Vascular-associated lymphoid tissue (VALT) involvement in aortic
aneurysm
Yuri V. Bobryshev *, Reginald S.A. Lord
Surgical Professorial Unit,Le6el17,O’Brien Building,St.Vincent’s Hospital,Uni6ersity of New South Wales,Darlinghurst,Sydney NSW, 2010,Australia
Received 1 November 1999; accepted 21 February 2000
Abstract
Vascular-associated lymphoid tissue (VALT) consisting of accumulations of immunocompetent and antigen presenting cells has recently been recognised in the arterial wall. In this study, we investigated the involvement of VALT in immune responses in abdominal aortic aneurysms (AAAs). Tissue samples were collected during operations from 31 patients with atherosclerotic infrarenal abdominal aortic aneurysms ranging in diameters from 5 to 8 cm. The specimens were immediately frozen and examined using single and double immunohistochemical staining. T-cell subpopulations, B-cells, dendritic cells and macrophages were identified using cell type specific antibodies. Cell contacts were examined by electron microscopy. Most inflammatory infiltrates were found in the adventitia. T-cells were the predominant cell type in a majority of inflammatory infiltrates but in seventeen cases, typical lymphoid follicles with B-cells forming germinative centres were also observed. In eight cases, the lymphoid follicles aggregated in lymph node-like structures. Dendritic cells were present within all inflammatory infiltrates and contacted lymphocytes. The present observations show that in aortic aneurysm, VALT is involved in immune responses and its activation mostly occurs in the adventitia. The formation of lymphoid follicles and lymph node-like structures in the adventitia suggests that VALT might locally serve the entire complex of both cellular and humoral immune responses in the aneurysmal wall. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Abdominal aortic aneurysm (AAA); Dendritic cells; Lymphocytes; Inflammation
www.elsevier.com/locate/atherosclerosis
1. Introduction
Vascular-associated lymphoid tissue (VALT) consist-ing of disseminated accumulations of immunocom-petent cells and antigen presenting cells has recently been recognised in the arterial intima [1,2]. In the non-diseased arterial wall, small numbers of immuno-competent and antigen presenting cells are distributed throughout the subendothelial layer of the arterial in-tima and some of these cells are also present around the vasa vasorum in the adventitia [1 – 5]. VALT is proba-bly analogous to mucosa-associated lymphoid tissue (MALT) of the respiratory and gastrointestinal tracts and like MALT, VALT probably screens ‘vascular tissue’ for potentially harmful antigens [1].
Based on the concept of VALT, Wick and co-workers [1] have proposed a new autoinimune hypo-thesis for atherogenesis which postulated that VALT activation by autoantigens is responsible for the initiation of immune responses in the arterial wall.
In primary atherosclerosis, immunocompetent cells accumulate within the arterial intima [6 – 9]. In contrast, in abdominal aortic aneurysms (AAAs), there is a dramatic change in the cellular composition of the outer aortic wall associated with massive infiltration of the external layer of the media and the entire adventitia by lymphocytes [10 – 12]. Furthermore, in primary atherosclerosis, T-lymphocytes exclusively infiltrate the intima [6 – 9] while the adventitial infiltrates in AAAs contain large numbers of both T-cells and B-cells [10 – 12]. Antigen-presenting dendritic cells have also been suggested to accumulate in the media and adventi-tia of the AAA wall [13].
* Corresponding author. Tel.:+1-617-726-1032; fax:+ 1-617-726-2874.
E-mail address:[email protected] (Y.V. Bobry-shev).
During the last decade, the mechanisms responsible for the weakening and destruction of the aortic wall, which lead to the formation of AAAs, have been intensively studied and the degradation of the extracel-lular matrix in the medial layer associated with in-creased proteolytic activity have been implicated in the breakdown of the structural integrity of the aortic wall [14 – 17]. In AAAs, inflammatory cells secrete cytokines that are the main source of enhanced proteolytic activ-ity responsible for weakening the aortic wall [18].
Despite the significance of immune and inflammatory cells accumulating in the AAA wall, the histopathologi-cal features of immune inflammation as well as the immunocompetent cell interactions in AAAs have re-ceived limited attention. We now report the involve-ment of VALT in immune responses in the aortic aneurysmal wall.
2. Methods
2.1. Tissue specimens
Material was collected in accordance with the princi-ples outlined in the Declaration of Helsinki [19]. The present study was approved by the institutional review board of St Vincent’s Hospital, Sydney.
Samples taken from the anterior wall were collected from 31 typical atherosclerotic infrarenal AAAs rang-ing in diameter from 5 to 8 cm. All the patients, whose ages ranged from 55 to 84 years, were operated upon on an elective basis and had no ruptured or rapidly expanding aneurysms. For immunohistochemical analy-sis, unfixed samples were immediately embedded in OCT compound, rapidly frozen in liquid nitrogen and stored at −70°C until cryostat sectioning. For electron
microscopy, small tissue pieces were fixed in 2.5% glu-taraldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4.
2.2. Immunohistochemical identification of cell types
T-cells were identified with anti-CD3 and their helper/inducer and suppressor/cytotoxic subtypes were identified with anti-CD4 and anti-CD8, respectively. B-cells were identified with anti-CD20. Dendritic cells were identified with S-100 and anti-CD1a. Macrophages were identified with anti-CD68 antibody. Granulocytes were identified with anti-CD15. Endothe-lial cells were identified with anti-von Willebrand factor antibody. Smooth muscle cells were identified with antibody to smooth muscle a-actin. The sources and
working concentrations of the antibodies used are given in Table 1.
2.3. Single immunostaining procedure
The tissue specimens were cut at 7mm thickness and
the sections air dried for 45 min. After eliminating endogenous peroxidase activity by 0.3% H2O2 for 5 min, the sections were preincubated with normal goat serum and then tested with avidin-biotin complex (ABC) using the peroxidase-anti-peroxidase (PAP) technique [20]. The sections were incubated for 30 min with the primary antibody and after washing in tris-phosphate buffered saline (TPBS), pH 7.6, for 10 min, the sections were incubated for 20 min with the appro-priate biotin-labeled secondary antibodies (horse anti-mouse-Vector BA-2000, or goat anti-rabbit-Vector BA1000). The sections were then washed in TPBS for 5 min and treated with avidin-biotin complex (Elite-ABC, Vector PK61 00) for 30 min. After washing for 10 min
Table 1
Antibodies used in the study
Working dilation Source
Type* Clone Specificity Cell types identified Designation
M NA1/34
CD1a CD1a Thymocytes, Langerhans cells, interdigitating cells, DAKO 1:50 vascular dendritic cells
–
P Glial cells, ependyma, Schwann cells, Langerhans cells,
S100 S-100A, S-100B DAKO 1:700
interdigitating cells, vascular dendritic cells P
CD3 – CD3 T-cells DAKO 1:100
M MT310 CD4
CD4 Helper/inducer subtype of T-cells DAKO 1:10
CD8 M DK25 CD8 Supressor/cytotoxic subtype of T-cells DAKO 1:50
CD20 B-cells DAKO
CD20 M L26 1:50
CD68 Macrophages DAKO 1:50
EBM11 M PG-MI
1:50 DAKO Endothelial cells
Von Willebrand M F8/86 Factor
factor VIII-related
antigen M 1A4 Smooth muscle
SMA Smooth muscle cells DAKO 1:400
a-actin
1:50 DAKO Granulocytes, Reed Stemberg cells
CD15
M MMA
CD15
in TPBS, visualisation of antigens was produced by treatment for 5 min with 3,3%-diaminobenzidine (DAB). The PAP system with DAB chromogen yielded a brown reaction product at the site of the target antigens. Alternatively, cell types were identified using the alka-line phosphatase anti-alkaalka-line phosphatase (APAAP) technique. Alkaline phosphatase-conjugated antibody (Dako) with Fast Red (Dako) as an enzyme substrate were used. The APAAP system with Fast Red chro-mogen resulted in a rose precipitate at the site of the identified antigens. All the incubations were completed at room temperature. For negative controls, the first antibodies were omitted or the sections were treated with an immunoglobulin fraction of non-immune goat serum (Vector S-1000) as a substitute for the primary antibody. None of the negative control sections showed positive immune staining. Counterstaining was per-formed with Mayer’s haematoxylin and the sections were examined in an Olympus microscope at 10×10 and 10×40 magnifications.
2.4. Double immunostaining procedure
The co-localisation of dendritic cells with lymphocytes was analysed by double immunostaining combining the PAP and APAAP techniques. After visualisation of dendritic cells by the PAP technique, the tissue sections were washed for 60 min with 0.1 M glycinehydrochloric buffer (pH 2.2) at 4°C. The sec-tions were then incubated with one of the second primary antibodies (anti-CD3 or anti-CD20) for 60 min at room temperature. Lymphocytes were visualised us-ing the APAAP technique, as described above for sus-ingle immunostaining. Negative controls were carded out as described above and none of the negative control sec-tions showed positive immune staining. Counterstaining was performed with Mayer’s haematoxylin.
2.5. Electron microscopic analysis
After fixation in 2.5% glutaraldehyde in PBS (pH 7.4), specimens were postfixed in 1% osmium tetroxide, dehydrated in graded ethanol and propylene oxide and were then embedded in Araldite resin. Ultrathin tions were cut on a LKB-III ultratome. Ultrathin sec-tions were stained with uranyl acetate and lead citrate and examined with the aid of a Hitachi H7000 electron microscope at an accelerating voltage of 100 kV.
3. Results
In all the specimens, atherosclerotic changes of the intima were present. Advanced atherosclerotic plaques containing a necrotic core were observed in 24 of the 31 cases. Atherosclerofic lesions contained CD68 positive
foam cells and CD68 negative foam cells, the later being immunoposifive for the antibody against smooth muscle a-actin. Amongst these foam cells, a large
num-ber of T-cells (CD3+) and a few dendritic cells
express-ing S-100 and CD1a were intermexpress-ingled.
Scattered between smooth muscle cells in the under-lying media were macrophages (CD68+) and T-cells
(CD3+). Some medial smooth muscle cells showed low
intensity staining for the antibody against smooth mus-cle (a-actin and the pycnotic appearance of their nuclei
suggested cellular destruction. The media contained many microvessels derived from the adventitial vasa vasorum, around which differing numbers of T-cells, dendritic cells and macrophages were observed. B-cells (CD20+) were seldom observed in the media.
In all the specimens, large numbers of immune and inflammatory cells were observed in the adventitia. There were diffusely distributed and intermingled T-cells (CD3+), B-cells (CD20+), dendritic cells (CD1a+;
S-100+), macrophages (CD68+) and granulocytes
(CD15+). T-cells (CD3+) were presented by their
CD4+ and CD8+ subpopulations and in all the
speci-mens, CD4+ cells were a prevalent cell subtype. The
numbers of CD4+ cells were estimated as exceeding the
numbers of CD8+ cells by four – 16 times. Massive
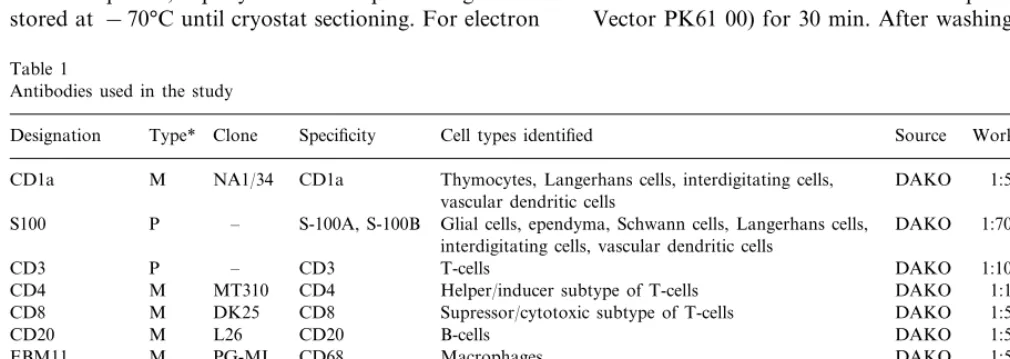
inflammatory cell accumulations were also observed in the adventitia (Fig. 1A – D). Within some of these infl-ammatory infiltrates, cells were distributed relatively evenly throughout (Fig. 1A) while within others, an uneven distribution of cells was prominent (Fig. 1C). Different patterns of cell distribution within the infiltrates corresponded to the peculiarities of the distri-bution of T-cells (CD3+) (Fig. 1B, D; Fig. 2A, B).
Infiltrates with a relatively even cell distribution con-sisted mostly of T-cells (CD3+) with only a few
den-dritic cells (S-100+; CD1a+) residing between them
(Fig. 2A, B).
Within inflammatory infiltrates where the cell distri-bution was uneven, one or several aggregates of CD3 negative cells were present and were usually located in the central areas of the infiltrates (Fig. 1D; Fig. 3A, B). These aggregates of CD3 negative cells were sur-rounded by CD3 positive cells (Fig. 1D; Fig. 3A, B). Cells which formed the aggregates of CD3 negative cells were found to stain positively with anti-CD20 antibody (Fig. 3C, D). This type of cell infiltrate was, therefore, identified as a typical lymphoid follicle as it contained a germinative centre(s) consisting of B-cells (Fig. 3A-D). In 17 of the 31 specimens, the lymphoid follicles with B-cells forming germinative centres were observed. In eight specimens, several follicles united to form lymph node-like structures (Fig. 1C, D). We were not able to establish a correlation between the degree of adventitilal inflammation and clinical data.
Fig. 1. Typical appearance of massive inflammatory infiltrates (A – C) and the distribution of CD3 positive cells (rose) (B, D) seen at a low magnification (×100) in the adventitia of the AAA wall. (B, D): Sections were stained with anti-CD3 antibody using the APAAP technique. (A) and (B) show a relatively even distribution of lymphocytes within the infiltrates while (C) and (D) represent consec-utive sections containing an infiltrate with an uneven distribution of lymphocytes. In (D), note aggregates of CD3 negative cells, which are surrounded by CD3 positive cells (rose) within the infiltrate. In (A), an atherosclerotic necrotic core is marked by an asterisk. M, media; A, adventitia. (A – C), counterstaining with Mayer’s haematoxylin.
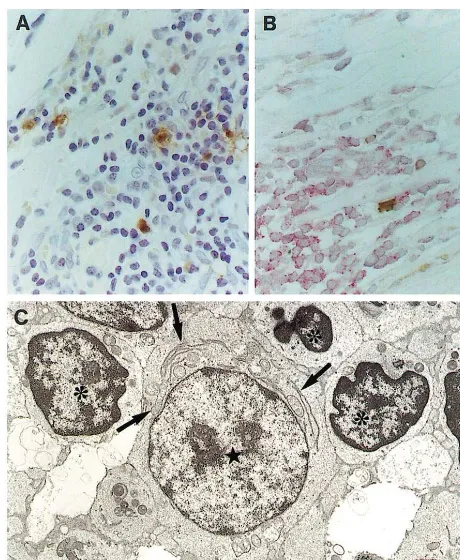
(Fig. 4A, B). Double immunostaining demonstrated that these dendritic cells contacted T-cells (Fig. 4B). Electron microscopic examination confirmed the forma-tion of contacts between dendritic cells and T-cells within lymphoid follicles (Fig. 4C). These contacts were mostly through dendritic cell processes extending from the cell body. In the cytoplasm of dendritic cells which clustered with T-cells, hypertrophied cisterns of the tubulovesicular system were prominent (Fig. 4C).
In some inflammatory infiltrates, B-cells represented a predominant cell type, estimated as being as much as 60 – 80% of the total cell population of the infiltrate (Fig. 5A, B). In the central areas of these infiltrates, B-cells were usually closely packed while on the periph-ery, B-cells were more diffusely disseminated (Fig. 5A, B). Sparse dendritic cells expressing S-100 and CD1a were distributed amongst lymphocytes in the peripheral zones of the infiltrates. Double immunostaining and electron microscopic examination of these infiltrates demonstrated that dendritic cells contacted B-cells (Fig. 5C, D).
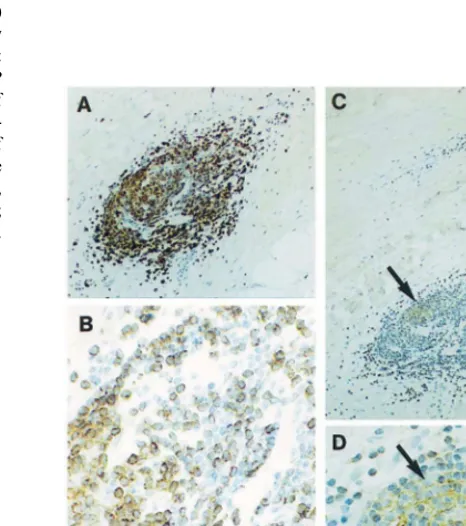
Fig. 3. Distribution of T-cells (A) and B-cells (C) in a lymphoid follicle formed in the AAA adventitia. T-cells (brown) were identified with anti-CD3 and B-cells (brown) were identified with anti-CD20 using the PAP technique. (A) and (C) are consecutive sections. (B) is a detail of (A). (D) is a detail of (C). In (C, D), a germinative centre formed by B-cells is shown by arrows. In (C), M, media; A, adventi-tia. (A – C), Counterstaining with Mayer’s haematoxylin. Magnifica-tions: ×100, ×400, ×100, ×400.
Fig. 2. Immunohistochemical identification of dendritic cells and visualisation of their contacts with T-cells within an adventitial inflammatory infiltrate. Dendritic cells (brown) were stained with S-100 antibody using the PAP technique and T-cells (rose) were identified with anti-CD3 antibody using the APAAP technique. (B) is a detail of (A). Note that the infiltrate consists of T-cells only. Note also that dendritic cells contact T-cells on the periphery of the infiltrate as well as in its centre. In (A), M, media; A, adventitia. Counterstaining with Mayer’s haematoxylin. Magnifications: ×100,
Fig. 4. Immunohistochemical (A, B) and electronmicroscopic (C) visualisation of dendritic cells and their contacts with T-cells at the peripheral zone of lymphoid follicles. In (A), dendritic cells (brown) were identified using anti-CD1a antibody and the PAP technique. Counterstaining with Mayer’s haematoxylin. Magnification: ×400. (B): Dendritic cells (brown) were stained with S-100 antibody using the PAP technique and T-cells (rose) were identified with anti-CD3 antibody using the APAAP technique. Counterstaining with Mayer’s haematoxylin. Magnification: ×400. (C), clustering of T-lymphocytes (asterisks) around a dendritic cell (star). Note that the dendritic cell posseses a well-developed tubulovesicular system while it lacks lysosomes and phagolysosomes. Cisterns of the tubulovesicu-lar system are shown by arrows. Electron micrograph. Magnification:
×8100.
processes extending from the cell body, lacked lyso-somes and phagolysolyso-somes and were characterised by the presence of a well developed tubulovesicular system which is a unique feature of cells from the dendritic cell family [21,22]. This unique tubulovesicular network may facilitate transport of antigens within the cell cytoplasm [21,23].
T-cells require antigen to be processed and presented to them by antigen-presenting cells [21,24,25]. Dendritic cells are involved in various diseases where they repre-sent a minor cell population [21,24,25]. Even though their numbers are small, dendritic cells provide very efficient antigen-presentation and are the most potent antigen-presenting cells known [21,24,25]. The associa-tion of T-cells with dendritic cells has been shown to occur in atherosclerosis [26]. Dendritic cells are proba-bly also an important cell element in VALT activation in AAAs. The present study is the first to demonstrate the formation of direct contacts between dendritic cells and lymphocytes within inflammatory infiltrates in the
Fig. 5. B-cell rich inflammatory infiltrates in the adventitia of the AAA wall. (A): A group of infiltrates containing large numbers of B-cells (CD20+). B-cells (brown) were identified using the PAP
technique. M, media; A, adventitia. Counterstaining with Mayer’s haematoxylin. Magnification:×100. (B) is a detail of (A). Magnifica-tion: ×400. (C), dendritic cell located within an infiltrate where it contacts B-cells. Double immunostaining in which dendritic cells were visualised with S-100 antibody by the PAP technique while B-cells were visualised with anti-CD20 by the APAAP technique. Counter-staining with Mayer’s haematoxylin. Magnification:×400. (D): Elec-tron micrograph demonstrating a contact of a dendritic cell (star) with a B-cells (plasma cells) (asterisks). Magnification: ×6000.
4. Discussion
The present study demonstrates that VALT is hyper-trophied in the wall of aortic aneurysms, which implies that VALT is involved in immune responses. VALT activation mostly occurs in the adventitia. In seventeen cases, the formation of lymphoid follicles with germina-tive centres consisting of densely packed B-cells were observed. These germinative centres were surrounded by numerous T-cells and in eight cases, the lymphoid follicles aggregated in lymph node-like structures.
adventitia of the AAA wall. That lymphocytes, includ-ing T- and B-cells, co-localise with dendritic cells within inflammatory infiltrates suggests that antigen-presenta-tion and activaantigen-presenta-tion of lymphocytes might occur directly in the AAA aortic wall. The formation of lymphoid follicles and lymph node-like structures with co-lo-calised lymphocytes and dendritic cells suggests that VALT might locally serve an entire complex of both cellular and humoral immune responses.
In the present study, intimal atheroma and adventital inflammation were features of all AAAs. These obser-vations agree with earlier publications of others [17,27,28], showing an association of adventitial im-mune-inflammatory response with intimal atherosclero-sis. In the diseased aortic wall, immunoglobulins have been found co-localised with deposits of the insoluble lipid, ceroid [29], and auto-antibodies to ceroid and oxidised low density lipoproteins (LDL) have been detected in the sera of patients with chronic periaortitis [30]. The insoluble ceroid and its component, oxidised LDL, have been suggested to initiate autoinimune reac-tion [30,31]. In this regard, the immune response occur-ring in chronic periaortitis has been thought to be likely mediated by a specific antigen-driven T- and B-cell activation within the diseased aortic wall [32,33].
Using a highly sensitive reverse transcription-poly-merase chain reaction amplification method, a recent work [32] investigated whether there is a selective acti-vation and expansion of a limited repertoire of T-cell antigen receptor (TCR)-specific T-lymphocytes. It has been shown that the TCR VBgene expression in aortic aneurysm is polyclonal and that there was no preferen-tial expression of any TCR VB gene in aortic tissue in comparison with that in peripheral blood in aneurysmal patients [32]. Similarly, an investigation which tested the hypothesis that the B-cell rich infiltrate concen-trated in the adventitia of AAAs is an autoinimune response to specific antigens, has demonstrated that in the vast majority of atherosclerotic AAAs the B-cell rich adventitial infiltrates are not an autoimmune re-sponse to a limited repertoire of tissue antigens [33]. It has been suggested, therefore, that multiple anti-genic epitopes may be present in ceroid or oxidised LDL and may be responsible for the recruitment and/
or expansion of diverse antigen specific T-cells [32,33]. Alternatively, it is possible that the specific TCR VB usaged T-cells might be masked by the infiltration of many unbiased T-cells recruited by lymphokines and other chemoattractants produced in the AAA wall [33]. These data also do not discount the hypotheses that adventitial infiltration is either a response to hypoxia or part of a viral/bacterial -induced inflammatory process [31 – 34].
While the mechanisms responsible for adventitial infl-ammation are still unclear, recent findings [35] unam-biguously show that the accumulation of immune cells
is crucial in the failure of the wall in AAAs. The breakdown in elastic laminae and the disappearance of a well organised medial smooth muscle cell layer has been shown to be caused by upregulation of proteolytic enzymes and, in particular, by matrix metallo-proteinases [14 – 17]. In AAAs, inflammatory cells secrete different cytokines, which are the main source of enhanced proteolytic activity and thus, might be re-sponsible for the weakening of the aortic wall [18]. Recent studies [36] also indicate that in aneurysmal tissue there is an increase in the level of both matrix metalloproteinases and their inhibitors, which suggests that the overall balance of enzyme activity may be preserved or only slightly altered during the AAA formation [35,36]. Decreased numbers of smooth mus-cle cells have been further suggested to be associated with smooth muscle cell apoptotic death which leads to impaired synthesis of the matrix proteins in the aneu-rysmal wall [35]. The local expression of death promot-ing mediators such as perforin, Fas and FasL by infiltrating immune cells has been shown to promote smooth muscle cell apoptosis [35]. Together, these ob-servations suggest that the accumulation of immune and inflammatory cells in the AAA wall is important. The present histopathological observations of VALT activation in AAAs might assist in understanding the mechanisms involved in the aortic wall failure.
Acknowledgements
This research was supported by St Vincents Clinic Foundation, Sydney.
References
[1] Wick G, Romen M, Amberger A, et al. Atherosclerosis, autoim-munity and vascular-associated lymphoid tissue (VALT). FASEB J 1997;11:1199 – 207.
[2] Waltner-Romen M, Falkensammer G, Rabl W, Wick G. A previously unrecognized site of local accumulation of mononu-clear cells: the vascular-associated lymphoid tissue. J Histochem Cytochem 1998;46:1347 – 50.
[3] Bobryshev YV, Lord RSA. Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contact-ing interactions of vascular dendritic cells in athero-resistant and athero-prone areas of the normal aorta. Arch Histol Cytol 1995;58:307 – 22.
[4] Bobryshey YV, Ikezawa T, Watanabe T. Formation of Birbeck granule-like structures in vascular dendritic cells in human atherosclerotic aorta. Lag-antibody to epidermal Langerhans cells recognizes cells in the aortic wall. Atherosclerosis 1997;133:193 – 202.
[5] Lord RSA, Bobryshev YV. Clustering of dendritic cells in athero-prone areas of the aorta. Atherosclerosis 1999;146:197 – 8 (letter to the Editors).
[7] Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulation of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Atherosclerosis 1986;6:131 – 8.
[8] Hansson GK, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis 1988;72:135 – 41.
[9] Ross R. Atherosclerosis-an infiammatory disease. New Eng J Med 1999;340:115 – 26.
[10] Ramshaw AL, Parums DV. Immunohistochemical characteriza-tion of inflammatory cells associated with advanced atheroscle-rosis. Histopathology 1990;17:543 – 52.
[11] Parums DV. The spectrum of chronic periaortitis. Histopathol-ogy 1990;16:423 – 31.
[12] Koch AE, Haines GK, Rizzo RJ, et al. Human abdominal aortic aneurysms: immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol 1990;137:1199 – 213.
[13] Bobryshev YV, Lord RSA, Parsson H. Immunophenotypic anal-ysis of the aortic aneurysm wall suggests that vascular dendritic cells are involved in immune responses. Cardiovasc Surg 1998;6:240 – 9.
[14] Vine N, Powell JT. Metalloproteinases in degenerative aortic disease. Clin Sci 1991;81:233 – 9.
[15] Newman KM, Ogata Y, Malon AM, et al. Identification of matrix metalloproteinases 3 and 9 in abdominal aortic aneurysm. Atherioscler Thromb 1994;14:1315 – 20.
[16] Newman KM, Jean-Claude J, Li R, et al. Cellular localisation of matrix metalloproteinases in abdominal aortic aneurysm wall. J Vasc Surg 1994;20:814 – 20.
[17] Freestone T, Turner RJ, Coady A, et al. Inflammation and matrix metalloproteinases in enlarging abdominal aortic aneu-rysm. Arterioscler Thromb Vasc Biol 1995;15:1145 – 51. [18] Koch AE, Kunkel SL, Pearce WH, et al. Enhanced production
of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneu-rysms. Am J Pathol 1993;142:1423 – 31.
[19] World Medical Association, Inc. World Medical Association Declaration of Helsinki: Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res 1997;35:2 – 3.
[20] Hsu S-M, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J His-tochem CyHis-tochem 1981;29:577 – 80.
[21] King PD, Katz DR. Mechanisms of dendritic cell function. Immunol Today 1990;11:206 – 11.
[22] Takahashi K, Naito M, Takeya M. Development and hetero-geneity of macrophages and their related cells through their differentiation pathways. Pathol Int 1996;46:473 – 85.
[23] Bobryshev YV, Lord RSA, Watanabe T. Structural peculiarities of vascular dendrific cell tubulovesicular system in human atherosclerofic aorta. J Submicrosc Cytol Pathol 1997;29:553 – 61.
[24] Steinman RM. The dendritic cell system and its role in immuno-genicity. Ann Rev Immunol 1991;9:271 – 96.
[25] Banchereau J, Steinman RM. Dendritic cells and control of immunity. Nature 1998;392:245 – 52.
[26] Bobryshev YV, Lord RSA. Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res 1998;37:799 – 810.
[27] Schwartz CJ, Mitchell JRA. Cellular infiltration of human arte-rial adventitia associated with atheromatous plaques. Circulation 1962;26:73 – 8.
[28] Rose AG, Dent DM. Inflammatory variant of abdominal atherosclerotic aneurysm. Arch Pathol Lab Med 1981;105:409 – 13.
[29] Parums DV, Chadwick DR, Mitchinson MJ. The localisation of immunoglobulin in chronic periaortitis. Atheroselerosis 1986;61:117 – 23.
[30] Parums DV, Brown DL, Mitchinson MJ. Serum antibodies to oxidized low-density lipoprotein and ceroid in chronic periaorti-tis. Arch Pathol Lab Med 1990;114:383 – 7.
[31] Gregory AK, Yin NX, Capella J, Xia S, Newman KM, Tilson IM. Features of autoimmunity in the abdominal aortic aneu-rysm. Arch Surg 1996;131:85 – 8.
[32] Yen HQ, Lee F-Y, Chau LY. Analysis of the T cell receptor VB
repertoire in human aortic aneurysms. Atherosclerosis 1997;135:29 – 36.
[33] Walton W, Powell JT, Parums DV. Unrestricted usage of im-munoglobulin heavy chain genes in B cells infiltrating the wall of atherosclerotic abdominal aortic aneurysms. Atherosclerosis 1997;135:65 – 71.
[34] Yonemitsu Y, Nakagawa K, Tanaka S, Mori R, Sugimachi K, Sueishi K. In situ detection of frequent and active infection of human cytomegalovirus in inflammatory abdominal aortic aneu-rysms: possible pathogenic role in sustained chronic inflamma-tory reaction. Lab Invest 1996;74:723 – 36.
[35] Henderson EL, Geng Y-J, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation 1999;99:96 – 104.
[36] Knox JB, Sukhova GK, Whittemore AD, Libby P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 1997;95:205 – 12.