Calcium and lipoprotein lipase synergistically enhance the binding
and uptake of native and oxidized LDL in mouse peritoneal
macrophages
Xiaosong Wang, Joachim Greilberger, Gu¨nther Ju¨rgens *
Institute of Medical Biochemistry,Karl-Franzens Uni6ersita¨t Graz,A-8010Graz,Austria
Received 7 June 1999; received in revised form 9 September 1999; accepted 29 September 1999
Abstract
The influence of Ca2+and Mg2+, together with lipoprotein lipase (LPL), on the binding and uptake of Eu3+-labeled native and oxidized low density lipoprotein (LDL) to mouse peritoneal macrophages (MPM), and on the deposition of esterified cholesterol in these macrophages, were studied. We found that both LPL and Ca2+ (but not Mg2+) increased the binding and uptake of native and mildly or moderately oxidized LDL, and the subsequent deposition of cholesterol esters in MPM. When added together, LPL and Ca2+synergistically increased the binding and uptake of native and oxidized LDL, and the deposition of esterified cholesterol derived from native and mildly or moderately oxidized LDL, in MPM. Since both calcium and LPL are found in the atherosclerotic lesions, our results suggest that Ca2+and LPL may synergistically promote foam cell formation and atherogenesis. Furthermore, future research in the metabolism of lipoproteins should take into account the calcium levels in the experimental conditions. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Low density lipoprotein; Lipoprotein lipase; Calcium; Macrophages; Oxidation
www.elsevier.com/locate/atherosclerosis
1. Introduction
Lipoprotein lipase (LPL) is the major enzyme re-sponsible for the hydrolysis of triglycerides in circulat-ing chylomicrons and very low density lipoproteins (VLDL). Aside from this enzymatic function, LPL was found to act as a bridge linking apoB of low density lipoprotein (LDL) with cell surface heparan sulfate proteoglycans (HSPG) and the LDL receptor related protein (LRP) [1 – 3]. In this way, LPL could enhance the binding and uptake of LDL by macrophages and smooth muscle cells.
It was shown that oxidation of LDL modified the structural and functional properties of this lipoprotein
[4,5]. Binding of oxidized LDL followed by the uptake in macrophages in an unregulated fashion would lead to the formation of foam cells, the hallmark of the formation of atherosclerotic lesions [6]. Recently, it was shown that exogenously added bovine LPL [7] and LPL produced by macrophages [8] also stimulated the bind-ing and uptake of moderately oxidized LDL by macrophages.
The deposition of calcium was found in human atherosclerotic lesions, and at the end stage of atherosclerosis some areas of the intima are calcified. Calcium was found to increase the binding of native and oxidized LDL to extracellular matrix [9] and the binding of VLDL to subendothelial cell matrix [10]. Therefore, it was of pathophysiological importance to
investigate whether Ca2+ could influence the foam cell
formation, and what is its effect on LPL-mediated binding and uptake of native and oxidized LDL in macrophages.
* Corresponding author. Tel.: +43-316-3804195, fax: + 43-316-3809615.
E-mail address:[email protected] (G. Ju¨rgens)
2. Materials and methods
2.1. Lipoprotein preparation
LDL and lipoprotein-deficient serum (LPDS) were isolated from the plasma of normolipidemic human donors with serum lipoprotein(a) levels lower than 1 mg/100 ml, by differential ultracentrifugation at density
ranges between 1.020 and 1.050 g/ml and \1.235,
respectively, as previously described [9]. Protein concen-tration of LDL was measured by the method of Lowry et al. [11] using bovine serum albumin (BSA) as stan-dard. LDL concentration in this study is referred to as its protein content.
2.2. Labeling of LDL with Europium3+ ( Eu3+)
Eu3+-labeling of LDL was performed as described
[9]. Briefly, 2 mg LDL in 50 mM NaHCO3 pH 8.5,
containing 20 mM Trolox
(2-carboxy-2,5,7,8-te-tramethyl-6-chromanol, a water-soluble vitamin E derivative from Hofmann LaRoche), was incubated with 0.2 mg Eu3+-chelate of N1-(p
-isothiocyanatoben-zyl)-diethylentriamine-N1, N2, N3, N3-tetraacetic acid
(DELFIA Eu-labeling kit; Wallac Oy) at 25°C in the dark for 12 h. Sephadex G-25 chromatography (Phar-macia Biotech) was used for the separation of the labeled protein from free europium in 50 mM Tris –
HCl pH 7.8, containing 0.05% NaN3 and 20 mM
Trolox. The labeling yield of Eu3+-LDL was between 4
and 22 Eu3+
/protein (mol/mol).
2.3. Cu2+-mediated oxidation of LDL and Eu3+-LDL
Prior to oxidation, LDL and Eu3+-LDL were
dia-lyzed against 10 mM PBS pH 7.4. Cu2+-mediated
oxidation of LDL (500 mg/ml) was performed at 37°C
with 30mM CuCl2. At intervals between 0 and 24 h the
reaction was terminated by adding a stop solution to achieve a final EDTA concentration of 0.27 mM. The samples were saturated with nitrogen and stored at 4°C. The degree of the oxidative modification of LDL was estimated as the relative electrophoretic mobility (REM) to the respective labeled and non-labeled native LDL on 1% agarose gels at pH 8.05 using the Lipi-dophor-system (Immuno AG). In our experiments,
LDL and Eu3+-LDL which were oxidized for 1 and
3 – 4 h revealed REM values around 1.3 and 2.4, respec-tively. They were designated mildly (1 h) and moder-ately (3 or 4 h) oxidized LDL, respectively. LDL which was oxidized for 8 h (REM value 3.0 – 3.3) and 24 h (REM value 3.5 – 3.8) were designated strongly and extensively oxidized LDL, respectively. In some sam-ples lipidperoxides (LPO) were estimated by a spec-trophotometric assay with CHOD-iodide color reagent (Merck) at 365 nm, as developed in this laboratory [12].
2.4. Cell cultures
Resident peritoneal macrophages from Balb/c mice
(MPM) were elicited by intraperitoneal injection of 2 ml of 3% thioglycollate medium (Gibco BRL) 3 days before harvesting. Primary cultures were prepared at a density of 1.5×105/well in 96-well plates (Costar,
Aus-tria), in RPMI-1640 medium (Gibco BRL) containing
10% fetal calf serum (FCS) (Gibco BRL), 100 units/ml
of penicillin and 100mg/ml of streptomycin. Cells were
maintained in a humidified incubator with 5% CO2 at
37°C. Three hours after the plating, non-adherent cells were washed away with 10 mM PBS pH 7.4. The cells were cultured in the above medium overnight before use. Cell viability was greater than 98% as assessed by trypan blue exclusion.
2.5. Purification and biotinylation of LPL
LPL was purified from fresh unpasteurized milk us-ing Heparin-Sepharose (Pharmacia) chromatography as described by Sexana et al. [13], followed by affinity chromatography on a HiTrap Heparin column (Phar-macia) eluted with 10 mM phosphate buffer pH 6.8, containing 0.75 – 2 M NaCl. Purified LPL showed a
major protein band of 55 kDa, and a minor band of
40 kDa in some fractions, when analyzed by
SDS-PAGE and stained with Coomassie Blue. Using an
anti-LPL monoclonal antibody the 55 kDa band
was visible in Western blot analysis, while the smaller
40 kDa band was not shown. This 40 kDa band
corresponds to the digested LPL fragment suggested by Hendriks et al. [14]. Purified LPL was biotinylated
using D-biotin-N-hydroxysuccinimide ester (Boehringer
Manheim, Germany), as described [15]. The
biotin-la-beled LPL was 55 kDa in size as checked with
SDS-PAGE, and enzymatically active. When biotin-la-beled LPL was transblotted to nitrocellulose after SDS-PAGE, and then detected with peroxidase-labeled
streptavidin, a major 55 kDa band was found.
2.6. Cellular binding and association studies
Cell binding and association studies were carried out
using Eu3+-labeled native and oxidized LDL [16]. This
non-radioactive time-resolved fluorometric assay can avoid the potential lipid peroxidation of LDL brought about by labeling lipoproteins with the widely used radioactive iodine, which is of special importance when one wants to compare the metabolism of native and mildly oxidized LDL [16], as in this study.
Cell binding and association studies were carried out in RPMI-1640 medium (Gibco) containing 10% LPDS, 25 mM HEPES, pH 7.4, by incubating the cells with
RPMI-1640 medium containing Eu3+
lipoproteins bound to the cell membrane) or 37°C (cell association, in which lipoproteins are associated with both cell membrane and intracellular compartments), in the absence or presence of exogenous LPL. Commer-cially purchased 1640 medium (normal
RPMI-1640 medium) contains 0.42 mM Ca2+ and 0.4 mM
Mg2+. CaCl
2· 2H2O and MgCl2· 6H2O were added to
this medium to achieve the desired final concentrations of Ca2+ and Mg2+. At the concentrations of Ca2+and
Mg2+ used, there was no precipitation in the medium
as checked by absorption at 450 nm. In some experi-ments, 1.34 mM EDTA was added to the medium to
chelate Ca2+ and Mg2+ in the medium. After washing,
the cells were dissolved with Triton X-100 (0.05%). Fluorescence in the cell lysate was measured in tripli-cates in enhancement solution with a VICTOR™ Mul-tilabel Fluorescence Counter (Wallac Oy). Cell protein content was measured in duplicate with the method of Lowry et al. [11] using BSA as standard. Specific cellu-lar binding and association were calculated by subtract-ing the non-specific bindsubtract-ing or association in the presence of an excess of unlabeled lipoproteins from those in the absence of unlabeled lipoproteins.
Binding of biotin-labeled LPL to MPM was carried out in RPMI-1640 medium containing different concen-trations of Ca2+ or Mg2+, for 2 h at 37°C. As control, biotin-labeled LPL was omitted from the medium.
Af-ter washing with PBS, Eu3+-labeled streptavidin in
RPMI-1640 medium was added to the cells and incu-bated for 30 min at 37°C. Cells were then washed and the fluorescence was measured as in cell binding and association studies. Specific binding was calculated by subtracting the fluorescence counts in the absence of labeled LPL from those in the presence of biotin-labeled LPL.
2.7. Measurement of cellular cholesterol
MPM were cultivated in 24-well plates (Costar) in RPMI-1640 medium containing 10% FCS. Twenty four hours before the experiment, RPMI-1640 medium sup-plemented with 10% human LPDS instead of FCS was added to the cells. At the start of the experiment, the cells were washed three times with RPMI-1640 medium.
RPMI-1640 medium containing 1% BSA, 100mg/ml of
native and oxidized LDL, various concentrations of
Ca2+ or Mg2+, in the presence or absence of 10
mg/ml
LPL, was then added to the cells and incubated for 24
h in a humidified incubator with 5% CO2 at 37°C.
Control incubations were performed in RPMI-1640 medium containing 1% BSA without other additions. At the end of the incubation, the cells were washed three times with PBS containing 0.1% BSA and two times with PBS. Cellular total and free cholesterol content were measured [17].
2.8. Binding of Eu3+
-labeled nati6e and oxidized LDL
to LPL
An aliquot of 1.5mg of LPL from bovine milk in 100
ml PBS was coated to each well of the microtitration
plates (Nunc) at 4°C for 18 h. After three washes with
PBS, each well was blocked with 200ml PBS containing
3% BSA for 1 h at room temperature. The plates were washed three times with PBS, and then RPMI-1640 medium or Tris – HCl buffer containing 50 mM NaCl,
1% BSA, Eu3+-labeled native and oxidized LDL, and
different concentrations of Ca2+ or Mg2+ was added
to each well. After incubation for 1 h at 37°C, the wells were washed with PBS, and the fluorescence of bound
Eu3+ was measured in the presence of enhancement
solution (200 ml/well). To measure the non-specific
binding, bovine serum album instead of bovine milk LPL was coated to the plates, and the binding was measured the same way. The non-specific binding of
Eu3+-labeled lipoproteins to BSA as compared to LPL
was below 5%, and was subtracted from the amount of binding to LPL.
3. Results
3.1. LPL-mediated binding and cell association of Eu3+
-labeled nati6e and oxidized LDL to MPM in the
presence of different concentrations of Ca2+ and Mg2+
The effect of LPL and increased concentrations of
Ca2+ in the medium on the binding of native and
differently oxidized LDL to MPM was studied (Table 1). In the original RPMI-1640 medium (containing 0.4
mM Ca2+), the binding of Eu3+-LDL to MPM
in-creased with the degree of oxidative modification. The percentage of the enhanced binding caused by addition
of LPL or 1.4 mM Ca2+ to this medium decreased with
the degree of oxidative modification of Eu3+-labeled
LDL. The combination of LPL supplement and the
addition of 1.4 mM Ca2+ in RPMI-1640 medium led
to a binding higher than the sum of the separate effects
of LPL and increased Ca2+ concentration for both
native and oxidized LDL, i.e. LPL and Ca2+
synergis-tically increased the binding of native and oxidized LDL to MPM (Table 1). It is noteworthy that LPL and
the increased Ca2+ concentrations, although each
sepa-rately had little effect on the binding of extensively oxidized LDL to MPM, when combined increased the binding of this oxidized LDL by 78.3%. When the increased binding due to the separate addition of LPL
and Ca2+ to the medium was subtracted from the
binding achieved by the simultaneous addition of LPL
and 1.4 mM Ca2+
Table 1
Effect of LPL and Ca2+concentrations on the binding of native and differently oxidized LDL to MPMa
RPMI+LPL RPMI+1.4 mM Ca2+ RPMI+LPL+1.4 mM Ca2+
RPMI (control) Synergistic effect
8.1390.2* (546.5%) 6.3890.36* (407.6%) 15.8591.22* (1159.5%)
LDL 1.2690.20 2.58 (205.4%)
11.1190.46* (117.9%) 10.8392.33* (112.3%)
5.1090.34 35.6090.15* (597.9%)
oxLDL (1 h) 18.73 (367.7%)
17.3690.39* (102.2%) 12.6291.30* (47.0%)
oxLDL (4 h) 8.5890.31 57.3392.11* (568.0%) 35.94 (418.8%)
55.73916.61* (30.8%) 57.0390.68* (33.9%)
42.5990.32 141.8292.70* (233.0%)
oxLDL (8 h) 71.66 (168.3%)
433.0299.51 (1.8%) 433.1092.85 (1.8%) 758.34917.66* (78.3%) 317.57 (74.7%) oxLDL (24 h) 425.35910.54
aBinding of Eu3+-labeled native and differently oxidized LDL to MPM was measured in the original RPMI-1640 medium containing 0.4 mM Ca2+(set as control), or in this medium with the addition of LPL (10
mg/ml), or with the addition of 1.4 mM Ca2+, or with the addition of both
LPL (10mg/ml) and 1.4 mM Ca2+. After 4 h of incubation at 4°C and washing the cells, the binding was measured (ng LDL protein/mg cell
protein). Synergistic effects were calculated by subtracting the separately increased binding in the presence of LPL and the binding in the presence of additional 1.4 mM Ca2+concentration from that in the presence of both LPL and additional 1.4 mM Ca2+. Values represent mean9SD of three experiments. Values in parentheses represent the percent increase compared with the controls, or the percent synergistic effect (the last column) of LPL and the increased Ca2+concentration.
*PB0.01 as compared with controls.
oxidized LDL. In contrast, the addition of 1.4 mM
Mg2+ in the medium neither influenced the binding of
native and differently oxidized LDL to MPM, nor affected the LPL-mediated binding of native and oxi-dized LDL to MPM (not shown). The results obtained from cell association studies were similar (not shown).
3.2. Binding of biotin-labeled LPL to MPM in the presence of different concentrations of Ca2+
and Mg2+
In order to evaluate the influence of Ca2+ and Mg2+
on the direct binding of LPL to MPM, biotin-labeled LPL was used. As shown in Fig. 1, there was no significant difference in the binding of biotin-labeled LPL to MPM, when either EDTA was used to chelate divalent cations in RPMI-1640 medium, or an
addi-tional 1.4 mM Ca2+ was added to this medium (P\
0.2). Moreover, the addition of 1.4 mM Mg2+ to the
medium decreased the binding of biotin-LPL to MPM
by 43% (PB0.05).
3.3. Effect of LPL on the accumulation in MPM of esterified cholesterol deri6ed from nati6e and oxidized
LDL in the presence of different concentrations of Ca2+
The effect of LPL on the accumulation of esterified cholesterol in MPM in the presence of different concen-trations of Ca2+was studied. Free and total cholesterol in MPM were measured after incubating the cells for 24
hours in RPMI-1640 medium containing 100 mg/ml of
native and differently oxidized LDL and different
con-centrations of Ca2+ in the absence or presence of LPL.
Esterified cholesterol was calculated by subtracting the free cholesterol from total cholesterol. As shown in Table 2, the addition of LPL or 1.4 mM calcium to RPMI-1640 medium both increased the accumulation
of esterified cholesterol derived from native LDL and mildly or moderately oxidized LDL in MPM, while both exerted only small effects in the case of extensively oxidized LDL. The combination of LPL and the in-creased calcium concentration in the medium led to a cholesterol deposition that was higher than the sum of the separate effects of LPL supplementation and
in-creased Ca2+ concentration. Thus, LPL and Ca2+
Fig. 1. Effect of the Ca2+and Mg2+concentrations on the binding of biotin-labeled LPL to MPM. Binding of biotin-labeled LPL to MPM was performed in the original RPMI-1640 medium containing 0.4 mM Ca2+and 0.4 mM Mg2+(control), or in this medium with the addition of 1.34 mM EDTA, or with additional 1.4 mM Ca2+or 2.6 mM Mg2+ by adding CaCl
2· 2H2O and MgCl2· 6H2O. After
Table 2
Deposition of cholesterol esters derived from native and differently oxidized LDL in MPM: effect of LPL and Ca2+concentrationsa
RPMI+LPL RPMI+1.4 mM Ca2+ RPMI+LPL+1.4 mM Ca2+ Synergistic effect RPMI (control)
2.3390.11* (356.9%) 2.2490.09* (339.2%)
LDL 0.5190.0.02 4.6290.25* (805.9%) 0.56 (109.8%)
oxLDL (1 h) 0.7290.03 1.8590.12* (156.9%) 5.9390.53* (723.6%) 9.8290.52* (597.9%) 2.76 (383.3%) 0.9690.05** (54.8%) 5.9590.62* (859.7%)
oxLDL (3 h) 0.6290.01 8.9890.93* (1348.5%) 2.69 (433.9%)
19.9890.36 (4.3%) 20.7793.6 (8.5%) 19.4192.52 (1.4%) −2.19 (−11.4%) 19.1590.52
oxLDL (24 h)
aTotal and free cholesterol were measured after incubating MPM with native and differently oxidized LDL (100
mg/ml) for 24 h in the original
RPMI-1640 medium containing 0.4 mM Ca2+(set as control), or in this medium with the addition of LPL (10
mg/ml), or with the addition of
1.4 mM Ca2+, or with the addition of both LPL (10
mg/ml) and 1.4 mM Ca2+. Cholesterol ester was calculated by subtracting the value of free
cholesterol from total cholesterol (mg cholesterol ester/mg cell protein). Synergistic effects were calculated by subtracting the separately increased
cholesterol ester deposition in the presence of LPL and that in the presence of additional 1.4 mM Ca2+concentration from that in the presence of both LPL and additional 1.4 mM Ca2+. Values represent mean9SD of three experiments. Values in parentheses represent the percent increase compared with the controls, or the percent synergistic effect (the last column) of LPL and the increased Ca2+concentration.
*PB0.01;
**PB0.05, as compared with the controls).
Table 3
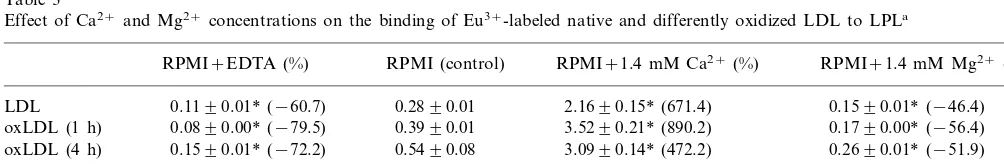
Effect of Ca2+and Mg2+concentrations on the binding of Eu3+-labeled native and differently oxidized LDL to LPLa
RPMI (control) RPMI+1.4 mM Ca2+(%)
RPMI+EDTA (%) RPMI+1.4 mM Mg2+(%)
0.2890.01 2.1690.15* (671.4)
0.1190.01* (−60.7) 0.1590.01* (−46.4)
LDL
oxLDL (1 h) 0.0890.00* (−79.5) 0.3990.01 3.5290.21* (890.2) 0.1790.00* (−56.4) 0.5490.08 3.0990.14* (472.2)
0.1590.01* (−72.2) 0.2690.01* (−51.9)
oxLDL (4 h)
1.8490.16 5.1290.30* (178.2)
oxLDL (8 h) 1.0690.02* (−42.4) 1.1690.01* (−36.9)
14.3090.65 18.4190.93** (28.8)
11.6190.12** (−18.8) 12.0490.08 (−15.8)
oxLDL (24 h)
aMicrotitration plates were coated with LPL (1.5 mg/well) at 4°C overnight. After blocking with BSA, Eu3+-labeled native and differently oxidized LDL in the original RPMI-1640 medium containing 0.4 mM Ca2+and 0.4 mM Mg2+(set as control), or in this medium containing 1.34 mM EDTA, or with the addition of 1.4 mM Ca2+, or with the addition of 1.4 mM Mg2+, were incubated in the wells at 37°C for 1 h. After washing, the fluorescence was measured. Values are expressed as ng LDL protein/well and represent mean9SD of three experiments. Values in parentheses represent the percent increase or decrease (−) compared with the respective controls.
**PB0.05,
*PB0.01 as compared with the controls.
synergistically increased the accumulation in MPM of cholesterol esters derived from native, mildly or moder-ately oxidized LDL (Table 2). This synergistic effect was more evident for mildly and moderately oxidized LDL, which is in agreement with the results from cell binding studies (Table 1), while it was not observed when extensively oxidized LDL (24 h oxidized LDL) was used.
3.4. Effect of Ca2+
on the binding of Eu3+ -labeled nati6e and oxidized LDL to LPL
Microtitration plates were coated with LPL, and the
binding of Eu3+-labeled native and oxidized LDL to
LPL in the presence of different concentrations of
Ca2+ and Mg2+ was studied (Table 3). The binding of
Eu3+-labeled native and oxidized LDL to LPL
de-creased when EDTA was added to the medium to chelate the divalent cations, and was enhanced with the
increase of the Ca2+ concentration. The percent
in-creased binding was more evident for native LDL and mildly or moderately oxidized LDL than heavily
oxi-dized LDL. In contrast, the addition of Mg2+ could
not increase, but decreased the binding of native and oxidized LDL to LPL. The results were similar when Tris – HCl buffer in stead of RPMI-1640 medium was used (not shown).
4. Discussion
Lipids deposited intra- and extracellularly in
atherosclerotic lesions are derived largely from plasma LDL. This lipoprotein is extremely susceptible to oxida-tive damage, and its oxidation results in recognition by various established or putative scavenger receptors on macrophages [6]. Apart from its enzymatic function, LPL was found to mediate the binding and uptake of
native LDL and moderately oxidized LDL in
macrophages [2,3,7,8,18], a process that may contribute to foam cell formation in atherosclerotic lesions. In the
present study, we found that LPL and Ca2+ separately
significance of our finding is evident, since atheroscle-rotic lesions become calcified with their development, and lesion areas contain much more calcium than non-lesion areas [19,20]. In addition, macrophages and smooth muscle cells in atherosclerotic lesions were found to produce LPL in situ [21,22]. So it is possible that LPL and calcium may cooperatively influence the process of atherosclerosis.
Ca2+ is required for the binding of LDL to the LDL
receptor [23]. Since both apolipoprotein B [24] and the
LDL receptor [25] are Ca2+ binding proteins, increased
Ca2+ concentrations may facilitate the binding of LDL
to LDL receptors on macrophages. Although the bind-ing of oxidized LDL to various scavenger receptors on
macrophages has not been found to be Ca2+
-depen-dent, its binding to some cell surface components may
require, or may be facilitated by, Ca2+. Heparan
sul-fate is able to compete with the binding of native and oxidized LDL to macrophages (unpublished observa-tions), and cell-surface HSPG, particularly syndecans and perlecan, are able to mediate the internalization of lipoproteins directly [26]. So it is possible that HSPG may be involved in the enhanced binding of native and
oxidized LDL to macrophages. With respect that Ca2+
could bind both HSPG [27] and apolipoprotein B [24] with high affinity, this divalent cation may bridge lipo-proteins to cell surface HSPG, thus facilitate the
inter-nalization of lipoproteins by macrophages.
Phospholipids in the cell membrane may also be linked
by Ca2+ to lipoproteins, since Ca2+ was found to
bridge certain proteins to phospholipids in the cell membrane [28].
LPL was found to bridge the binding of native and mildly or moderately oxidized LDL, but not heavily oxidized LDL, to HSPG on macrophages. We found that LPL alone could not influence the binding and cell association of heavily oxidized LDL (oxLDL-24 h) in macrophages. This was not due to the weakened bind-ing of heavily oxidized LDL to LPL compared with less oxidized LDL; on the contrary, heavily oxidized LDL bound much more to LPL than less oxidized LDL did, as shown in Table 3. We propose that the reasons for the discrepancy are: (1) When LDL is oxidized, its negative charge increases. Thus, there might be a repul-sion between the negatively charged oxidized LDL and the negatively charged heparan sulfate proteoglycans on macrophages when they bind LPL at the same time, or they compete the same or closely located binding site(s) on LPL; (2) The direct binding of heavily oxi-dized LDL to macrophages is much stronger than that of less oxidized LDL or native LDL (unpublished data). Large amount of heavily oxidized LDL could bind to the scavenger receptors on macrophages, and in addition, this kind of oxidized LDL might have a higher affinity for scavenger receptors than for LPL. So the LPL-mediated effect of heavily oxidized LDL was
overwhelmed by the latter’s binding to scavenger recep-tors on macrophages. We found that LPL could also enhance the binding of more extensively oxidized LDL
to macrophages in an environment where the Ca2+
concentration is elevated, such as in atherosclerotic lesions.
The synergistic effect of LPL and Ca2+ in enhancing
the binding and uptake of native and oxidized LDL in macrophages may be explained in two ways. First, the
structural stability of LPL is dependent on Ca2+ [29].
Second, Ca2+ is able to increase the binding of native
and oxidized LDL to LPL, thus enhancing the bridging effect of LPL, as shown in this study. This possibility is
further supported by the fact that Mg2+, another
diva-lent cation that could not increase the binding of native and oxidized LDL to LPL, has no synergistic effect with LPL in enhancing the binding and uptake of native and oxidized LDL in macrophages. The syner-gistic effect seems less likely to be the enhanced binding
of LPL to macrophages by Ca2+, when LPL bridges
the binding of lipoproteins to macrophages, since the
increased Ca2+ concentration was not able to
signifi-cantly increase the binding of LPL to macrophages in our study.
Expression of LPL in tissues other than the arterial wall may be beneficial, since LPL markedly improves lipid profile and helps to clear atherogenic lipoproteins such as LDL, VLDL and VLDL remnants [30]. Our
results suggest that Ca2+ may expedite this clearance
process, thus being anti-atherogenic. On the other hand, LPL, which can be produced by macrophages and smooth muscle cells in atherosclerotic lesions [21,22], was found to stimulate the binding and uptake
of mildly and moderately oxidized LDL in
macrophages. Thus, LPL may promote foam cell for-mation locally in the lesion area and act there as a
pro-atherogenic factor. Our findings suggest that Ca2+
in the lesions may be an additional factor to enhance the LPL-mediated foam cell formation.
Our results also suggest that calcium concentrations in the experimental conditions (for example, different cell culture media contain different concentrations of calcium) should be taken into consideration in future research, when studying the influence of LPL on the metabolism of lipoproteins by cells. The calcium con-centration in the experimental conditions should be similar to that in the local environment where the physiological or pathological lipoprotein metabolism being studied is taking place.
Acknowledgements
This work was supported by the Austrian Science Fund, special research center ‘Biomembranes’, project
F 00710, and by the Jubila¨umsfonds der
References
[1] Beisiegel U, Weber W, Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc Natl Acad Sci USA 1991;88:8342 – 6.
[2] Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low den-sity lipoproteins by fibroblasts and macrophages. J Clin Invest 1992;90:1504 – 12.
[3] Mulder M, Lombardi P, Jansen H, van Berkel TJC, Frant RR, Havekes LM. Heparan sulphate proteoglycans are involved in the lipoprotein lipase-mediated enhancement of the cellular bind-ing of very low density and low density lipoproteins. Biochem Biophys Res Commun 1992;185:582 – 7.
[4] Ju¨rgens G, Hoff HF, Chisolm GM, Esterbauer H. Modification of human serum low density lipoprotein by oxidation-characteri-zation and pathophysiological implications. Chem Phys Lipids 1987;45:315 – 36.
[5] Esterbauer H, Gebicki J, Puhl H, Ju¨rgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Rad Biol Med 1992;13:341 – 90.
[6] Steinberg D. Low density lipoprotein oxidation and its pathobio-logical significance. J Biol Chem 1997;272:20963 – 6.
[7] Hendriks WL, van der Boom H, van Vark LC, Havekes LM. Lipoprotein lipase stimulates the binding and uptake of moder-ately oxidized low-density lipoprotein by J774 macrophages. Biochem J 1996;314:563 – 8.
[8] Wang X, Greilberger J, Levak-Frank S, Zimmermann R, Zech-ner R, Ju¨rgens G. Endogenous produced lipoprotein lipase en-hances the binding and cell association of native, mildly oxidized and moderately oxidized low-density lipoprotein in mouse peri-toneal macrophages. Biochem J 1999;343:347 – 53.
[9] Greilberger J, Schmut O, Ju¨rgens G. In vitro interactions of oxidatively modified LDL with type I, II, III, IV and V collagen, laminin, fibronectin, and poly-D-lysine. Arterioscler Thromb Vasc Biol 1997;17:2721 – 8.
[10] Saxena U, Ferguson E, Auerbach BJ, Bisgaier CL. Lipoprotein lipase facilitates very low density lipoprotein binding to the subendothelial cell matrix. Biochem Biophys Res Comm 1993;194:769 – 74.
[11] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;1951:265 – 75.
[12] El-Saadani M, Esterbauer H, El-Sayed M, Goher M, Nassar AY, Ju¨rgens G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res 1989;30:627 – 30.
[13] Saxena U, Witte LD, Goldberg IJ. Release of endothelial cell lipoprotein lipase by plasma lipoproteins and free fatty acids. J Biol Chem 1989;264:4349 – 55.
[14] Hendriks WL, van Vark LC, Schoonderwoerd K, Jansen H, Havekes LM. Not the mature 56 kDa lipoprotein lipase protein but a 37 kDa protein co-purifying with the lipase mediates the
binding of low density lipoproteins to J774 macrophages. Biochem J 1998;330:765 – 76.
[15] Sivaram P, Wadhwani S, Klein MG, Sasaki A, Goldberg IJ. Biotinylation of lipoprotein lipase and hepatic triglyceride lipase: application in the assessment of cell binding sites. Anal Biochem 1993;214:511 – 6.
[16] Wang X, Greilberger J, Ju¨rgens G. Time resolved fluorometric assay for measuring cell binding and association of native and oxidized low-density lipoproteins to macrophages. Anal Biochem 1998;267:271 – 8.
[17] Gamble W, Vaughan M, Kruth HS, Avigan J. Procedure for determination of free and total cholesterol in micro- and nanogram amounts suitable for studies with cultured cells. J Lipid Res 1978;19:1968 – 70.
[18] Obunike JC, Edwards IJ, Rumsey SC, Curtiss LK, Wagner WD, Deckelbaum RJ, Goldberg IJ. Cellular differences in lipoprotein lipase-mediated uptake of low density lipoproteins. J Biol Chem 1994;269:13129 – 35.
[19] Daoud AS, Frank AS, Jarmolych J, Fritz KE. The effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) on necrosis of atherosclerotic lesions. Atherosclerosis 1987;67:41 – 8.
[20] Elliott RJ, McGrath LT. Calcification of the human thoracic aorta during aging. Calcif Tissue Int 1994;54:268 – 73.
[21] O’Brien KD, Gordon D, Deeb S, Ferguson M, Chait A. Lipo-protein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest 1992;89:1544 – 50.
[22] Yla¨-Herttuala S, Lipton BA, Rosenfeld ME, Goldberg IJ, Stein-berg D, Witztum JL. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA 1991;88:10143 – 7.
[23] Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Ann Rev Biochem 1977;46:897 – 930.
[24] Dashti N, Lee DM, Mok T. Apolipoprotein B is a calcium binding protein. Biochem Biophys Res Comm 1986;137:493 – 9. [25] Dirlam-Schatz KA, Attie AD. Calcium induces a conformational
change in the ligand binding domain of the low density lipo-protein receptor. J Lipid Res 1998;39:402 – 11.
[26] Williams KJ, Fuki IV. Cell-surface heparan sulfate proteogly-cans: dynamic molecules mediating ligand catabolism. Curr Opin Lipidol 1997;8:253 – 62.
[27] Hunter GK, Wong KS, Kim JJ. Binding of calcium to gly-cosaminoglycans: an equilibrium dialysis study. Arch Biochem Biophys 1988;260:161 – 7.
[28] Bazzi MD, Nelsestuen GL. Extensive segregation of acidic phos-pholipids in membranes induced by protein kinase C and related proteins. Biochemistry 1991;30:7961 – 9.
[29] Goldman R. Control of lipoprotein lipase secretion by macrophages: effect of macrophage differentiation agents. J Leuk Biol 1990;47:79 – 86.
[30] Santamarina-Fojo S, Dugi KA. Structure, function and role of lipoprotein lipase in lipoprotein metabolism. Curr Opin Lipidol 1994;5:117 – 25.
.