Receptiveness of some tropical soils from banana fields in
Martinique to the arbuscular fungus Glomus intraradices
C. Plenchette
∗Station d’Agronomie, INRA, 17 rue Sully, 21034 Dijon Cedex, France
Received 26 November 1999; received in revised form 10 March 2000; accepted 14 March 2000
Abstract
Tropical crops of great economic importance such as banana are known to benefit from mycorrhizal association. Develop-ment and survival of introduced mycorrhizal propagules depend not only on the crops but mainly on the edaphic conditions and soil types where the symbiosis is established. Seven soils from banana fields of Martinique were sampled and tested to determine their receptiveness to mycorrhizal inoculation. Biological tests involved cultivation of 10 leek plantlets in pots containing soil inoculated with a range of mycorrhizal propagule densities (3, 10, 30, 100, 300 propagules/100 g soil). My-corrhizal colonisation was recorded after 2 weeks of growth and tentatively correlated with inoculum density. For each soil regression equations enable determination of the number of propagules needed to induce mycorrhizal colonisation of 50% of the leek plantlets. This measurement unit parameter value was called ‘the mycorrhizal soil receptiveness50(MSR50) unit’.
Values recorded varied from 22.7 (for Fafane, Enterrement, Parc à veaux soils) to 96.7 propagules/100 g (for the Morne-vidou soil). Soil receptiveness tended to decrease when the number of propagules increased. Multiple regression tests between MSR50unit values and soil parameters (pH, P, Cu, Zn, CEC, N) permitted development of mathematical equations enabling
prediction of mycorrhizal soil receptiveness. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Bioassay; Glomus intraradices; Inoculation; Mycorrhiza; Propagule; Receptiveness
1. Introduction
Although nearly all soils contain indigenous arbus-cular mycorrhizal (AM) fungi (Abbott and Robson, 1982), their distribution and their abundance (i.e. the indigenous inoculum potential) vary greatly among regions, soil types and crop production systems (Gianinazzi-Pearson et al., 1985; Declerck, 1996). Moreover, the association between a given plant species and the indigenous fungal population may not always be optimal. Improvement of plant growth response to AM inoculum may be achieved through
∗Tel.:+33-3-8069-3032; fax:+33-3-8069-3222.
E-mail address: [email protected] (C. Plenchette).
better management of introduced fungi (Sieverding, 1991).
Under tropical conditions AM fungi could be highly beneficial to some perennial crops which require nurs-ery production before transplantation to the field (Feld-mann and Idzak, 1992). Among these crops, banana is considered promising due to its economic impor-tance for farmer’s production (Delvaux et al., 1990). Moreover, recent studies on the response of banana to AM symbiosis were very promising (Jaizme-Vega and Azcon, 1995). It is well-known that inoculation with AM fungi gives better results when performed at the early stages of plant development. Significant growth response of micropropagated banana were obtained when plants were inoculated at the beginning of the
weaning phase (Declerck et al., 1994), depending on varieties and AM fungi used (Declerck et al., 1995).
Thus, under tropical stress conditions in soils which are strongly P-deficient and where crop production is largely dependent on mycorrhizal status of plants, the management of AM fungi is fundamental (Sieverding, 1991). In such environments where the indigenous in-oculum potential is low and/or AM fungi poorly or not efficient, adding AM fungi to soil may be considered as highly beneficial to long-term plant production. De-velopment of AM symbiosis, however, is dependent on host plant and edaphic conditions (Plenchette et al., 1983). Thus, the quantity of inoculum needed for op-timum functioning of the association varies according to soil type and other factors. So, it is necessary to determine the soil receptiveness to AM fungi (Duvert et al., 1990), i.e. the capacity of the soil to favor their development before proceeding to inoculation.
AM symbiosis has been shown to be of great value in improving sustainable agriculture in banana crop-ping systems (Declerck, 1996). Since AM symbiosis proved to be more efficient if inoculation or outplant-ing mycorrhizal plants are performed in disinfected soil, the aim of this work was to determine and com-pare receptiveness of disinfected tropical soils from banana fields of Martinique to AM inoculation.
2. Materials and methods
2.1. Principle of the method
Soil receptiveness to AM fungi can be estimated by a standard bioassay from a dose (quantity of inoculum)–response (mycorrhizal status of test plants) relationship according to the biological assay
princi-Table 1
Location of the sampling areas in Martinique
No. Field Farm Locality Type of soila
1 Fafane Bellevue Marigot Andesitic ash and tuff (humitropept)
2 Ti-Bois2 Chalvet Basse-Pointe Recent volcanic on ashes and pumices (udivitrand) 3 Morne-Vidou Grands-Fonds Le François Old volcanic conglomerate (dystropept)
4 Enterrement Le Simon Le François Alluvial deposit of basaltic origin (ustert) 5 Parc `a veaux Longchamp Morne-Rouge Andosol on pumices (hapludand) 6 Abricot Rivi`ere L´ezarde Saint Joseph Brunisolic with halloysite 7 Romulde Saint Michel Gros-Morne Andesitic tuff (humitropept)
aUS soil classification system (soil taxonomy).
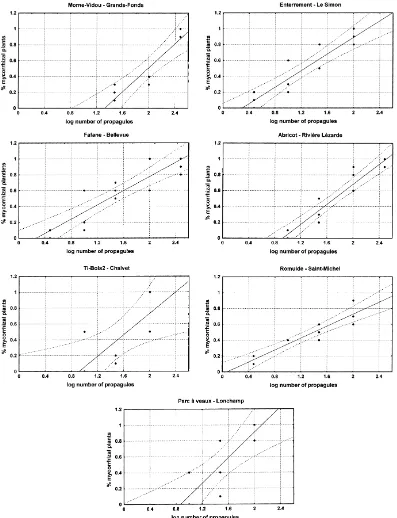
Fig. 1. Map of Martinique with the locations of the sampling areas.
ple (Finney, 1971). The method involves cultivation of a population of susceptible plantlets in controlled environmental conditions on a range of inoculum quantity in the soil studied (Duvert et al., 1990).
2.2. Soils
Table 2
Physical and chemical properties of the soils studied
Soil
then air-dried prior to being shipped to France for test-ing. After sieving to a maximum particle size of 2 mm, soils were oven-dried (65◦C, 48 h) and gamma irra-diated (>1 kGy). Physico-chemical properties of the soil samples are given in Table 2. Bioavailability of phosphorus (Table 3) was also determined using the isotopic kinetic method (Fardeau, 1993).
2.3. Inoculum
The inoculant fungus Glomus intraradices was cho-sen because of its ability to form abundant internal vesicles which proved to be very infective propag-ules (Duvert et al., 1990). This isolate (CP-103/CP) from our laboratory collection is propagated on leeks growing on calcined clay and was deposited in the Canada Department of Agriculture Herbarium, Ottawa (DAOM 181602). The inoculum consisted of infected leek roots produced according to the method devel-oped by Plenchette (1982). Infected roots were hand
Table 3
Isotopic exchange kinetics parameters characterising the P avail-ability of the soilsa
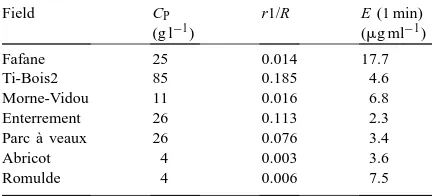
Field CP
Parc `a veaux 26 0.076 3.4
Abricot 4 0.003 3.6
Romulde 4 0.006 7.5
aC
P(g l−1)=P concentration of the soil solution; r1/R=P fixing
power of the soil; E (1 min)=isotopically exchangeable P in 1 min (Fardeau, 1993).
cut with a scalpel in 1–3 mm pieces, each considered as one propagule (Duvert et al., 1990). To provide a log-arithmic scale of inoculum density (300, 100, 30, 10, 3 infective propagules/100 g of soil) inoculum pieces were counted under a dissecting microscope and thor-oughly mixed with the soil. Direct observation of a sample of this inoculum was made after clearing (10% KOH) and staining (0.05% acid fuchsin in lactoglyc-erol) in order to evaluate the proportion of pieces with-out vesicles or hyphae. This proportion was taken into account to be sure to have the required number of in-fective propagules for each treatment.
For each inoculum density, 100 g of soil containing the required number of AM propagules was weighed and poured into plastic multipots (Somapo-Sopirec, Diemeringen, France). Three replicates were done for each density. Seeds of leek (Allium porrum L. var Bleu de Solaise) were surface disinfected (6% cal-cium hypochlorite for 2 min) and germinated on wet filter paper in Petri dishes. After 1 week, 10 plantlets were transplanted in each pot. Pots were then placed in a growth chamber for 2 weeks, under the follow-ing conditions: 24◦C/20◦C day/night, 16 h photope-riod and 200mmol m−2s−2PAR. Plants were watered with deionized water without addition of nutrients.
2.4. Mycorrhizal assessment
entry point was considered as a record of mycorrhizal infection to give an all or nothing quantal response. Results were expressed as the percentage of mycor-rhizal plants per pot.
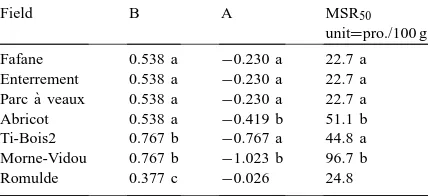
2.4.1. Determination of mycorrhizal receptiveness For each soil, the percentage of mycorrhizal plants was plotted against the logarithm of the number of propagules introduced in the soil. Regression curves (model Y=BX+A) were calculated for each soil and variance analysis was performed to test the non-equality of their slope. The Mycorrhizal soil re-ceptiveness unit was calculated using a regression line equation (Duvert et al., 1990) and defined as the number of propagules of a given inoculum re-quired to infect 50% of the plant population under the bioassay conditions and thus was called the my-corrhizal soil receptiveness50 (MSR50) unit. MSR50 values varied among soils; the lower the value (num-ber of propagules), the higher the receptiveness of the soil.
Variance analyses were performed to compare slopes of regression lines between propagule con-centrations and percentage of mycorrhizal plantlets. Soils with statistically similar regression slopes were grouped and a common slope (Table 4) was com-puted to compare their y-intercepts using a t-test (Dagnelie, 1975). The programme used was written in Fortran by André Carteron, Station de Génétique et d’Amélioration des Plantes, INRA, Dijon, France. Simple and multiple regressions were calculated
Table 4
Regression curve characteristics (model Y=A+BX) and estimation
of mycorrhizal soil receptiveness to Glomus intraradicesa
Field B A MSR50
unit=pro./100 g
Fafane 0.538 a −0.230 a 22.7 a Enterrement 0.538 a −0.230 a 22.7 a Parc `a veaux 0.538 a −0.230 a 22.7 a Abricot 0.538 a −0.419 b 51.1 b Ti-Bois2 0.767 b −0.767 a 44.8 a Morne-Vidou 0.767 b −1.023 b 96.7 b Romulde 0.377 c −0.026 24.8
aB comparison determine three group of plots, B values not
followed by the same letter are significantly different (p<0.05); for A and MSR50unit comparisons were made within a group and
values not followed by the same letter are significantly different (p<0.05).
between MSR50 values and physico-chemical soil characteristics.
3. Results and discussion
To quantify soil receptiveness, linear regression curves between percentages of mycorrhizal plantlets obtained in response to inoculation with increased quantities of infectious propagules (Fig. 2) were cal-culated as previously described (Plenchette et al., 1989). Percentage values of 0 and 100 were not taken into account since the low number of inoculum levels (five) used does not permit accurate calculation of the number of propagules needed to reach these two levels, respectively (Bouhot and Joannes, 1979). Re-ceptiveness expressed as MSR50 units, i.e. numbers of propagules (pro.) per 100 g of soil needed to in-fect 50% of plantlets, varied greatly among the soils tested. The lowest MSR50 unit value was 22.7 pro. for the Fafane, Enterrement and Parc à veaux soils and the highest was 96.7 for Morne Vidou (Table 4). This means that the receptiveness of the soils from Fafane, Enterrement and Parc à veaux was four times higher than that of the soil from Morne Vidou, i.e. successful inoculation of the Fafane, Enterrement and Parc à veaux soils requires four times less inoculum than the Morne Vidou soil.
Table 5
Correlation of soil receptiveness to Glomus intraradices with soil variables
Soil variables Correlation coefficient (r)
Linear Reciprocal
The results showed that soils from Fafane, Enter-rement, Parc à veaux and Romulde have high recep-tiveness compared to those of the soils from Ti-Bois2 and Abricot, which themselves have moderate recep-tiveness compared to that of Morne Vidou, which is the lowest.
Receptiveness did not correlate with chemical or isotopical parameters of P availability, nor with any other soil physico-chemical parameter characteristics (Table 5). Consequently, none of these soil variables can be used individually to predict receptiveness of a soil to mycorrhizal inoculation. Menge et al. (1982) working with 26 soils and nursery mixes observed re-ciprocal correlations between mycorrhizal dependency of citrus and extractable P, Zn, Mn, K, Cu, pH, CEC and organic matter, but found that any of them was ad-equate individually to predict mycorrhizal dependency of citrus.
Using multiple regression analysis to predict soil re-ceptiveness, including soil variables having individu-ally higher linear or reciprocal correlation coefficients, it was determined that the soil variables with the most predictive value were P, pH, Cu, Zn, clay content, N, CEC.
Equations listed in Table 6 showed that soil P and Zn contents accounted for more than 50% of the variability of soil receptiveness to mycorrhizal inocu-lation. Eq. (1) expressing soil receptiveness as a func-tion of soil pH, P, Zn, Cu and clay was found the most
Table 6
Multifactor reciprocal equations calculated to predict receptiveness of soil from banana fields in Martinique to mycorrhizal inoculation
r2
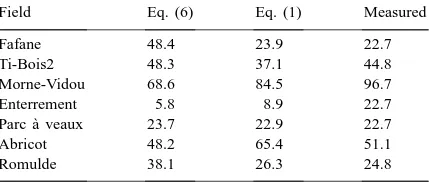
useful since it takes into account 97% of the variabil-ity. Eq. (6) which takes into account only soil P and Zn contents tended to overestimate or underestimate MSR50unit values with the exception of soil from the Ti-Bois and Parc à Veaux plots whose MSR50 unit values obtained with Eq. (6) fitted well with measured values (Table 7). Values obtained with Eq. (1) were strongly correlated (r=0.93) with measured values.
Soil variables cannot be used individually to pre-dict the receptiveness of soil from Martinique to my-corrhiza. However, it was possible to determine some equations including variables which account for vari-ability of soil receptiveness. Among these variables pH, P, Zn have already been used to predict mycor-rhizal dependency of plants (Menge et al., 1982) in Californian soils where pH and Zn were also inversely correlated with mycorrhizal dependency. Results ob-tained corroborated these previous findings since it is likely that soil variables which determine the influence of mycorrhizas on the growth response of host plants
Table 7
Comparison between measured and calculated (Eqs. (1) and (6) from Table 6) values of soil receptiveness to Glomus intraradices expressed as MSR50units (a MSR50unit is defined as the number
of propagules required to infect 50% of the plant’s population under the bioassay conditions)
Field Eq. (6) Eq. (1) Measured
Fafane 48.4 23.9 22.7
Ti-Bois2 48.3 37.1 44.8
Morne-Vidou 68.6 84.5 96.7
Enterrement 5.8 8.9 22.7
Parc `a veaux 23.7 22.9 22.7
Abricot 48.2 65.4 51.1
could also govern early stages of development of the symbiosis, such as propagule germination.
Additional experimentation with a greater num-ber of soil samples would help to ascertain the main factors influencing soil receptiveness to mycorrhizas. The equations obtained here need also to be tested with data from other soils from different agropedo-logical situations and cropping history. Most of the soils studied present a high receptiveness to mycor-rhizas. Parameters of the isotopic exchange kinetics (Table 3) which characterize the phosphorus bioavail-ability show that all the soils studied had a very high P fixing power (r1/R<0.2) and a low P concentration in soil solution (CP<0.1mg ml−1). This situation was shown to be favorable to mycorrhizal development and efficiency of mycorrhizal symbiosis (Plenchette and Fardeau, 1988; Plenchette and Morel, 1996). From a practical stand point, the results obtained al-lowed us to identify plots and soil types suitable to undertake field inoculation experiments. Data also indicate that the quantity of inoculum needed, in this test, may be reduced by a factor of four between soils of low or high receptiveness. Such a result is of great importance for developing practical applications of mycorrhizas since the main factors limiting these applications are always the cost and the large scale production of inoculum.
Many works have shown that mycorrhizal inocula-tion is of most benefit to plants when introduced to pre-viously disinfected soil. However, disinfection of soil is mainly used in nurseries and not currently practiced for large crops. So it will be necessary to develop bi-ological tests, without altering the biotic environment factors, to determine the ability of an introduced my-corrhizal fungus to establish and colonize host roots in natural soil. The problem remains to distinguish the introduced fungus from the indigenous mycorrhizal flora. Recent progress in molecular biology could be of great interest to develop molecular diagnosis which would contribute to efficient management of AM fungi in agro-ecosystems.
Acknowledgements
The author wishes to thank C. Chabrier and C. Melin from CIRAD-FLHOR in Martinique, for col-lecting and sending soil samples, and R. Perrin, INRA,
Dijon, for their critical comments and discussions. This work was supported by a grant from CEE STD3 No. 92104 and a contract with the University (UCL) of Louvain, Belgique.
References
Abbott, L.K., Robson, A.D., 1982. The role of vesicular–arbuscular mycorrhizal fungi in agriculture and the selection of fungi for inoculation. Aust. J. Agric. Res. 33, 389–408.
Bouhot, D., Joannes, H., 1979. Recherches sur l’écologie des champignons parasites dans le sol. IX. Mesure du potentiel infectieux des sols naturellement infestés par Pythium. Soil Biol. Biochem. 11, 417–429.
Dagnelie, P., 1975. Théorie et méthodes statistiques: applications agronomiques, 2nd Edition. Les presses agronomiques de Gembloux, Belgique, 472 pp.
Declerck, S., 1996. Biologie des champignons mycorhiziens à arbuscules associés au bananier en Martinique. Thèse de Doctorat de l’Université d’Angers, 175 pp.
Declerck, S., Devos, B., Delvaux, B., Plenchette, C., 1994. Growth response of micropropagated banana plants to VAM inoculation. Fruits 49, 103–109.
Declerck, S., Plenchette, C., Strullu, D.G., 1995. Mycorrhizal dependency of banana (Musa acuminata, AAA group) cultivar. Plant Soil 176, 183–187.
Delvaux, B., Perrier, X., Guyot, Ph., 1990. Diagnostic de la fertilité de systèmes culturaux intensifs en bananeraies à la Martinique. Fruits 45, 223–235.
Duvert, P., Perrin, R., Plenchette, C., 1990. Soil receptiveness to VA mycorrhizal association: concept and method. Plant Soil 124, 1–6.
Fardeau, J.C., 1993. Le phosphore assimilable des sols: sa représentation par un modèle fonctionnel à plusieurs compartiments. Agronomie 13, 317–331.
Feldmann, F., Idzak, E., 1992. Inoculum production of vesicular–arbuscular mycorrhizal fungi for use in tropical nurseries. Methods Microb. 24, 339–357.
Finney, D.J., 1971. Statistical Method in Biological Assay. Charles Griffin and Co. Ltd., London, 668 pp.
Gianinazzi-Pearson, V., Gianinazzi, S., Trouvelot, A., 1985. Evaluation of the infectivity and the effectiveness of indigenous vesicular–arbuscular fungal populations in some agricultural soils in Burgundy. Can. J. Bot. 63, 1521–1524.
Jaizme-Vega, M.C., Azcon, R., 1995. Responses of some tropical and subtropical cultures to endomycorrhizal fungi. Mycorrhiza 5, 213–217.
Menge, J.A., Jarrel, W.M., Labanauskas, C.K., Ojala, J.C., Johnson, E.L.V., Sivert, D., 1982. Predicting mycorrhizal dependency of Troyer citrange on Glomus fasciculatus in California citrus soils and nursery mixes. Soil Sci. Soc. Am. J. 46, 762–768. Plenchette, C., 1982. Recherches sur les endomycorhizes à
Plenchette, C., Fardeau, J.C., 1988. Effet du pouvoir fixateur du sol sur le prélèvement de phosphore par les racines et les mycorhizes. C. R. Acad. Sci. III 306, 201–206.
Plenchette, C., Morel, C., 1996. External phosphorus requirement of mycorrhizal and non mycorrhizal barley and soybean plants. Biol. Fertil. Soils 21, 303–308.
Plenchette, C., Fortin, J.A., Furlan, V., 1983. Growth response of several plant species to mycorrhizae in a soil of moderate
P-fertility. I. Mycorrhizal dependency under field conditions. Plant Soil 70, 199–209.
Plenchette, C., Perrin, R., Duvert, P., 1989. The concept of soil infectivity and a method as applied to endomycorrhizas. Can. J. Bot. 67, 12–115.