Multiple cytokines regulate the expression of extracellular
superoxide dismutase in human vascular smooth muscle cells
Pontus Stra˚lin, Stefan L. Marklund *
Department of Medical Biosciences,Clinical Chemistry,Umea˚ Uni6ersity Hospital,S-901 85 Umea˚, Sweden
Received 12 February 1999; received in revised form 20 September 1999; accepted 4 October 1999
Abstract
Oxygen free radicals as well as immunological reactions have been suggested to play important roles in atherogenesis and other pathological processes of the blood vessel wall. We have previously shown that the vascular wall contains exceptionally large amounts of extracellular superoxide dismutase (EC-SOD) and that the enzyme is produced and secreted to the extracellular space by the smooth muscle cells. In this work, we studied the influence of inflammatory cytokines on vascular smooth muscle cell expression of EC-SOD, the mitochondrial manganese superoxide dismutase (Mn-SOD) and the cytosolic copper zinc superoxide dismutase (CuZn-SOD). The expression of EC-SOD was up-regulated by interferon-g(IFN-g) and interleukin 4 (IL-4), and was down-regulated by tumor necrosis factor-a (TNF-a). The ratio between the maximal stimulation and depression observed was around 20-fold. The responses were slow and developed over periods of several days. The Mn-SOD activity was strongly up-regulated by TNF-a and IL-1a and moderately by IFN-g. The CuZn-SOD activity of the smooth muscle cells was not significantly influenced by any of the cytokines. The findings suggest that large changes in the SOD isoenzymes might occur in vascular diseases, significantly altering the susceptibility of the vascular wall to adverse effects of the superoxide radical. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Superoxide dismutase; Superoxide radical; Atherosclerosis; Vascular smooth muscle cells; Free radicals
www.elsevier.com/locate/atherosclerosis
1. Introduction
The superoxide anion radical has increasingly been implicated in pathologies of the vascular wall. Many potential sources in the wall have been established including membrane-bound NADH-oxidases [1], xan-thine oxidase [2], the NADPH-oxidase of macrophages [3] and other phagocytes, and formation as by-products by nitric oxide synthase [4] and by enzymes synthesizing prostanoids [5]. Increased formation of superoxide in the vascular wall has been shown in a variety of patho-logical situations such as hypertension [6], diabetes [7] and hyperlipidemia [8]. The superoxide radical reacts rapidly with nitric oxide (NO) to form peroxynitrite
[9,10]. This impairs potentially protective actions of NO such as vasodilatation [6], platelet deactivation [11], leukocyte deactivation [12], and antioxidant effects [13]. The noxious peroxynitrite formed may in turn cause adverse actions such as inducing peroxidation of lipo-proteins [14] and nitration of protein tyrosines in vital proteins [15]. Extensive protein tyrosine nitration has been found in human atherosclerotic lesions [16]. The superoxide radical has itself been implicated in the induction of oxidation of LDL by cultured cells [17 – 19], and in the marked enhancement of LDL oxidation caused by ceruloplasmin [20]. The superoxide radical may thus play a significant role in atherogenesis [21,22]. It has recently been reported [23,24] that the human arterial wall contains exceptionally large amounts of the secreted SOD isoenzyme EC-SOD [25], while the contents of the cytosolic CuZn-SOD [26] and the mito-chondrial matrix Mn-SOD [27] are low compared to other tissues. The EC-SOD was found to be evenly distributed in the wall, including large amounts in the
* Corresponding author. Tel.: +46-90-7851239; fax: + 46-90-777296.
E-mail address: [email protected] (S.L. Marklund)
intima. The principal source of EC-SOD is the smooth muscle cells [23]. The strategic location sug-gests that EC-SOD exerts an important protective role against pathologies induced by the superoxide radical in the vascular wall.
Both inflammatory and immunological responses are integral parts of the atherosclerotic process, and also occur in other diseases of the vascular wall. Infl-ammatory cytokines have previously been found to influence the synthesis of EC-SOD by human dermal fibroblasts [28]. In the present study we examined the effects of cytokines involved in inflammatory and im-munological responses on SOD isoenzyme expression by human arterial smooth muscle cells. We found large effects on EC-SOD and Mn-SOD synthesis, sug-gesting that cytokines formed in vascular diseases may significantly alter the response of the vascular wall to superoxide radicals.
2. Methods
2.1. Cell culture and regulation experiments
The arterial smooth muscle cell (SMC) lines were initiated from pieces of human uterine artery as de-scribed [29]. Cell lines from five different persons were established (Au-1 – 5) and were used between the 5th and 8th passages. Their identity as smooth muscle cells was assessed by morphological appearance and
by their expression of smooth muscle a-actin [30]
(Boehringer Mannheim GmbH, Mannheim, Germany) as analyzed by flow cytometry. Uterine arteries
con-tained around 33 mg/g wet weight of EC-SOD, a
value similar to those previously found in coronary arteries and aorta [23]. The cells were maintained in
Waymouth MB 752/1 medium containing 15% fetal
calf serum (FCS), 105 units/l bensylpenicillin, 100 mg/l
streptomycin, 2 mmol/l glutamine and 1 mmol/l Na
pyruvate.
For synthesis regulation experiments the cells were seeded into 12-well culture plates, bottom area 3.80
cm2, and grown into near confluence. During the
ex-periments, the culture media were supplemented with either 15% FCS or 1% bovine serum albumin (BSA), as indicated. When supplemented with 1% BSA, the medium was exchanged twice to medium with 1% BSA about 20 h before the start of the experiments. The experiments were started by exchange to 0.5 ml medium with either 1% BSA or 15% FCS containing indicated concentrations of cytokines or only medium with 1% BSA or 15% FCS (controls). Every 24 h the media were collected and replaced with fresh media containing cytokines. At the end of the experiments, after 4 days, the media were collected and the wells
were washed three times with 0.15 mol/l NaCl. To
collect and homogenize the cells, 0.5 ml ice-cold 50
mmol/l Na phosphate, pH 7.4, containing 0.3 mol/l
KBr, 10 mmol/l diethylene-triamine pentaacetic acid,
0.5 mmol/l phenylmethylsulfonyl fluoride and 100
KIU/ml aprotinin (the latter three additions to inhibit
proteases) was added to the wells. After sonication in the wells, with the plate bathed in ice water, the
ho-mogenates were centrifuged (20 000×g for 10 min)
and the supernatants were collected for analysis. All
samples were kept at −80°C until assay.
2.2. Analysis of SOD isoenzymes
EC-SOD protein in culture media and cell ho-mogenates was determined with an ELISA [31]. EC-SOD is throughout presented as amount of enzyme protein in figures and tables.
In the cell homogenates, the CuZn-SOD and Mn-SOD enzymatic activities were also measured, using
the direct spectrophotometric method employing KO2
as previously described [32,33]. 3 mmol/l cyanide was
used for primary distinction between the cyanide-sen-sitive isoenzymes CuZn-SOD and EC-SOD and the nearly resistant Mn-SOD. One unit in the assay corre-sponds to 4.3 ng human CuZn-SOD [34], 8.6 ng hu-man EC-SOD [35], and 65 ng bovine Mn-SOD. For final calculation of the activity of the SOD isoen-zymes in the cell homogenates, the EC-SOD activity was calculated from the amount of protein as
deter-mined by ELISA and the specific activity (8.6 ng/
unit). The Mn-SOD activity was then taken as the cyanide resistant activity corrected for a minor in-hibitory effect of the cyanide minus remaining
mini-mal activity of CuZn-SOD and EC-SOD. The
CuZn-SOD activity was finally calculated as total SOD activity minus the calculated EC-SOD activity and the Mn-SOD activity.
2.3. Turno6er of EC-SOD in cell cultures
To assess the rate of uptake of EC-SOD by cul-tured cells, conditioned media from SMCs containing EC-SOD were incubated with human cell lines not producing EC-SOD. Thus, Waymouth medium sup-plemented with 1% BSA was incubated with confluent SMCs for 4 days, after which it was diluted with
unconditioned medium to around 3 ng/ml of
2.4. Protein and DNA analysis
For protein analysis, Coomassie Brilliant Blue G-250 was employed [36], standardized with human serum
albumin. The DNA concentration was determined with
fluorimetry as a complex with bisbenzimidazol
(Hoechst 33 258) [37] using calf thymus DNA as a standard.
Fig. 1. (Continued)
2.5. Incorporation of 35S-methionine into protein
Smooth muscle cells were cultured in 12-well culture
plates (3.8 cm2) for 4 days as described above. At the
end of the experiment, the cells were incubated in
methionine free medium with35S-methionine added for
1 h, followed by homogenization, protein precipitation
with 10% trichloroacetic acid, and analysis of 35S
in-corporation into protein, as previously described [28]. Aliquots of the cell homogenates were also analyzed for EC-SOD, protein and DNA as described above.
2.6. RNA extraction and Northern blot analysis
Smooth muscle cells of the Au-1 line were cultured in medium containing 1% BSA as described above. At the end of the experiment, RNA was isolated by TRI-zol reagent (Gibco BRL, Life Sciences Inc,
Gaithers-burg, USA). Total RNA (10 mg per lane) was
electrophoresed in formaldehyde-containing 1.2%
agarose gels, transferred to nylon filters (Hybond N, Amersham plc, Amersham, UK) and immobilized by UV-linkage. The filters were then prehybridized for 15 min and hybridized for 1 h with Quickhyb solution (Stratagene Inc, La Jolla, CA, USA) at 65°C in a hybridization oven. For EC-SOD mRNA detection, a DNA probe was used, which corresponded to nucle-otides 1018 – 1211 in the cDNA sequence (22). For glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA detection, a 1100 base pairs cDNA sequence
(Clontech, Palo Alto, CA, USA) was used.32
P-labeling was achieved by random priming (Megaprime-DNA labeling systems, Amersham, UK). To allow rehy-bridization, boiling 0.1% SDS was twice poured onto the filters. Radiolabeled filters were exposed to imaging screens for 3 – 4 days and analyzed in a Molecular Imager (BioRad, Life Technologies, Hercules, CA, USA).
2.7. Materials
The following recombinant human cytokines were obtained from Genzyme Corp, Boston, MA, USA:
IFN-g, TNF-a, IL-1a, IL-3, IL-4, granulocyte
macrophage colony stimulating factor
(monocyte-derived). Recombinant human IFN-a-2b was obtained
from Schering Corp, Kenilworth, NJ, USA; recombi-nant human IL-6 and IL-8 were obtained from R&D, Minneapolis, MN, USA; recombinant human IL-2 was obtained from Boehringer Mannheim GmbH, Ger-many, and recombinant human growth hormone was obtained from Pharmacia – Upjohn Ltd., Stockholm,
Sweden. Recombinant human TGF-b, E. coli
3. Results
3.1. Design of the experiments
Smooth muscle cell lines from human uterine arteries were cultured for 4 days with media containing active substances exchanged and collected each day. At the end, the cell layers were collected and homogenized. EC-SOD was analyzed in the culture media and all SOD isoenzymes in the cell homogenates. To compen-sate for effects of the cytokines on overall cell growth and protein synthesis, the contents of protein and DNA were measured in the cell homogenates. In a set of separate experiments the effects of the cytokines on
incorporation of 35S-methionine into protein were also
determined, and in another set of experiments the ef-fects of cytokines on the level of EC-SOD mRNA was determined. Since smooth muscle cell lines are hetero-geneous and show heterohetero-geneous responses [38], effects of the cytokines were mostly tested on four different cell lines. Experiments were carried out in media sup-plemented both with 15% FCS or 1% BSA. Up-regulat-ing effects on EC-SOD synthesis were generally less pronounced in media supplemented with 15% FCS. Down-regulating effects were equally pronounced with the two media supplements.
Experiments were generally carried out on two wells for each data point. Based on 132 sets of 2-wells-per-datapoint measurements, the following relative stan-dard deviations for the analyses were calculated: EC-SOD in media and cell homogenates 13%, CuZn-SOD 8%, Mn-CuZn-SOD 15%, protein 14% and DNA 7%. Based on the highest relative standard deviation found (15%) it was calculated that a factor between groups in 2-well experiments of 1.35 or more is statistically
signifi-cant (PB0.05).
To determine the uptake of EC-SOD by cultured cells, we added medium conditioned by smooth muscle cells to contain EC-SOD to cultures of four different human cell lines that do not produce EC-SOD. The cultures contained the following levels of cell protein:
Du 145 285mg/well, MG-251 135mg/well, Hep G2 140
mg/well and PL-3 50 mg/well and were found to
inter-nalize 33, 21, 24 and 18% of the EC-SOD present in the medium in 24 h, c.f. Section 2. It cannot be excluded that some of the tested factors to a certain extent influence the internalization of EC-SOD by the SMCs. Based on these considerations and the statistical calcu-lations, we decided only to consider differences larger than 50% in the content of this enzyme in the culture media between groups.
3.2. Effects of cytokines on EC-SOD expression
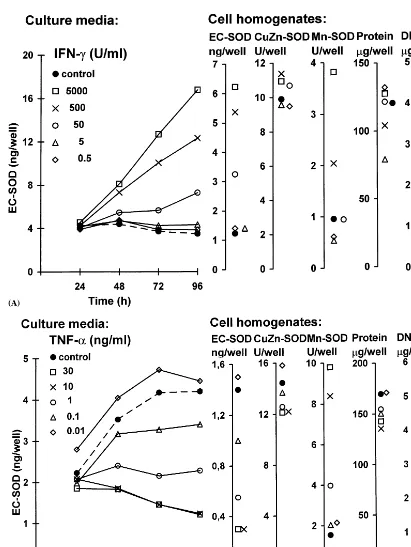
IFN-g up-regulated the EC-SOD expression in all
smooth muscle cell lines. Half maximal effect was seen
at 50 U/ml and maximal expression near 5000 U/ml,
increasing the level of secreted protein about 5-fold in the Au-2 cell line (Fig. 1A and Table 1). The response
to IFN-g was slow, and appeared to evolve during the
entire 4-day long experiment. There was also a similar increase in EC-SOD content of the cells homogenized at the end of the experiment. The increase in the level of EC-SOD synthesis was not due to stimulation of cell growth, since there was no significant difference
be-tween controls and IFN-g-treated cultures with regard
to protein and DNA content in the cell layer. 35
S-me-thionine incorporation studies suggested a minor
reduc-tion in protein synthesis by the IFN-g (Table 1). Very
similar patterns were seen in all cell lines tested. North-ern blot analysis revealed a relative increase in EC-SOD
mRNA as compared to GAPDH mRNA on IFN-g
stimulated cells, similar in magnitude to the relative increase in secreted EC-SOD protein (Fig. 2).
TNF-adown-regulated the expression of EC-SOD in
all cell lines tested (Fig. 1B, Table 1). Maximal re-sponses, depressing the level to about 30% in the Au-1
cell line, were reached near 10 ng/ml. TNF-aappeared
not to influence the general protein synthesis in our cell lines (Table 1). Northern blot analysis revealed a rela-tive decrease in EC-SOD mRNA as compared to
GAPDH mRNA on TNF-a stimulated cells similar in
magnitude to the relative decrease in secreted EC-SOD protein (Fig. 2).
IL-1awas found to influence the EC-SOD expression
to some extent, but the direction varied between the cell lines (Table 1).
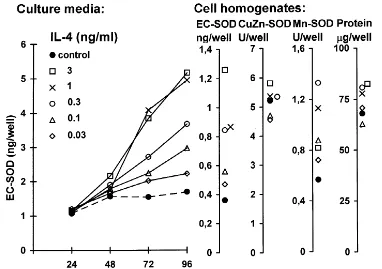
IL-4 up-regulated the EC-SOD synthesis in all cell
lines tested (Table 1, Fig. 1C). As with IFN-g, the
response was slow in all cell lines. The maximal re-sponse, reaching around a 3-fold stimulation in the
culture media, was seen at 1 ng/ml and half maximal at
P
Collection of data for cytokine treatments influencing SOD expressiona
Concentration
Medium Concentration
Cell line
Concentrations Content in cultures, change (-fold) versus controls
additive at maximum at half tested
maximum effect Culture media Cell homogenates effect
EC-SOD EC-SOD Mn-SOD CuZn-SOD DNA Protein 35S count
1.5 2.11 1.3 0.84 0.8
IFN-g(U/ml) 0.5;5;50;500;5000 Au-1 15% FCS 5000 50 0.95
1.4 1.42 2.4 0.91 0.76 0.85
0.5;5;50;500;5000 Au-1 1% BSA 5000 50 2.9 3.61 3.7 1.2 1.0 1.0 0.65
4.8 5.08 4.0 1.1 1.0 1.1
0.01;0.1;1;10;30 Au-1 15% FCS 1 0.29 0.21 5.4 0.85 0.93 0.8
TNF-a(ng/ml) 10
0.59 0.56 11 1.1 1.2 1.0
\1 0.01;0.1;1;10;30 Au-2 15% FCS 10
\1
0.005;0.05;0.5;5;50Au-2 15% FCS 5 1.0 1.39 7.6 1.1 1.1 1.6
5 1.1 1.37 4.2 1.1 1.1
1% BSA 1.3
0.005;0.05;0.5;5;50Au-1 50 1.0
0.005;0.05;0.5;5;50Au-2 1% BSA 50 5 1.8 1.93 16.3 0.89 1.0 1.1 1.2
0.77 1.0 5.4 0.89 1.1 1.2
50 Au-4 1% BSA
0.49 0.32 13.2 1.2 1.2 1.7
50 Au-5 1% BSA
1.7 1.1 n.d. n.d. 1.0
IL-4 (ng/ml) 0.03;0.1;0.3;1;3 Au-1 15% FCS 1.2
3.0 3.6 1.4 1.1 1.2 1.2
0.1 – 0.3 ng/ml (Table 1). Effects on cell growth and general protein synthesis were small and not significant.
As with IFN-g, Northern blot analysis revealed a
rela-tive increase in EC-SOD mRNA as compared to GAPDH mRNA on IL-4 stimulated cells similar in magnitude to the relative increase in secreted EC-SOD protein (Fig. 2).
3.3. Regulation of CuZn-SOD and Mn-SOD expression by cytokines
The CuZn-SOD activity of the smooth muscle cell lines was not significantly affected by any of the cytoki-nes. The Mn-SOD activity was up-regulated about
4-fold by IFN-g. The effect was half-maximal at 50
U/ml and maximal at 5000 U/ml, similar to the effect
on the EC-SOD (Table 1, Fig. 1A). TNF-a markedly
up-regulated the Mn-SOD activity in all cell lines. The
effect was half-maximal at around 0.5 ng/ml and
maxi-mal at 10 ng/ml, reaching about a 6-fold stimulation.
IL-1aalso markedly up-regulated the Mn-SOD activity
in all cell lines, though less than TNF-a. The effect was
half-maximal at 3 U/ml and near maximal at 50 U/ml
(Table 1).
3.4. Other factors tested
The Au-1 and Au-2 cell lines were also exposed to
the following factors: IFN-a (500 U/ml), IL-2 (1000
U/ml), IL-3 (30 ng/ml), IL-6 (2 and 20 ng/ml), IL-8 (60
and 800 ng/ml), TGF-b (0.005; 0.05; 0.5; 5; and 25
ng/ml), E. coli lipopolysacharide (1 mg/ml),
granulo-cyte-macrophage colony-stimulating factor (10 ng/ml),
human growth hormone (100 and 1000 ng/ml), platelet
activating factor (20 and 200 ng/ml), and complement
5A (0.1 and 1mmol/l). None of these factors influenced
the EC-SOD, the CuZn-SOD or the Mn-SOD activities of the cells.
4. Discussion
In this study we show extensive regulation of EC-SOD and Mn-EC-SOD expression in smooth muscle cells by cytokines involved in inflammatory and immunolog-ical responses. In contrast, none of the examined fac-tors influenced CuZn-SOD. Our findings on smooth muscle cells confirm observations made on other cell types, that the three SOD isoenzymes are differently
regulated, indicating different physiological roles
[28,39]. This is in accordance with the fact that the superoxide anion radical crosses membranes poorly [40], which implies that the SOD isoenzymes exert their protective actions in their distinct compartments. The changes in EC-SOD synthesis evolved over several days suggesting multi-step signaling. It is possible that the
modulations of SOD isoenzyme synthesis reflect paral-lel modulations in the phenotype of the smooth muscle cells. The response of the cell lines differed to some extent when cultured in the presence and absence of FCS. From a study of the expression of 8600 genes in fibroblasts it was found that serum induced a pattern expected in wound repair [41]. Possibly serum may induce a more proliferative ‘response to injury’ pheno-type in the smooth muscle cells, whereas in its absence the cells are modulated more towards a contractile phenotype.
The EC-SOD synthesis was up-regulated by both
IFN-gand IL-4, while TNF-asuppressed the synthesis.
Changes in the levels of EC-SOD protein were reflected in similar changes in the levels of EC-SOD mRNA, indicating a pretranslational level of regulation of the
protein synthesis. The effect of IFN-g was similar to
that previously seen in human dermal fibroblasts, which however did not respond to IL-4 [28]. IL-4 has previ-ously been shown to inhibit human arterial smooth muscle cell proliferation [42], a finding not reproduced in our study. The fibroblasts down-regulated EC-SOD
in response to both TGF-b and TNF-a, while the
smooth muscle cells only responded to TNF-a. As
previously shown with so many other cell types [43],
TNF-a and IL-1 markedly up-regulated Mn-SOD
syn-thesis in smooth muscle cells. Similar to previous
find-ings in fibroblasts [28], IFN-g also up-regulated the
synthesis. Unlike the case with EC-SOD, IL-4 did not consistently influence the Mn-SOD expression in the smooth muscle cells (Table 1).
All cytokines shown here to influence SOD isoen-zyme synthesis have been shown to occur in diseased vessel walls. Activated CD4 cells and their product
IFN-g have been detected in atherosclerotic plaques
[44]. IL-4 is primarily synthesized by T-helper type 2 cells [45] as well as by mast cells and basophils [46]. IL-4 expression has been detected in human
atheroscle-rotic aortic aneurysms [47]. TNF-a, which can be
syn-thesized by a variety of cell types in the vascular wall, has been demonstrated in atherosclerotic lesions [48] as
have both IL-1a and IL-1b [49].
All the tested cytokines show pleiotropic and often overlapping effects in vitro and in vivo. It is difficult to discern a relation between all these effects and the influences on SOD isoenzyme synthesis. Regarding
ef-fects on superoxide radical production, IFN-g[50], IL-1
and TNF-a [51] enhance secretion by monocytes and
macrophages, while IL-4 [52] inhibits the secretion. Neutrophils are primed to produce superoxide by
IFN-g [53] and by TNF-a and IFN-g combined [54].
Fur-thermore, IL-1 and TNF-a enhance superoxide
relation between the effects of the individual cytokines on superoxide production and the effects on EC-SOD secretion by smooth muscle cells. Regarding regulation
of Mn-SOD, TNF-a has been shown to increase
super-oxide formation in the mitochondria [39], while there is no information on the effects of the other cytokines on mitochondrial superoxide formation.
In conclusion, we here show that inflammatory cy-tokines occurring in the atherosclerotic vascular wall, in vitro markedly up- or down-regulate secretion of EC-SOD and expression of mitochondrial matrix Mn-EC-SOD in smooth muscle cells. The findings suggest that large increases or decreases in these enzymes might occur in vascular disease, significantly altering the susceptibility of the vascular wall to adverse effects of the superoxide radical. Indeed, the EC-SOD content in atherosclerotic lesions in human aorta was considerably reduced com-pared with non-affected tissue [56]
Acknowledgements
The skillful technical assistance of Ms E Bern, Ms K
Hjertkvist, Ms Lotta Nilsson, Ms A O8berg and Ms E
M O8 hman is gratefully acknowledged. We thank Mr
Peter Nilsson for valuable advice concerning the North-ern blot experiments. The study was supported by the Swedish Medical Science Research Council (grant no 12 566).
References
[1] Griendling KK, Minieri CA, Ollerenshaw JD, Alexander AW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994;74:1141 – 8.
[2] White CR, Darley Usmar V, Berrington WR, et al. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci USA 1996;93:8745 – 9.
[3] Chanock SJ, El Benna J, Smith RM, Babior BM. The respira-tory burst oxidase. J Biol Chem 1994;269:24519 – 22.
[4] Pritchard KA, Groszek L, Smalley DM, et al. Native low-density lipoprotein increases endothelial cell nitric oxide synthase gener-ation of superoxide anion. Circ Res 1995;77:510 – 8.
[5] Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH and NADPH. Circ Res 1986;59:612 – 9.
[6] Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA 1991;88:10045 – 8.
[7] Hattori Y, Kawasaki H, Abe K, Kanno M. Superoxide dismu-tase recovers altered endothelium-dependent relaxation in dia-betic rat aorta. Am J Physiol 1991;261:H1086 – 94.
[8] White RC, Brock TA, Chang LY, et al. Superoxide and perox-ynitrite in atherosclerosis. Proc Natl Acad Sci USA 1994;91:1044 – 8.
[9] Huie TE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun 1993;18:195 – 9.
[10] Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beck-man JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 1992;5:834 – 42.
[11] Radomski MW, Palmer RMJ, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987;8567:1057 – 8.
[12] Gaboury J, Woodman RC, Granger DN, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol 1993;265:H862 – 7.
[13] Rubbo H, Radi R, Trujillo M, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation: for-mation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 1994;269:26066 – 75.
[14] Darley-Usmar VM, Hogg N, O’Leary VJ, Wilson MT, Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low-density lipoprotein. Free Radic Res Commun 1992;17:9 – 20.
[15] Ischiropoulos H, Zhu L, Chen J, et al. Peroxynitrite-mediated tyrosine nitration catalysed by superoxide dismutase. Arch Biochem Biophys 1992;298:431 – 7.
[16] Beckman JS, Yao ZY, Anderson PG, et al. Extensive nitration of protein tyrosines in human atherosclerosis detected by im-munohistochemistry. Biol Chem Hoppe-Seyler 1994;175:81 – 8. [17] Heinecke JW, Baker L, Rosen H, Chait A. Superoxide-mediated
modification of low-density lipoprotein by arterial smooth mus-cle cells. J Clin Invest 1986;77:757 – 61.
[18] Kawamura M, Heinecke JW, Chait A. Pathophysiological con-centrations of glucose promote oxidative modification of low-density lipoprotein by a superoxide-dependent pathway. J Clin Invest 1994;94:771 – 8.
[19] Heinecke JW, Kawamura M, Suzuki L, Chait A. Oxidation of low-density lipoprotein by thiols: superoxide-dependent and -in-dependent mechanisms. J Lipid Res 1993;34:2051 – 61.
[20] Mukhopadhyay CK, Ehrenwald E, Fox PL. Ceruloplasmin en-hances smooth muscle cell- and endothelial cell-mediated low-density lipoprotein oxidation by a superoxide-dependent mechanism. J Biol Chem 1996;271:14773 – 8.
[21] Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801 – 9.
[22] Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increases its atherogenicity. New Engl J Med 1989;320:915 – 24. [23] Stra˚lin P, Karlsson K, Johansson BO, Marklund SL. The inter-stitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol 1995;15:2032 – 6.
[24] Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismu-tase in vessels and airways of humans and baboons. Free Radic Biol Med 1996;20:957 – 65.
[25] Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci USA 1982;79:7634 – 8.
[26] McCord JM, Fridovich I. Superoxide dismutase, an enzymic function for erythrocuprein. J Biol Chem 1969;244:6049 – 55. [27] Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase.
Site of synthesis and intramitochondrial localisation. J Biol Chem 1973;248:4793 – 6.
[28] Marklund SL. Regulation by cytokines of extracellular superox-ide dismutase and other superoxsuperox-ide dismutase isoenzymes in fibroblasts. J Biol Chem 1992;267:6696 – 701.
[29] Fager G, Hansson GK, Ottosson P, Dahllo¨f B, Bondjers G. Human arterial smooth muscle cells in culture: effects of platelet-derived growth factor and heparin on growth in vitro. Exp Cell Res 1988;176:319 – 35.
[31] Karlsson K, Marklund SL. Plasma clearance of human extracel-lular-superoxide dismutase C in rabbits. J Clin Invest 1988;82:762 – 6.
[32] Marklund SL. Spectrophotometric study of spontaneous dispro-portionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem 1976;251:7504 – 7. [33] Marklund SL. Direct assay of superoxide dismutase with
potas-sium superoxide. In: Greenwald RE, editor. Handbook of Meth-ods for Oxygen Radical Research. Boca Raton, FL: CRC Press, 1985:249 – 55.
[34] Andersen PM, Nilsson P, Forsgren L, Marklund SL. CuZn-su-peroxide dismutase, extracellular suCuZn-su-peroxide dismutase, and glu-tathione peroxidase in blood from individuals homozygous for Asp90Ala CuZu-superoxide dismutase mutation. J Neurochem 1998;70:715 – 20.
[35] Tibell L, Hjalmarsson K, Edlund T, Skogman G, Engstro¨m A,, Marklund SL. Expression of human extracellular-superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci USA 1987;84:6634 – 8. [36] Bradford MM. A rapid and sensitive method for the
quantita-tion of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248 – 54.
[37] Labarca C, Paigen K. A simple, rapid and sensitive DNA assay procedure. Anal Biochem 1980;102:344 – 52.
[38] Frid MG, Dempsey CE, Durmowicz AG, Stenmark KR. Smooth muscle cell heterogeneity in pulmonary and systemic vessels: importance in vascular disease. Arterioscler Thromb Vasc Biol 1997;17:1203 – 9.
[39] Wong GHW, Elwell JH, Oberley LW, Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cyto-toxicity of tumor necrosis factor. Cell 1989;58:923 – 31. [40] Winterbourn CC, Stern A. Human red cells scavenge
extracellu-lar hydrogen peroxide and inhibit formation of hypochlorous acid and hydroxyl radical. J Clin Invest 1987;80:1486 – 91. [41] Iyer VR, Eisen MB, Ross DT, et al. The transcriptional program
in the response of human fibroblasts to serum. Science 1999;283:83 – 7.
[42] Vadiveloo PK, Stanton HR, Cochran FW, Hamilton JA. Inter-leukin-4 inhibits human smooth muscle cell proliferation. Artery 1994;21:161 – 81.
[43] Harris CA, Derbin KS, Hunte McDonough B, et al. Manganese superoxide dismutase is induced by IFN-gamma in multiple cell types. Synergistic induction by IFN-gamma and tumor necrosis factor or IL-1. J Immunol 1991;147:149 – 54.
[44] Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 1989;135:169 – 75.
[45] Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145 – 73.
[46] Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in responce to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature 1989;339:64 – 7.
[47] Ramshaw AL, Roskell DE, Parums DV. Cytokine gene expres-sion in aortic adventitial inflammation associated with advanced atherosclerosis. J Clin Pathol 1994;47:721 – 7.
[48] Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, For-rester JS. Detection and localisation of tumor necrosis factor in human atheroma. Am J Cardiol 1990;65:297 – 302.
[49] Moyer CV, Sajuthi D, Tulli H, Williams JK. Synthesis of IL-1a
and IL-1b by arterial cells in atherosclerosis. Am J Pathol 1991;138:951 – 60.
[50] Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 1983;158:670 – 89.
[51] Warren JS, Kunkel SL, Cunningham TW, Johnson KJ, Ward PA. Macrophage-derived cytokines amplify immune complex-triggered O2− responses by rat alveolar macrophages. Am J Pathol 1988;130:489 – 95.
[52] Zhou Y, Lin G, Murtaugh MP. Interleukin-4 suppresses the expression of macrophage NADPH oxidase heavy chain subunit (gp91-phox). Biochim Biophys Acta 1995;1265:40 – 8.
[53] Tennenberg SD, Fey DE, Lieser MJ. Oxidative priming of neutrophils by Interferon-g. J Leukoc Biol 1993;53:301 – 8. [54] Berkow RL, Wang D, Larrick JW, Dodson RW, Howard TH.
Enhancement of neutrophil superoxide production by preincuba-tion with recombinant human tumor necrosis factor. J Immunol 1987;139:3783 – 91.
[55] Matsubara T, Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol 1986;137:3295 – 8.
[56] Luoma JS, Stralin P, Marklund SL, Hiltunen TP, Sarkioja T, Yla-Herttuala S. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arte-rioscler Thromb Vasc Biol 1998;18:157 – 67.