Production of fertile transgenic wheat plants via tissue
electroporation
Alexander P. Sorokin, Xia-Yi Ke, Dong-Fang Chen, Malcolm C. Elliott *
The Norman Borlaug Institute for Plant Science Research,De Montfort Uni6ersity,Scraptoft,Leicester,LE7 9SU, UK
Received 14 October 1999; received in revised form 31 January 2000; accepted 20 March 2000
Abstract
Electroporation has been used effectively to deliver DNA into the tissue of intact wheat immature embryos. Transformed plantlets have been recovered after electroporation using field strengths of 275 and 750 V/cm, 960-mF capacitor and 50mg/ml of linear plasmid DNA, containingbaranduidAgenes. The field strength of 750 V/cm proved to be more effective for DNA delivery (estimated by transient GUS expression) and for recovery of transformed plants (two transgenic plants were recovered with an efficiency of 0.4%). After application of a field strength of 275 V/cm there was no visual evidence of transient GUS expression, but one transgenic plant was recovered with an efficiency of 0.2%, based on the number of electroporated embryos. This indicates that the amount of DNA delivered into the cells was too low for visual identification of transient GUS expression and that GUS expression may not provide an appropriate assessment of the efficiency of DNA delivery. Southern blot hybridisation has revealed a low copy number of transgene integration with some rearrangements in integrated loci. None of the transgenic plants has shown any visual GUS expression, although we could amplify the transcript of theuidAgene in T0progeny using RT-PCR. This may
indicate that suppression ofuidAexpression occurred at the post-transcriptional level. The efficiency of tissue electroporation is still dependent on the quality of the plant material which is used but the transformation events were reproducible from one group of experiments to another. At present, this technique is dependent on a combination of factors including pretreatments of the recipient tissue, quality of tissue culture and optimisation of electroporation conditions. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Immature embryo;bar/uidAGenes; Tissue electroporation; Transformation; Wheat
www.elsevier.com/locate/plantsci
1. Introduction
Cereals are the main source of food for human beings [1]. Wheat is the most significant of the cereals in terms of the total area committed to the crop worldwide and the volume of grain produc-tion [2]. Wheat breeding programmes will benefit from new procedures for generation of varieties with improved disease resistance, enhanced stress tolerance and increased yield. Genetic manipula-tion of cereals promises to enhance resistance to insects, viruses and herbicides, and hence to make significant contributions to those programmes.
A number of DNA delivery techniques have been developed for wheat transformation. They include direct gene transfer by microprojectile bombardment [3,4], and Agrobacterium-mediated transformation [5]. Such successes have been
achieved with model genotypes, but the
methods are still not sufficiently reliable to be applied to a wide spectrum of commercial wheat varieties.
Electroporation is a DNA delivery technique, which utilises a high intensity electric pulse to create transient pores in the cell membrane and, hence, facilitates the uptake of molecules such as DNA and proteins. The simplicity and efficiency of DNA delivery into plant protoplasts by this technique [6 – 8] has encouraged its application for targeting intact cells in whole tissues of maize and
* Corresponding author. Tel.: +44-116-2577715; fax: + 44-116-2577752.
E-mail address:[email protected] (M.C. Elliott).
rice [9 – 12] leading to the successful production of stably transformed plants. In wheat, however, tis-sue electroporation has produced, to date, only
limited success. Klo¨ti et al. reported transient gene expression in intact scutellum cells [13], while Ke et al. have achieved a low frequency of wheat
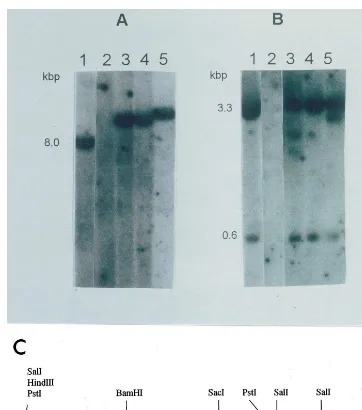
Fig. 1. Southern blot hybridisation of genomic DNA digested withHind III (A) andSal I (B). A mixture ofbaranduidAgene fragments was used as a probe. (1) pDB1 plasmid was the positive control; (2) DNA of non-transgenic plants was the negative control; (3 – 5) DNA of the transgenic plants. (C) Map of plasmid pDB1 and restriction enzymes used for digestion and Southern hybridisation analysis.
Table 1
Transient expression of theuidAgene after tissue electroporation with linear and circular DNA
State of Number of embryos with visible GUS
Field strength Mean number of GUS foci per embryo,
plasmid
(V/cm) expression (%) (9S.E.M.)
No DNA
275 0 0
No DNA 0
750 0
0
Linear 0
275
65 (32.5%) 0.9890.11
Linear 750
Circular 0 0
275
0.4190.06 Circular 49 (24.5%)
Table 2
Efficiency of wheat transformation via tissue electroporation
Number of embryos
Field Number of plants after Number of transgenic Transformation selection
strength, electroporated plants frequency (%)
V/cm
12
275 540 1 0.2
540
750 9 2 0.4
Table 3
Segregation of thebar-resistance trait in the T1generation
Line number T1seedlings used Resistant seedlings Sensitive seedlings x2, df=1a
1 29 23 6 0.29
18 7
25 0.12
2
24 7
3 31 0.10
aChi-square test for departure from expected 3:1 ratio,P\0.05 for all lines.
transformation using an electroporation device which they built themselves [14].
Tissue electroporation offers several potential advantages over microprojectile bombardment and Agrobacterium-mediated transformation, including:
high viability of cells after application of the
electric pulse (up to 50% of the treated cells survive the treatment) [15];
higher DNA delivery rate (40 – 60% of the cell
population received DNA under optimal elec-troporation conditions) [16];
applicability to single cells and cell clusters,
which are susceptible to damage by other techniques;
permitting equivalent conditions to be used for
development of both transfected and non-trans-fected cells hence increasing the efficiency of selection. This is in contrast to microprojectile bombardment where the targeted cells require time to recover from particle damage and are, therefore, at a disadvantage with respect to adjacent undamaged cells which can overgrow the potential transformants;
technical simplicity and low cost.
This paper reports stable transformation of wheat via tissue electroporation of intact imma-ture embryos.
2. Materials and methods
Plants of the spring wheat variety Scamp, were grown in the greenhouse at 18°C day and 15°C night temperatures, with photoperiod of 16 h. The 14-day-old embryos were isolated from material previously sterilised in 15% Domestos solution (Diversey Lever) for 20 min, rinsed three times with sterile water, and then subcultivated on MS medium [17] at 26°C in the dark overnight.
electric pulse of 275 or 750 V/cm (based on condi-tions optimised by others [13,19]), discharged from a 960-mF capacitor. Electroporation of immature embryos without DNA was used as a control.
After electroporation, the embryos were incu-bated on MS-induction medium [17], supplemented with 2.5 mg/l of 2,4-D, 3% of maltose and 3 g/l of
Gelrite for 3 – 4 weeks, after which they were trans-ferred to bialaphos containing medium (5 mg/l), without hormones, for selection and regeneration. GUS assays were carried out 48 h after electropo-ration using the procedures described by McCabe et al. [20].
Total genomic DNA was isolated from leaf tissue as described by Michaels et al. [21]. A total of 15
mg of DNA were digested with Hind III or Sal I restriction enzymes and electrophoresed in 1% agarose. DNA was transferred to a Hybond N+ membrane (Amersham) and radioactive Southern hybridisation was carried out according to the protocol provided by the supplier.
The pDB1 plasmid DNA was digested withBam HI/Sac I and Sal I restriction enzymes to cut out fragments of theuidAandbargenes and a mixture of these fragments was used as a probe for hybridi-sation.
RNA isolation and RT-PCR were carried out using a QIAGEN RNeasy Plant Mini Kit and an Omniscript™ Reverse Transcriptase Kit according to the manufacturer’s instructions. The following oligonucleotides were used for RT-PCR amplifica-tion of the uidA gene: 5% -CAGGAAGTGATG-GAGCATCAG-3%(position 603 – 623 onuidA) and 5%-TCGTGCACCATCAGCACGTTA-3% (position 1220 – 1240 on uidA).
Segregation ratios for the transgenes in T1 progeny were estimated on the basis of leaf resis-tance to bialaphos. The 5-day-old seedlings were briefly dipped in the bialaphos solution (0.25 mg/ ml) and resistance was scored after 4 – 5 days.
3. Results and discussion
The efficiency of DNA uptake of linear or circu-lar plasmids at 275 or 750 V/cm field strengths has been estimated, 48 h after electroporation, by analysing transient expression of the uidAgene in the cells of immature wheat embryos. A direct comparison between linear and circular DNA indi-cated that the former facilitated DNA uptake and gene expression (t=4.55, PB0.001; Table 1). There was no visible GUS activity after a pulse of 275 V/cm had been applied. This implies that a very low amount of DNA was delivered into the cells. Electroporation with linear DNA at 750 V/cm yielded the best results for DNA delivery into embryo tissue. GUS activity was evident in 65 of the 200 embryos which had been subjected to
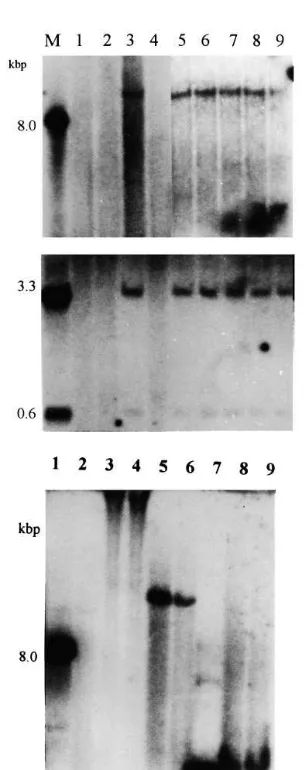
electropo-Fig. 2. Southern hybridisation analysis of T1 progeny from
line 2, digested with Hind III (A) and Sal I (B), and T0/T1
progeny digested with Hind III and Bam HI/SacI enzymes (C). A mixture ofuidAandbargene fragments was used for hybridisation in (A) and (B). M, plasmid DNA digested with the corresponding enzyme. Lane 1, DNA of a non-transgenic plant as control; lanes 2 – 9, DNA of T1 lines from a
trans-genic plant. TheuidAfragment (BamHI/SacI) was used for hybridisation in (C). Lane 1, plasmid DNA digested with Hind III as a positive control; lane 2, undigested DNA of a non-transgenic plant as a negative control; lanes 3 and 4, undigested DNA of T0and T1 progeny from line 2; lanes 5
and 6,Hind III digested DNA of T0and T1progeny; lane 7,
plasmid DNA digested withBam HI/Sac I enzymes; lanes 8 and 9, DNA of T0and T1progeny digested withBamHI/Sac
Fig. 3. Regeneration of transgenic wheat plants (cv. Scamp) after tissue electroporation: (A) Transient GUS assay 7 days after electroporation; (B) shoot formation on regeneration medium; (C) root development on bialaphos containing medium (1 mg/l); (D) resistance of transgenic plants to spraying with ammonium glufosinate (30 mg/m2); three control non-transgenic plants died
after spraying, while the transgenic plants continued their normal development.
ration, with one to six foci per embryo. The number of cells per embryo with visual GUS activity was twice as high after electroporation with linear DNA as with circular. No GUS ex-pression has been detected in the control, which was electroporated without DNA.
On the basis of the results of the transient gene expression experiments, linear plasmid DNA was used in all subsequent experiments. Transformed plantlets were recovered after electroporation at field strengths of either 275 or 750 V/cm (Table 2). Although the regeneration capacity of embryos was reduced after electroporation at 750 V/cm, the efficiency of transformation was higher than that after DNA delivery at a field strength of 275 V/cm. The overall efficiency of gene transfer was
0.3%, based on the total number of electropo-rated embryos. Three independent transgenic plants were obtained. They were resistant to bialaphos at a concentration of 5 mg/l in the
medium and survived spraying with Basta (30 – 50 mg/m2), but there was no visual evidence of GUS expression in any tissues of the T0and T1progeny. There were no morphological differences between T0 transgenic and control non-transgenic plants, although transgenic plants were partially sterile.
Fig. 4. RT-PCR analysis ofuidAgene expression. M, marker. Lane 1, positive control with pDB1 plasmid; lane 2, negative control with DNA of a non-transgenic plant; lanes 3 – 5, T0
Each transgenic plant produced from 40 to 60 seeds which were used for further molecular-bio-logical analyses.
Southern blot hybridisation in T0 plants has confirmed that there was a low copy number of transgene integration in all of the plants which were resistant to bialaphos and Basta (Fig. 1A). Bothbar(0.6-kb fragment) and uidA(3.3-kb frag-ment) genes were present in the genome (Fig. 1B). Some DNA rearrangements were observed in two of the transgenic plants (Fig. 1B, lanes 3 and 5). This may indicate that multiple copies of the transgene were present in the integration locus.
These experiments have demonstrated that DNA has been delivered into the wheat embryos via electroporation and transgenic plants have been recovered. The efficiency of this technique is still dependent on a combination of factors includ-ing the characteristics of the recipient tissue, the culture efficiency and the electroporation conditions.
It is important to note that in our experiments application of a field strength of 750 V/cm resulted in more efficient DNA delivery into the cells of immature embryos than that which occurred at a field strength of 275 V/cm. This result conflicts with the data of Klo¨ti et al. [13], who reported that application of a pulse with a field strength higher than 325 V/cm resulted in lower efficiency of DNA delivery. This might be a consequence of the different construction of the electroporation cuvette used in their experiments.
Although there was no visual indication of GUS expression after DNA delivery at 275-V/cm field strength (Table 1), a transgenic plant was recov-ered under these conditions. This is probably be-cause the quantity of DNA delivered by the electroporation technique was too low to permit visual detection of GUS activity in contrast to the biolistic method where a relatively higher amount of DNA is delivered into the cells. The uniform distribution of DNA in the electroporation buffer restricts the delivery of DNA into the cells after electroporation and, as a result, reduces the possi-bility of visual detection of the marker gene’s product.
Songstad et al. [9] showed transient expression of the GUS gene after electroporation in a buffer containing spermidine. It has been shown that spermidine can induce condensation and clustering of DNA molecules [22] and this may facilitate
uptake of more DNA by the cells, and as a result, more intensive transgene expression.
Segregation analysis of the T1 progeny has re-vealed a 3:1 segregation ratio (Table 3). This indi-cates that transgene integration has occurred into a single locus of the genome. Southern analysis of T1progeny from line 2 has confirmed the presence of both uidAandbargenes, as well as the segrega-tion ratio of 3:1 (Fig. 2). Delivery of a low copy number of the transgenes into the wheat genome has been confirmed by the results of further exper-iments in other transgenic plants (data not shown). The uptake of a relatively small amount of plasmid DNA into the cells may explain both the low copy number transgene integration and the low efficiency of transformation. The endo-and exonuclease activities of the cells determine the low concentration and short life-time of plas-mid DNA and hence limit the exposure of the cell nucleus to the plasmid DNA, and reduce the efficiency of transformation.
High levels of GUS activity were observed dur-ing the early stages of callus induction and shoot formation on the selection medium (Fig. 3A,B), but this activity was not detected in adult plants. A lower level ofuidA gene expression was already evident during the early stages of shoot formation after transfer to regeneration medium, and expres-sion was not observed at all as the shoots devel-oped. Despite the absence of visually detectable GUS activity in T0and T1plants the transcript of theuidAgene has been amplified using RT-PCR in T0 progeny (Fig. 4). This implies that expression may be suppressed at the post-transcriptional level. We recognise, however, that the histochemi-cal method of GUS assay is not very sensitive, hence, a small amount of protein could have been translated from the transcript, but not detected by the histochemical GUS assay. It must be empha-sised that all the T0 plants have survived spraying with Basta, while control plants died within 2 weeks (Fig. 3D).
The factors influencing DNA condensation near the cell membrane and facilitating more efficient DNA uptake (which might be dependent on the cell cytoskeleton) are under investigation.
Acknowledgements
We thank Dr John Hall for his very helpful comments and friendly support, Dr Alex McCor-mac for useful discussions, Dr Dirk Becker and Professor Horst Lo¨rz for the pDB1 vector con-struct and Professor John Snape for his careful, constructively critical reading of the manuscript. The project was supported by the MAFF Crop Molecular Genetics Programme.
References
[1] D. Deutscher, The current status of breeding for protein quality in corn, in: M. Friedman (Ed.), Nutritional Improvement of Food and Feed Proteins, Plenum, New York, 1978, pp. 281 – 300.
[2] M.J. Gooding, Wheat Production and Utilization: Sys-tem, Quality and the Environment, CAB International, New York, 1997.
[3] V. Vasil, A.M. Castillo, M.E. Fromm, I.K. Vasil, Herbi-cide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryo-genic callus, Bio/Technology 10 (1992) 667 – 674. [4] D. Becker, R. Brettschneider, H. Lo¨rz, Fertile transgenic
wheat from microprojectile bombardment of scutellar tissue, Plant J. 5 (1994) 299 – 307.
[5] M. Cheng, J.E. Fry, S. Pang, H. Zhou, C.M. Hironaka, D.R. Duncan, T.W. Conner, Y. Wan, Genetic transfor-mation of wheat mediated by Agrobacterium tumefa -ciens, Plant Physiol. 115 (1997) 971 – 980.
[6] S.B. Ha, F.S. Wu, T. Thorne, Transgenic turf-type tall fescue (Festuca arundinacea Schreb.) plants regenerated from protoplasts, Plant Cell Rep. 11 (1992) 601 – 604. [7] C.A. Rhodes, D.A. Pierce, I.J. Mettler, D. Mascarenhas,
J.J. Detmer, Genetically transformed maize plants from protoplasts, Science 240 (1988) 204 – 207.
[8] K. Shimamoto, R. Terada, T. Izawa, H. Fujimoto, Fer-tile transgenic plants regenerated from transformed pro-toplasts, Nature 338 (1989) 274 – 276.
[9] D.D. Songstad, F.G. Halaka, D.L. DeBoer, C.L. Arm-strong, M.A.W. Hinchee, C.G. Ford-Santino, S.M. Brown, M.E. Fromm, R.B. Horsch, Transient expres-sion of GUS and anthocyanin constructs in intact maize
immature embryos following electroporation, Plant Cell. Tiss. Organ. Culture 33 (1993) 195 – 201.
[10] S.M. Pescitelli, K. Sukhapinda, Stable transformation via electroporation into maize type-II callus and regener-ation of fertile transgenic plants, Plant Cell Rep. 14 (1995) 712 – 716.
[11] K. D’Halluin, E. Bonne, M. Bossut, M. De Beuckeleer, J. Leemans, Transgenic maize plants by tissue electropo-ration, Plant Cell 4 (1992) 1495 – 1505.
[12] A. Arencibia, E. Gentinetta, E. Cuzzoni, S. Castiglione, A. Kohli, P. Vain, M. Leech, P. Christou, F. Sala, Molecular analysis of the genome of transgenic rice (Oryza sati6aL.) plants produced via particle
bombard-ment or intact cell electroporation, Mol. Breeding 4 (1998) 99 – 109.
[13] A. Klo¨ti, V.A. Iglesias, J. Wu¨nn, P.K. Burkhardt, S.K. Datta, I. Potrykus, Gene transfer by electroporation into intact scutellum cells of wheat embryos, Plant Cell Rep. 12 (1993) 671 – 675.
[14] X-Y. Ke, D-F. Chen, Y. Huang, H.P. Shi, M.C. Elliott, B.J. Li, Commercial wheat transformed by electropora-tion of immature embryos, Biotechnol. Biotechnol. Equip. 11 (1997) 28 – 31.
[15] C.H. Lin, L. Xiao, B.H. Hou, S.B. Ha, J.A. Saunders, Optimization of electroporation conditions for expres-sion of GUS activity in electroporated protoplasts and intact plant cells, Plant Physiol. Biochem. 35 (1997) 959 – 968.
[16] J.A. Saunders, C.H. Lin, B.H. Hou, J. Cheng, N. Tsen-gwa, J.J. Lin, C.R. Smith, M.S. McIntosh, S.V. Wert, Rapid optimization of electroporation conditions for plant cells, protoplasts and pollen, Mol. Biotechnol. 3 (1995) 181 – 190.
[17] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassay with tobacco tissue culture, Phys-iol. Plant. 15 (1962) 473 – 497.
[18] Y. Tada, M. Sakamoto, T. Fujimura, Efficient gene introduction into rice by electroporation and analysis of transgenic plants — use of electroporation buffer lack-ing chloride-ions, Theor. Appl. Genet. 80 (1990) 475 – 480.
[19] H.T. Luong, P.R. Shewry, P.A. Lazzeri, Transient gene expression in cassava somatic embryos by tissue electro-poration, Plant Sci. 107 (1995) 105 – 115.
[20] D.E. McCabe, W.F. Swain, B.J. Martinell, P. Christou, Stable transformation of Soybean (Glycine max) by par-ticle acceleration, Bio/Technology 6 (1988) 923 – 926. [21] S.D. Michaels, M.C. John, R.M. Amasino, Removal of
polysaccharides from plant DNA by ethanol precipita-tion, Biotechniques 17 (1994) 274 – 276.
[22] H.G. Hansma, R. Golan, W. Hsieh, C.P. Lollo, P. Mullen-Ley, D. Knoh, DNA condensation for gene therapy as monitored by atomic force microscopy, Nu-cleic Acids Res. 26 (1998) 2481 – 2487.