Summary Root elongation was measured in mature Euca-lyptus pauciflora Sieber ex Sprengel subsp. pauciflora trees in a high-elevation stand, and in seedlings of E. pauciflora and E. nitens (Deane & Maiden) grown in a glasshouse. Elongation of non-mycorrhizal roots (>10 mm long) was measured at 0600 and 1800 h on several consecutive days. Root elongation of seedlings of both species in the glasshouse was greater than that of mature E. pauciflora trees in the forest. For seedlings of comparable size, roots of E. nitens elongated faster than roots of E. pauciflora. Root elongation was always greater during the night than during the day in both species, in both the glasshouse and forest environment. Both water and osmotic potentials of root tips of E. pauciflora seedlings were lower during the night than during the day. The derived turgor pres-sure value of root tips was greater during the night than during the day and there was no difference in turgor pressure above the yield threshold.
Keywords: Eucalyptus pauciflora, Eucalyptus nitens, root elongation, turgor pressure, yield threshold.
Introduction
Circadian rhythms in plant growth have been studied for many years (see Bünning 1977). Reed (1939) found that more than 60% of the primary shoot growth of Pinus echinata Mill. and P. taeda L. occurred during the night. Growth rates of several parts of plants are known to have daily variation, including: stem diameters of P. canariensis C. Smith (Holmes and Shim 1968) and Prunus cerasus L. (Kozlowski 1968), leaf extension of Salix viminalis L. (McDonald et al. 1992), root diameters of Gossypium hirsutum L. cv. Auburn 56 (Huck et al. 1970), and fruit diameters of P. cerasus (Kozlowski 1968). In a range of tree species grown in rhizotrons and pots, nocturnal root elon-gation rates were greater than diurnal rates (Head 1965, Lyr and Hoffman 1967, Kolesnikov 1971, Hilton and Khatamian 1973, Hurshudian 1974). In contrast to these findings, Stahel (1972) found that, irrespective of short- or long-day treatments with constant light intensities, root growth of Picea sitchensis (Bong.) Carr. seedlings was greater during the day than during
the night. Little further is known about the phenomenon of variation in day/night root elongation or the factors that may control such variation.
The overall objective of our current research is to gain a better understanding of root physiology of subalpine Eucalyp-tus pauciflora Sieber ex Sprengel subsp. pauciflora and mon-tane Eucalyptus nitens (Deane & Maiden) in relation to belowground temperature in Victoria, Australia. As a part of this project, we examined the day/night variation and factors that influence root elongation of E. pauciflora and E. nitens. Root tip water potential (Ψ), osmotic potential (Ψπ), water potential at yield threshold (Ψy), and derived turgor pressure
(P) were measured to seek an explanation for differences in day/night root elongation rates.
Materials and methods Glasshouse studies
Seeds were collected from an E. pauciflora stand on Mt. Stirling, Victoria (37°07′ S, 146°48′ E) at an elevation of 1545 m and from an E. nitens stand from the Toorongo Plateau, Victoria (37°54′ S, 146°02′ E) at an elevation of 1000 m. Seeds were stratified for 3 weeks at 3 °C and then sown in pots (150 mm diameter × 200 mm height) filled with 4 kg of heat-steril-ized soil (110 °C for 48 h) from the A2 horizon of a podzolized
sandy soil. The pH of the soil was 5.2 in water. No mycorrhizae were found throughout the experiment. Four grams of a slow release fertilizer (Osmocote® N,P,K 17,4.4,10 (w/v) with trace amounts of micronutrients) were added to each pot before sowing. Plants were grown in a glasshouse with day/night temperatures ranging from 32 to 10 °C and a mean of 26/16 °C. Seedlings were grown in an 18-h photoperiod where natural day length was supplemented with three incandescent 150 W bulbs.
A wooden Muromtsev rhizotron was modified by removing the panels used for split-root experiments (see Kolesnikov 1971, p 169). The rhizotron had eight observation windows that were each 200 mm wide and 330 mm deep (total surface observation area of the eight windows was 0.53 m2). The inside
Elongation of Eucalyptus roots during day and night
REESE HALTER,
1,4ROGER SANDS,
1,4E. K. SADANANDAN NAMBIAR
2and
DAVID H. ASHTON
31
School of Forestry, The University of Melbourne, Creswick, Victoria, 3363, Australia
2 Commonwealth Scientific and Industrial Research Organization, Division of Forestry, PO Box 4008, Canberra, ACT, 2600, Australia
3
Department of Botany, La Trobe University, Bundoora, Victoria, 3083, Australia
4
Present address: School of Forestry, University of Canterbury, Private Bag 4800, Christchurch, New Zealand
Received October 25, 1995
distance between opposite windows was 85 mm. The bottom of the rhizotron was perforated for drainage. The rhizotron volume of 0.30 m3 was filled with 36 kg of soil mixed with 36 g of Osmocote®. The bulk density was 12 kg m−3.
Four seedlings (about 400 mm tall with five sets of paired leaves) were transplanted to the rhizotron. Each seedling was planted at the midpoint of the two corresponding opposite windows to maximize the number of observations of elongat-ing roots. Seedlelongat-ings were watered to field capacity every third day, and the seedlings were grown in a natural photoperiod. Measurements began one month after transplanting, during the winters of August 1992 and May 1994, when seedlings were well established.
Elongation of the same two root tips was measured at 0600 and 1800 h for 7 consecutive days. At the beginning of the measurements all roots measured were longer than 10 mm. Positions of root tips were marked on the windows of the rhizotron with fine tipped indelible markers. Distances be-tween successive marks were then measured with a hand lens. During the measurement period, plants were watered daily to field capacity after the 0600 h measurement. Soil temperatures were recorded at 50 mm depth before day/night root elonga-tion measurements. Soil temperatures for E. pauciflora ranged from 15 to 18 °C at 0600 h and from 22 to 26 °C at 1800 h and for E. nitens, they ranged from 14 to 19 °C at 0600 h and from 23 to 26 °C at 1800 h.
Root tips (8.5 mm in length) were harvested at 1200 and 2400 h on the first and second days from eight seedlings of E. pauciflora grown under conditions identical to those used for root measurements. Their total water potential, water po-tential at yield threshold, and osmotic popo-tential were deter-mined with a psychrometer, as described by Sands et al. (1992). Total water potential was determined by extrapolating the plot of psychrometer output against time, back to zero time.
Field studies
A mature stand of E. pauciflora (about 235 stems ha−1 and a mean height of 9 m) with an understory of Oxylobium alpestre F. Muell., Prostantheraa cuneata Benth, and Poa sp., at an elevation of 1545 m, was selected on Mt. Stirling, Victoria. The soil was an alpine uniform humus (Northcote 1979) with a dark loamy, 350-mm deep, sandy A horizon and a bulk density of 6.4 kg m−3. It contained 15% coarse fragments and had a pH of 4.1 in water. Within the stand, an area of 20 × 20 m was marked as an experimental plot. Within the plot, eight sam-pling points were marked at random, along a slope of 20° with a southerly aspect. Soil profile (A horizon) was cut vertically and a wedged surface 200 mm wide and 200 mm deep was exposed. There were abundant roots, with many root intercepts of E. pauciflora at all sampling points. Eucalypt roots can be readily distinguished from the roots of understory species (cf. Chilvers 1972). Sheets of Perspex (Plexiglas) 100 mm wide × 150 mm deep were pressed against the soil surface and retained by triangular iron fittings, held by the side walls of exposed soil wedges. Perspex sheets were covered with 15-mm styro-foam and back-filled with the soil removed previously. Root elongation measurements were made of the new apices from
non-mycorrhizal roots longer than 10 mm that emerged on the Perspex front. The same two root apices from each Perspex sheet were measured at 0600 and 1800 h for 7 days in late summer between January 31 and February 6. Another two root apices were monitored consecutively for 6 days in mid-autumn between April 1 and 6. At the beginning of a measurement period, the position of the root apex was marked on the Perspex and subsequent elongation was recorded from this fiducial point. A hand lens, vernier calipers and a head torch were used to facilitate measurements. Gravimetric water content of the surface 200 mm of soil was determined from eight cores (70 mm diameter × 200 mm long) positioned at random on each day of measurement. A Lambrecht soil thermograph was installed to record soil temperatures continuously at 50 and 200 mm soil depth.
An ANOVA was performed for each species on seedlings and mature trees, on day and night root growth data, daily water potential, osmotic potential, and water potential at yield threshold. Least Squared Differences were used to evaluate differences set at 0.05 probability (Zar 1984).
Results and discussion
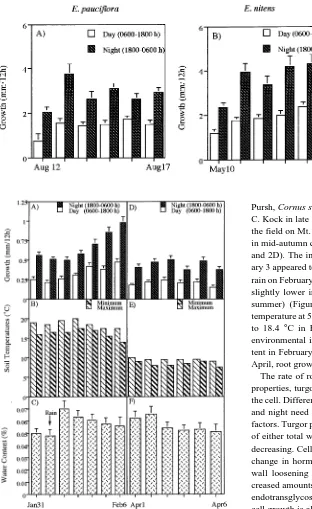
Seedlings of E. pauciflora and E. nitens had six pairs of leaves and an average height of 450 mm (± 43 SE) at the time of root measurements. Root elongation of seedlings of both species was significantly greater during the night (1800--0600 h) than during the day (0600--1800 h), and roots of E. nitens grew significantly faster than those of E. pauciflora during both night and day (Figures 1A and 1B). Values for nocturnal and diurnal elongation of roots of E. nitens seedlings were 3.7 and 1.9 mm per 12 h, respectively, whereas the corresponding values for E. pauciflora roots were 2.9 and 1.4 mm per 12 h, respectively. Head (1965) measured root elongation of Prunus avium (L.) at 4-h intervals and found that the maxi-mum rate always occurred during the night but not necessarily during the same 4-h period. Lyr and Hoffman (1967) recorded greater root elongation rates at night than during the day. Percent nighttime increases for the following species were: Populus trichocarpa (Torr. and Gray) 60%, Quercus borealis var. maxima (Marsh.) Ashe 37%, P. sylvestris L. 36%, and P. abies (L). Karst. 30%. Over a 30-day period of measure-ment, root elongation rates of a Quercus sp. were 64% greater during the night than during the day (Hurshudian 1974). We found that root elongation rates in E. nitens and E. pauciflora seedlings were 60 and 67% higher, respectively, during the night than during the day (Figures 1A and 1B).
Cydonia oblonga Mill., P. resinosa Ait., P. × canadensis Moench, and Vitis vinifera L. × V. rupestris Sch. in rhizotrons were 17% greater at night than during the day from spring to midsummer. However, in late summer and early autumn, only C. oblonga had greater root elongation rates at night. Burnett (1978) reported a similar pattern of greater root elongation in a rhizotron at night than during the day in Alnus crispa (Ait.)
Pursh, Cornus stolonifera Michx. and Larix larcinia (DuRoi) C. Kock in late spring. We found that root elongation rates in the field on Mt. Stirling were reduced by approximately 40% in mid-autumn compared to rates in late summer (Figures 2A and 2D). The increase in overall elongation rate after Febru-ary 3 appeared to follow the peak soil water content following rain on February 1 (Figure 2C). Gravimetric water content was slightly lower in April (mid-autumn) than in February (late summer) (Figures 2C and 2F). Also, the mean maximum temperature at 50 mm soil depth in April was 9.5 °C compared to 18.4 °C in February (Figures 2B and 2E). Because the environmental influences of soil temperature and water con-tent in February were more conducive to root growth than in April, root growth rate was highest in late summer at the site.
The rate of root elongation probably depends on cell wall properties, turgor pressure and the conductivity of water into the cell. Differences in the rate of root elongation between day and night need to be explained in terms of changes in these factors. Turgor pressures could be increased at night as a result of either total water potential increasing or osmotic potential decreasing. Cell wall loosening at night could be caused by a change in hormone concentrations, temperature or pH. Cell wall loosening in roots at low water potential requires in-creased amounts of both abscisic acid (ABA) and xyloglucan endotransglycosylase (XET) (Wu et al. 1994). Biophysics of cell growth is also dependent on temperature (Pritchard et al. 1990, Boyer 1993) and pH (Cosgrove 1993). Webb (1976) found that when seedlings of Acer saccharum Marsh. were exposed to high light intensities, root elongation was greater during the night than during the day. However when the same seedlings were exposed to low light intensities, differences between day and night elongation rates gradually disappeared. Excess assimilate appears to be preferentially directed toward night elongation but the mechanism(s) for this remains un-clear. Ferris and Taylor (1994) found no difference between day and night elongation in three species of herb at ambient atmospheric carbon dioxide concentrations, but found greater night elongation at enriched carbon dioxide concentrations. However, the situation was reversed in another species.
Figure 1. Root elongation measured every 12 h at 0600 and 1800 h on consecutive days in a glasshouse for (A)
E. pauciflora seedlings in
August 1992 and (B) E.
ni-tens seedlings in May 1994.
Vertical bars indicate ± SE of each mean.
Figure 2. Root elongation measured every 12 h at 0600 and 1800 h for
E. pauciflora roots, daily maximum and minimum soil temperatures
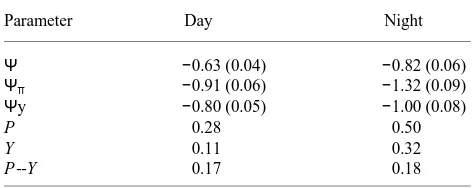
We examined the relationship between water potential (Ψ), osmotic potential (Ψπ), and derived turgor (Ψ−Ψπ) in the E. pauciflora root tips grown in the rhizotron under the same conditions as those for which elongation rates are shown in Figure 1A. Water potentials of roots were significantly lower during the night (−0.82 MPa) than during the day (−0.63 MPa) (Table 1). Usually, tissue water potentials of aboveground parts of plants are higher during the night than the day (e.g., Kramer 1983). It is to be expected that the difference between night and day potentials would be less for roots than for shoots, but no explanation can be offered as to why water potentials of root tips were higher during the day than at night in our study. However, osmotic adjustment was greater at night than during the day to the extent that the calculated turgor pressure was greater during the night (0.50 MPa) than the day (0.28 MPa). Therefore, the greater elongation at night can perhaps be ex-plained by greater turgor pressure at night.
Some recent work suggests that there is no direct relation-ship between turgor pressure and elongation (Zhu and Boyer 1992, Tomos and Pritchard 1994), so the relationship that we have found here may be coincidental. Also, cell elongation has frequently been considered in terms of Lockhart’s (1965) model, which takes into account the yield turgor (the turgor pressure above which the cell will commence to elongate) as well as turgor pressure and cell wall extensibility:
δV/δt =Φ(P − Y) (1)
where δV/δt = relative expansion rate, Φ = cell wall extensibil-ity, P = turgor pressure, and Y = yield turgor.
This may be expanded to
δV /δt =Φ[(Ψ−Ψπ)−(Ψy−Ψπ)]
=Φ(Ψ−Ψy) (2)
where Ψ = water potential of the root, Ψπ = osmotic potential of the root, and Ψy = water potential of the root at the yield
threshold.
Recent reviews by Passioura and Fry (1992) and Passioura (1994), have criticized the Lockhart model for not taking into
account how cell wall extensibility and yield threshold vary to maintain a constant growth rate despite a change in turgor.
Derived values for P, Y, and (P --Y) are given in Table 1. These were calculated from measurements of Ψ, Ψy, and Ψπ
using the psychrometric techniques, and attention has recently been drawn to possible sources of error in psychrometric measurements of water potential components (Sands et al. 1992, Ferris and Taylor 1994). Ideally, turgor pressures should be measured directly with a pressure probe. However, relative changes should still be valid. Yield turgor (calculated as Ψy--Ψπ) was greater at night (0.32 MPa) than during the day
(0.11 MPa) and the residual turgor pressure above the yield threshold (P --Y) was the same during night and day. Therefore, differences in elongation cannot be explained in terms of differences in (P --Y) and, if the model is correct, would need to be explained by differences in cell wall extensibility (Φ), which was not measured directly here. Pritchard et al. (1990) found that both Y and Φ influenced Triticum aestivum L. cv. Flanders root growth. By contrast, Ferris and Taylor (1994) found that when greater night than day elongation was induced in the roots of three herbs by atmospheric carbon dioxide enrichment, the increase in root elongation at night was asso-ciated with increases in both Φ and P but not (P --Y). In our study, increased root elongation was related to increased P but not to increased (P --Y).
Conclusion
Evidence is presented of the existence of day/night variation in root elongation of both seedling and mature eucalypts. Rates of root elongation differed between species, but, in general, were 60% faster at night than during the day. Faster root elongation during the night was associated with decreased root tip osmotic potentials and increased turgor pressures.
Acknowledgments
R. Halter is the recipient of a postgraduate scholarship from Global Forest, Canada. We acknowledge research grants from The University of Melbourne and The Commonwealth Scientific and Industrial Re-search Organization (CSIRO), Division of Forestry. We thank Dr. David Sheriff (CSIRO) for helpful comments and suggestions on the manuscript. We also thank Mr. Scott Fair of the Alpine Resorts Com-mission (ARC), Mt. Buller, Victoria, for assistance on Mt. Stirling.
References
Boyer, J.S. 1993. Temperature and growth-induced water potential. Plant Cell Environ. 16:1099--1106.
Bünning, E. 1977. Fifty years of research in the wake of Wilhelm Pfeffer. Annu. Rev. Plant Physiol. 28:1--22.
Burnett, M.A. 1978. Studies on the seasonal periodicity of root growth in certain woody plants. M.Sc. Thesis, Univ. Guelph, Guelph. Chilvers, G.A. 1972. Tree root pattern in a mixed eucalypt forest. Aust.
J. Bot. 20:229--234.
Cosgrove, D.J. 1993. How do plant cell walls extend? Plant Physiol. 102:1--6.
Table 1. Water potential (Ψ), osmotic potential (Ψπ), water potential at
yield threshold (Ψy), derived turgor pressure (P), derived yield turgor
Ferris, R. and G. Taylor. 1994. Increased root growth in elevated CO2:
a biophysical analysis of root cell elongation. J. Exp. Bot. 45:1603--1612.
Head, G.C. 1965. Studies of diurnal changes in cherry root growth and nutational movements of apple root tips by time-lapse cinematog-raphy. Ann. Bot. 29:219--224.
Hilton, R.J. and H. Khatamian. 1973. Diurnal variation in elongation rates of roots of woody plants. Can. J. Plant Sci. 53:699--700. Holmes, J.W. and S.Y. Shim. 1968. Diurnal changes in stem
diame-ter of Canary Island pine trees (Pinus canariensis C. Smith) caused by soil water stress and varying microclimate. J. Exp. Bot. 19:219--232.
Huck, M.G., B. Klepper and H.M. Taylor. 1970. Diurnal variation in root diameter. Plant Physiol. 45:529--530.
Hurshudian, P.H. 1974. Periodicity of root growth in trees. In Interna-tional Symposium on Ecology and Physiology of Root Growth. Potsdam, D.D.R., pp 297--308.
Kolesnikov, V.K. 1971. The root system of fruit plants. Mir Publishers, Moscow, 269 p.
Kozlowski,T.T. 1968. Diurnal changes in diameter of fruits and tree stems of Montmorency cherry. J. Hort. Sci. 43:1--15.
Kramer, P.J. 1983. Water relations in plants. Academic Press, New York, 489 p.
Lockhart, J.A. 1965. An analysis of irreversible plant cell elongation. J. Theor. Biol. 8:264--275.
Lyr, H. and G. Hoffman. 1967. Growth rates and growth periodicity of tree roots. In International Review of Forest Research, Vol. 2. Eds. J.A. Romberger and P. Mikola. Academic Press, New York, pp 181--236.
McDonald, A.J.S., I. Stanenberg and R. Sands. 1992. Diurnal variation in extension growth of leaves of Salix viminalis. Tree Physiol. 11:123--132.
Northcote, K.N. 1979. A factual key for the recognition of Australian soils. Rellim Technical Publications, Coffs Harbour, 122 p. Passioura, J.B. and S.C. Fry. 1992. Turgor and cell expansion: beyond
the Lockhart equation. Aust. J. Plant Physiol. 19:565--576. Passioura, J.B. 1994. The physical chemistry of the primary cell wall:
implications for the control of expansion rate. J. Exp. Bot. 45:1675--1682.
Pritchard, J., R.G. Wyn Jones and A.D. Tomos. 1990. Measurement of yield threshold and cell wall extensibility of intact wheat roots under different ionic, osmotic and temperature treatments. J. Exp. Bot. 41:669--675.
Reed, J.F. 1939. Root and shoot growth of shortleaf and loblolly pines in relation to certain environmental conditions. Duke Univ. School of Forestry Bull. 4:5--51.
Sands, R., A.J.S. McDonald and I. Standenberg. 1992. An evaluation of techniques for measuring yield turgor in excised Salix leaves. Plant Cell Environ. 15:107--114.
Stahel, J.B. 1972. The effect of day length on root growth of Sitka spruce. For. Sci. 18:27--31.
Tomos, D. and J. Pritchard. 1994. Biophysical and biochemical control of cell expansion in roots and leaves. J. Exp. Bot. 45:1721--1732. Webb, D.P. 1976. Root growth in Acer saccharum Marsh. seedlings:
effects of light intensity and photoperiod on root elongation rates. Bot. Gaz. 137:211--217.
Wu, Y., W.G. Spollen, R.E. Sharp, P.R. Hetherington and S.C. Fry. 1994. Root growth maintenance at low water potentials. Increased activity of xyloglucan endotransglycosylase and its possible regu-lation by abscisic acid. Plant Physiol. 106:607--615.
Zar, J. H. 1984. Biostatistical analysis. Prentice-Hall, London, 718 p. Zhu, G.L. and J.S. Boyer. 1992. Enlargement in Chara studied with a