TREE vol. 15, no. 6 June 2000 0169-5347/00/$ – see front matter © 2000 Elsevier Science Ltd. All rights reserved. PII: S0169-5347(00)01837-1 2 2 5
E
cological complexity results from thecoexistence of many species. With this in mind, Will Provine (Cornell Uni-versity, Ithaca, NY, USA) began a recent workshop*by pointing out that ecologic-al divergence must be involved in specia-tion, otherwise new species could not coexist. This focused attention on the central question of the meeting: how, and under what circumstances, does selec-tion reduce gene flow to the extent that it precipitates speciation?
Darwin and Wallace believed that new species arose as a direct result of adap-tation. However, during the latter half of this century, the fragmentation of a species’ range by a physical barrier to gene flow became seen as essential to the speciation process1. Under this scenario, speciation
occurs during geographical isolation and is potentially followed by ecological co-existence once the physical barrier to gene flow is removed. Today, however, nu-merous examples of adaptive divergence along ecological gradients2,3demonstrate
that selection can be effective in the face of gene flow. In addition, theory predicts that selection alone might cause speci-ation within a single populspeci-ation4.
Neverthe-less, because allopatric separation might make speciation easier, the actual impor-tance of physical barriers to gene flow in the formation of new species remains an important issue.
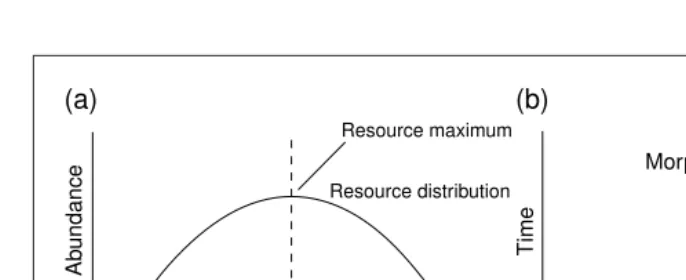
What surprised many people at this meeting was how easily models of ‘adap-tive dynamics’ predict the splitting of a single population under selection. In a simple clonal population with a unimodal resource distribution, Hans Metz (Inter-national Institute for Applied Systems Analysis, Laxenburg, Austria) modelled the exploration of phenotypic space through the sequential fixation of mu-tations of small effect (Fig. 1). Initially, the mean phenotype of a population evolves to exploit the most abundant resource, which then becomes depleted. Adaptation to this resource, therefore, comes to rep-resent a ‘fitness minimum’. Disruptive selection then begins to act, because mu-tants that differ in either direction from this phenotype can invade by avoiding density-dependent competition with the rest of the population. Branching is an emergent property of the model, dividing
a continuous resource into distinct niches occupied by daughter populations. Such adaptive branching is predicted whenever the resource distribution is wider than the distribution of competitive effects between individuals.
Subsequent talks extended this simple scenario to include more realistic assump-tions. Eva Kisdi (University of Turku, Finland) showed that diploid populations also evolve into the region of phenotypic space where selection is disruptive, mean-ing that conditions for the maintenance of adaptive polymorphisms are less restrict-ive than previously thought. In another model, Ulf Dieckmann (International Insti-tute for Applied Systems Analysis) consid-ered a sexually reproducing population, where the independence of newly derived lineages depends on assortative mating. This occurs most easily if assortment is based on a trait that is associated with resource utilization (e.g. body size). How-ever, if mate choice is based on an unre-lated trait, genetic drift is necessary to generate a genetic association between the signal and the resource-use trait, thus allowing evolutionary branching. Although the average ‘waiting-time’ for such link-age disequilibrium to arise depends on the number of loci involved in each trait and on the effective size of the popu-lation, competition-driven speciation is likely to occur rapidly and under a wide range of biologically plausible conditions5.
Similarly rapid evolutionary branching is also predicted for other types of eco-logical interaction, including predation and mutualism, as shown by Michael Doebeli (University of British Columbia, Vancouver, Canada).
Palaeontological evidence suggests that adaptive speciation can occur rapidly in nature. Amy McCune (Cornell Univer-sity) showed that repeated radiations of semionotid fish have occurred during cycles of lake formation in Mesozoic sediments in eastern USA. In many cases, large numbers of new species appear within the first 7000 years after lake formation.
Other examples showed that speci-ation in distant lineages often results in strikingly similar adaptations. Jonathan Losos (Washington University, St Louis, MO, USA) showed that on large Caribbean islands, repeated radiations of Anolis
lizards involve changes in similar traits between species in similar habitats. Eric Knox (Rutgers University, Newark, NJ, USA) described adaptive radiations of the
giant Senecio species (Compositae) in east Africa, and showed that the coloni-zation of different mountain tops by alpine forms was followed by similar adaptations to lower altitude niches. However, since these repeated adaptations are observed in relatively old lineages, it remains pos-sible that such adaptation follows specia-tion, rather than being involved in the actual process of evolutionary branching. Probably the best evidence for adap-tive speciation comes from very young lineages, where the phylogenetic signa-ture of rapid diversification can still be seen, along with intermediate pheno-types between extant species. Howard Rundle (University of British Columbia) showed that, in the Canadian stickle-backs, feeding morphs adapted to lim-netic and benthic habitats within a single lake display strong assortative mating, whereas mating between the same feed-ing morphs from different lakes is random. This suggests that ecological adaptation has led directly to reproductive isolation, possibly after double invasion by partially isolated ancestral forms.
By contrast, in the African lake cich-lids, patterns of colour variation (Ole Seehausen and Jacques van Alphen, Uni-versity of Leiden, The Netherlands) sug-gest that disruptive sexual selection might act to generate assortative mating even before ecological divergence occurs. This view is supported by a recent theo-retical model by Higashi et al.6
Although theoretical models predict that adaptive speciation should occur under a wide range of conditions, empir-ical evidence suggests that such evolution-ary branching might be restricted to cer-tain ecological or demographic situations. For example, in the sticklebacks, both feeding morphs are found together only in glacial lakes of intermediate size and depth, where their different habitats are more or less equally represented. One interpretation of this is that differences in the population size of different feeding morphs increase the risk of extinction of one or other morph during the early stages of branching. Similarly, Roger Thorpe (Uni-versity of Wales, Bangor, UK) described how Anolislizards on the small islands of the Lesser Antilles do not show the marked adaptive radiation observed on the larger Caribbean islands. This might point to some minimum population size or spatial extent required for population branching to successfully lead to speciation.
Given that ecological interactions are often transient in nature, François Bonhomme (Université Montpellier 2, France) was concerned that the condi-tions that cause population branching might not persist for long enough to allow speciation. This focused attention
NEWS & COMMENT
Adaptive dynamics: is speciation too easy?
on the rate at which genetic isolation evolves as a by-product of adaptation or because of the fixation of neutral, or nearly neutral, mutations. The latter pro-cess was discussed by Sergey Gavrilets (University of Tennessee, Knoxville, USA), who showed that even without adaptive divergence, postzygotic isolation can evolve under continued gene flow. At present, however, it is difficult to know how rapidly such genetic barriers would develop in real situations, or how much this process could be accelerated by divergence under selection. This latter question depends on the genetic archi-tecture of traits involved during adap-tation and remains an important challenge for future work.
A further contrast between empirical and theoretical work was highlighted by Guy Bush (Michigan State University, East Lansing, USA). Although adaptive dynamic models describe an initially unimodal resource distribution, most empirical ex-amples involve an initially bimodal re-source, which probably makes adaptive speciation easier. For example, many of the host shifts observed in insects have resulted from the introduction of a new agricultural crop, generating a new re-source peak at high abundance that is rela-tively similar to the original host. Does this mean that evolutionary branching under a single resource peak does not occur or simply that it is difficult to observe?
One important line of evidence is the genetic architecture of the adaptations that differentiate species. If speciation occurs in response to sudden changes in resource distribution, trait divergence is likely to occur by fixation of relatively few
loci of large effect – as originally proposed for mimicry7 and recently explored in
detail8. By contrast, if adaptation occurs in
gradually changing, spatially distinct envi-ronments, trait divergence might involve the sequential fixation of loci of small effect. For example, Roger Butlin (Univer-sity of Leeds, UK) showed that host adap-tation between races of the planthopper,
Nilaparvata lugens, is probably controlled by two loci of large effect9. Conversely,
mate-choice divergence (male signal and female response) between these races is influenced by many loci of small effect, suggesting gradual divergence, possibly as a result of drift.
The mapping of quantitative trait loci will soon generate more data on the genetic architecture of species differ-ences. However, as with phenotypic pat-terns, genetic changes immediately fol-lowing the evolution of a new trait or species could quickly alter the distribu-tion of observed gene effects. For exam-ple, the sequential substitution of alleles of small effect at the same locus, or the fixation of other alleles at interacting loci, might generate major gene effects even when none were important during adaptation. Once again, the study of very young lineages is necessary to investi-gate the process of adaptive speciation.
Theoretical models tend to look for general conditions that allow different kinds of speciation to occur. However, na-ture is complex and, because many differ-ent processes are infludiffer-ential in the history of a given trait, population or species, we cannot expect their evolution to conform to any single model. Instead, empirical studies of speciation rely on contemporary
patterns to infer which processes were important in the past, even though they might not be important today. Having es-tablished that adaptive speciation is the-oretically probable, the next challenge is to marry these two approaches and generate predictions that can be tested using the kind of data that is the currency of empiricists.
Acknowledgements
We would like to thank Hans Metz, Ulf Dieckmann, Michael Doebeli and Diethard Tautz for organizing this workshop and for useful comments, and Roger Butlin for interesting discussions. Additional financial support was provided by the Theoretical Biology of Adaptation Programme of the European Science Foundation. Edited contributions from the workshop will be published in 2001.
Jon R. Bridle
Biodiversity and Environmental Processes Group, School of Biosciences, University of Cardiff, UK CF1 3TL ([email protected])
Chris D. Jiggins
The Galton Laboratory, Dept of Biology, University College London, UK NW1 2HE ([email protected])
References
1 Mayr, E. (1963) Animal Species and Evolution,
Harvard University Press
2 Haldane, J.B.S. (1948) The theory of a cline.
J. Genet. 48, 277–284
3 Ehrlich, P.R. and Raven, P.H. (1969) The
differentiation of populations. Science 165, 1228–1232
4 Maynard Smith, J. (1966) Sympatric speciation.
Am. Nat. 100, 637–650
5 Dieckmann, U. and Doebeli, M. (1999) On the
origin of species by sympatric speciation. Nature 400, 354–357
6 Higashi, M. et al. (1999) Sympatric speciation by
sexual selection. Nature 402, 523–526
7 Turner, J.R.G. (1977) Butterfly mimicry: the
genetical evolution of an adaptation. Evol. Biol. 10, 163–206
8 Orr, H.A. (1998) The population genetics of
adaptation: the distribution of factors fixed during adaptive evolution. Evolution 52, 935–949
9 Sezer, M. and Butlin, R.K. (1998) The genetic
basis of oviposition preferences differences between sympatric host races of the brown planthopper (Nilaparvata lugens). Proc. R. Soc. London Ser. B 265, 2399–2405
NEWS & COMMENT
2 2 6 TREE vol. 15, no. 6 June 2000
Fig. 1.A simple adaptive dynamics model showing evolutionary branching as an emergent property
of competition. In (a) the distribution of a continuous resource (e.g. seed size) is represented along the relevant trait axis (e.g. beak size in seed-eating birds). In (b) the evolution of the relevant trait is shown over time. Initially, the population mean phenotype, represented by black squares in (a), evolves by the sequential fixation of mutations to exploit the most common resource. Once the population has reached this resource maximum, density-dependent competition turns it into a ‘ fit-ness minimum’. At this point, the population is susceptible to invasion by new mutations that avoid competition by exploiting resources on either side of the fitness minimum. The population therefore evolves to the point where population branching is likely. In a sexual population, the evolution of assortative mating is necessary to maintain these independent lineages, often requiring the genera-tion of linkage disequilibrium between the resource-utilizagenera-tion trait and traits involved in mate choice5.
Trends in Ecology & Evolution
Ab
undance
Resource distribution Resource maximum
(a) (b)
Time
Morph 1
Fitness minimum
Morph 2
Resource use phenotype Trait
Students
Did you know that you are entitled to a 50% discount on
a subscription to Trends in Ecology & Evolution? See the subscription order card in