., Li, Z., Wang, C., Jia, F., Foresman, L., Adany, I., Pinson, D.M., Stephens, E.B., Narayan, O., 1998. Chimeric SHIV that causes CD4qT cell loss and AIDS in rhesus macaques. J. Med. Primatol. 27, 59–64.
Kaplan, J.R., Heise, E.R., Manuck, S.B., Shively, C.A., Cohen, S., Rabin, B.S., Kasprowicz, A.L., 1991. The relationship of agonistic and affiliative behavior patterns to cellular immune function among cynomolgus
Ž .
monkeys Macaca fascicularis living in unstable social groups. Am. J. Primatol. 25, 157–173. Laudenslager, M.L., Berger, C.L., Boccia, M.L., Reite, M.L., 1996. Natural cytotoxicity toward K562 cells by
macaque lymphocytes from infancy through puberty: effects of early social challenge. Brain Behav. Immun. 10, 275–287.
Laudenslager, M.L., Capitanio, J.P., Reite, M.L., 1985. Possible effects of early separation experiences on subsequent immune function in adult macaque monkeys. Am. J. Psychiatry 142, 862–865.
Laudenslager, M.L., Held, P.E., Boccia, M.L., Reite, M.L., Cohen, J.J., 1990. Behavioral and immunological consequences of brief mother–infant separation: a species comparison. Dev. Psychobiol. 23, 247–264. Lilly, A.A., Mehlman, P.T., Higley, J.D., 1999. Trait-like immunological and hematological measures in
female rhesus across varied environmental conditions. Am. J. Primatol. 48, 197–223.
Line, S.W., Kaplan, J.R., Heise, E.R., Hilliard, J.K., Cohen, S., Rabin, B.S., Manuck, S.B., 1996. Effects of social reorganization on cellular immunity in male cynomolgus monkeys. Am. J. Primatol. 39, 235–249. Lubach, G.R., Coe, C.L., Ershler, W.B., 1995. Effects of early rearing environment on immune responses of
infant rhesus monkeys. Brain Behav. Immun. 9, 31–46.
Marcario, J.K., Raymond, L.A.M., McKiernan, B.J., Foresman, L.L., Joag, S.V., Raghavan, R., Narayan, O., Hershberger, S., Cheney, P.D., 1999. Simple and choice reaction time performance in SIV-infected rhesus macaques. AIDS Res. Hum. Retroviruses 15, 571–583.
Mason, W.A., 1991. Effects of social interaction on well-being: development aspects. Lab. Anim. Sci. 41, 323–328.
peptide from the first conserved region in the envelope protein gp160 is a strong T-cell epitope in HIV-infected chimpanzees and humans. Viral Immunol. 11, 147–158.
Novak, M.A., Suomi, S.J., 1991. Social interaction in nonhuman primates: an underlying theme for primate research. Lab. Anim. Sci. 41, 308–314.
Reinhardt, V., 1989. Behavioral response of unrelated adult male rhesus monkeys familiarized and paired for the purpose of environmental enrichment. Am. J. Primatol. 17, 243–248.
Reinhardt, V., 1993. Empirical use of metronidazole for treatment of diarrhea in laboratory rhesus macaques: an evaluation. J. Med. Primatol. 22, 280–283.
Reinhardt, V., 1994. Pair-housing rather than single-housing for laboratory rhesus macaques. J. Med. Primatol. 23, 426–431.
Reinhardt, V., Houser, D., Eisele, S., Cowley, D., Vertein, R., 1988. Behavioral responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. Am. J. Primatol. 14, 135–140. Reinhardt, V., Liss, C., Stevens, C., 1995. Social housing of previously single-caged macaques: what are the
options and the risks? Anim. Welfare 4, 307–328.
Ž .
Ruppenthal, G.C., Walker, C.G., Sackett, G.P., 1991. Rearing infant monkeys Macaca nemestrina in pairs produces deficient social development compared with rearing in single cages. Am. J. Primatol. 25, 103–113.
Sarkar, A.K., Mitchell, M.F., Hamada, K., Buchl, S.J., Satterfield, W.C., Schapiro, S.J., Keeling, M.E., Sastry, K.J., 1999. Evaluation of cellular immune responses in rhesus monkeys subjected to adenovirus-mediated gene transfer into cervix. Cancer Gene Ther. 6, 220–227.
Schapiro, S.J., Bloomsmith, M.A., 1994. Behavioral effects of enrichment on pair-housed juvenile rhesus monkeys. Am. J. Primatol. 32, 159–170.
Schapiro, S.J., Bloomsmith, M.A., Porter, L.M., Suarez, S.A., 1996a. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl. Anim. Behav. Sci. 48, 159–171.
Schapiro, S.J., Bloomsmith, M.A., Suarez, S.A., Porter, L.M., 1996b. Effects of social and inanimate enrichment on the behavior of yearling rhesus monkeys. Am. J. Primatol. 40, 247–260.
Schapiro, S.J., Bushong, D., 1994. Effects of enrichment on veterinary treatment of laboratory rhesus
Ž .
macaques Macaca mulatta . Anim. Welfare 3, 25–36.
Schapiro, S.J., Lee-Parritz, D.E., Taylor, L.L., Watson, L., Bloomsmith, M.A., Petto, A., 1994. Behavioral
Ž .
management of specific pathogen-free SPF rhesus macaques: group formation, reproduction, and parental competence. Lab. Anim. Sci. 44, 229–234.
Schapiro, S.J., Nehete, P.N., Perlman, J.E., Bloomsmith, M.A., Sastry, K.J., 1998. Effects of dominance status and environmental enrichment on cell-mediated immunity in rhesus macaques. Appl. Anim. Behav. Sci. 56, 319–332.
Terao, K., Hamano, M., Koyama, T., 1995. The repeated procedure of weaning and peer group formation causes accumulation of stress and changes of plasma cortisol level and natural killer cell activity in squirrel
Ž .
monkeys Saimiri sciureus . Primates 36, 121–127.
Villinger, F., Hunt, D., Mayne, A., Vuchetich, M., Findley, H., Ansari, A.A., 1993. Qualitative and quantitative studies of cytokines synthesized and secreted by non-human primate peripheral blood mononuclear cells. Cytokine 5, 469–479.