Belfiore F, Mogensen CE (eds): New Concepts in Diabetes and Its Treatment. Basel, Karger, 2000, pp 1–2
. . . .
Introduction
Diabetes mellitus and its complications are clinical conditions of growing importance both from the clinical as well as epidemiological standpoint. The relevance of diabetes at clinical and individual level is given by its life-threatening acute complications and, especially, by its chronic complications affecting several organs and systems, with increased risk for ocular, renal, cardiac, cerebral, nervous and peripheral vascular diseases. The high preva-lence of diabetes in many developed countries or in special ethnic groups, entailing premature disability and mortality, points to its relevance at popula-tion level. It is, therefore, mandatory for both the specialist and the practipopula-tioner to be acquainted with the pathophysiological mechanisms, clinical manifesta-tions and, above all, therapy of diabetes mellitus.
Recent data showing that control of hyperglycemia may prevent the onset or slow down the progression of complications point to the importance of an appropriate and efficacious treatment. Indeed, the aim of this book is to serve as a tool to provide physicians with the latest views on diagnostic aspects and pathophysiological mechanisms as a premise to go deep into the various facets of the modern management of diabetes.
The mechanisms of complications are treated as an introduction to the understanding of possible therapeutic strategies. Then retinopathy, nephrop-athy, hypertension and cardiovascular disease are considered in their clinical aspects and therapeutic interventions. Extensive space is devoted to the various neuropathic manifestations, including erectile dysfunction, as well as to the foot problems. Final chapters highlight the need for multifactorial treatment and the clinical and therapeutic problems of diabetic pregnancy.
The international panel of authors has made any effort to condense this rich content into a relatively short text and to present it in a clear and smooth-to-read form. While more extensive information may be found in larger treatises (see Suggested Reading, below), we hope that this medium-size book will be useful to all physicians interested in the management of diabetic patients by providing them with a simple yet updated source of information concerning
theNew Concepts in Diabetes and Its Treatment.
Francesco Belfiore Carl Erik Mogensen
Suggested Reading
Alberti KGMM, Zimmet P, DeFronzo RA: International Textbook of Diabetes mellitus, ed 2. Chichester, Wiley, 1999.
Belfiore F (ed): Frontiers in Diabetes. Basel, Karger, vol 8/1987, vol 9/1990, vol 10/1990, vol 11/1992, vol 12/1993, vol 14/1998.
Bray G, Bouchard C, James WPT (eds): Handbook of Obesity. New York, Dekker, 1997. Kakn CR, Weir GC (eds): Joslin’s Diabetes mellitus, ed 13. Malvern, Lea & Febiger, 1994.
Mogensen CE (ed): The Kidney and Hypertension in Diabetes mellitus, ed 5. Boston, Kluwer Academic, 2000.
Pickup JC, Williams G (eds): Textbook of Diabetes, ed 2. Oxford, Blackwell, 1997.
Chapter I
Belfiore F, Mogensen CE (eds): New Concepts in Diabetes and Its Treatment. Basel, Karger, 2000, pp 3–19
. . . .
Etiological Classification,
Pathophysiology and Diagnosis
F. Belfiore, S. Iannello
Institute of Internal Medicine, University of Catania, Ospedale Garibaldi, Catania, Italy
Introduction
According to the classical definition, diabetes mellitus is a disorder re-sulting from both genetic predisposition and favoring environmental factors, and is characterized by alterations in the metabolism of carbohydrate, fat and protein, which are caused by a relative or absolute deficiency of insulin secretion and different levels of insulin resistance. In the patients with long-standing diabetes, late complications develop consisting of alterations and failure of various organs (especially the noninsulin-sensitive ones) including the eyes (retinopathy with vision loss), kidneys (nephropathy leading to renal failure), nerves (peripheral and autonomic neuropathy), heart and blood vessels (preco-cious and severe cardiovascular, cerebrovascular and peripheral vascular ath-erosclerosis). Diabetes mellitus includes etiologically and clinically different diseases that have hyperglycemia in common, representing a syndrome rather than a single disease.
published in 1997, divided into four sections (definition and description of diabetes, classification of diabetes, diagnostic criteria and testing for diabetes), which we summarize in this chapter.
Definition and Description of Diabetes mellitus
The basis of the metabolic alterations in diabetes is the reduction (to a various degree) of insulin action on insulin-sensitive tissues, due to deficiency of insulin secretion or to insulin resistance or both. The majority of cases of diabetes mellitus falls into two major forms: type 1 and type 2 diabetes.
Type 1 Diabetes
Immune-Mediated Type 1 Diabetes
Type 1 diabetes (previously also named insulin-dependent diabetes mel-litus – IDDM – or juvenile-onset diabetes) is an immune-mediated form of diabetes, which accounts for approximately 5–10% of all diabetics in the West-ern world. It occurs mainly in healthy nonobese children or young adults but may also affect subjects at any age, and results from an absolute deficiency of insulin secretion (evidenced by low or undetectable levels of plasma C-peptide), caused by a cellular-mediated autoimmune destruction of pancreatic b-cells. Although the affected subjects are usually nonobese, the presence of obesity is not incompatible with the diagnosis of type 1 diabetes. The course may be rapid in children and young adults, slower in older patients. Adult patients can retain for some time a residualb-cell function while children and adolescents often show early the effects of severe insulin lack, with a diabetes appearing abruptly over days or weeks and rapidly progressing to acute life-threatening complication (ketoacidotic coma), which may be the first mani-festation of the disease, particularly in presence of precipitating factors such as infections or other stress.
suggested to occur in chromosomes 2, 6, 11 and 15. However, the major gene seems to be located at the HLA locus in the chromosome 6. Indeed, it is now largely accepted that type 1 diabetes is strongly associated to HLA system, especially with the class II molecules which encode for the D allele. Patients who express the DR3 or DR4 alleles or those who are heterozygous (DR3/ DR4) are especially susceptible to type 1 diabetes. Class I alleles (B8, B15) also seem to be associated to type 1 diabetes as they show linkage disequilibrium, i.e. show nonrandom association with the D alleles. Recently, great importance has been attributed to the DQ locus. It has been shown that DQb1*0301 and DQb1*0302 segregate with DR4 and that DQb1*0201 segregates with DR3. Presence of DQb1*0201 and DQb1*0302 or, especially, the heterozygous state DQb1*0201/0302 entails high risk. On the other hand, DQb1*0502 and DQb1*0602 are associated with the DR2 haplotypes and would be protective.
Immunologic Mechanisms. Class II molecules are expressed by
nonendo-crine autoimmune diseases (Grave’s disease, Hashimoto’s thyroiditis, Addison’s disease, primary gonadal failure, vitiligo, pernicious anemia, connective tissue disease, celiac disease, myasthenia gravis, etc.). This primary autoimmune pathogenesis seems to be confirmed by a persistence of islet cell autoantibodies (ICAs) forever. In 85–90% of patients, diabetes is early associated with one or more serological genetic markers such as ICAs, IAAs (insulin autoantibodies), GAD65(autoantibodies to glutamic acid decarboxylase) and IA-2 or IA-2b (autoantibodies to tyrosine phosphatase). These autoantibodies disappear over the course of a few years in the majority of patients, and may be the result rather than the cause of the autoimmune process.
Clinical Picture. Manifest type 1 diabetes is characterized by symptoms linked to the marked hyperglycemia, such as polyuria (due to the osmotic effect of glucose), polydipsia (to compensate for the water lost with polyuria), polyphagia (to compensate for the energetic substrate glucose lost in the urine), weight loss and fatigue (due to loss of glucose in urine and to dehydration), and blurred vision (due to lens osmotic disturbances). These patients are insulin-dependent for their survival and prone to ketosis; impairment of growth, susceptibility to certain infections, hypertension, lipoprotein metabolism al-terations, periodontal disease and psychosocial dysfunctions are frequent.
Idiopathic Type 1 Diabetes
The idiopathic diabetes includes some forms of type 1 diabetes (common in individuals of African and Asian origin) due to unknown etiology, with strong genetic inheritance (not HLA-associated), without markers of autoim-munity. There is severe deficit of insulin secretion and tendency to ketoacidosis, with absolute requirement of insulin therapy.
Pathophysiology of Type 1 Diabetes
utiliza-Fig. 1.Scheme showing the main metabolic pathways of intermediate metabolism in the three insulin-sensitive tissues (liver, muscle and adipose tissue) participating in the metabolic homeostasis. Note that most metabolic pathways are opposed to each other to form couples composed of a ‘forward pathway’ and a ‘backward pathway’, thus allowing substrate cycling. Examples are: glycogen synthesis and glycogenolysis (steps 1 and 2 in liver, 11 and 12 in muscle), glycolysis and gluconeogenesis (steps 5 and 6), triglyceride synthesis and hydrolysis (lipolysis) (steps 17 and 18 in adipose tissue; 26 and 27 in liver), protein synthesis and proteolysis (steps 13 and 14), etc. Some cycles are ‘inter-tissular’, linking liver and muscle, such as the Cori cycle (expanded to include alanine in addition to lactate and pyruvate), composed of steps 10, 6, 3, 8 and 9, pertaining to carbohydrate metabolism, as well as the cycle linking liver and adipose tissue (steps 19, 22, 26, 28 and 29), pertaining to lipid metabolism. In the normal state, blood glucose is kept at the normal level through a balance between hepatic glucose production (step 3) and glucose utilization by peripheral tissues, mainly the muscle (step 8). VLDL and triglycerides are kept normal through a balance between hepatic production (step 28) and peripheral degradation by LPL, primarily at adipose tissue level (step 29). Ketones are not present because Ac-CoA is entirely oxidized to CO2(or utilized for the synthesis of FFA – step 24).
Fig. 2.Scheme of the main metabolic pathways (similar to that outlined in figure 1) and of their changes in activity rate occurring in states of severe insulin deficiency, such as decompensated type 1 diabetes (thick or thin arrows indicate increased or decreased activity,
respectively). Note the prevalence of the catabolic pathways over the anabolic ones:
Type 2 Diabetes
Type 2 diabetes (previously also named non-insulin-dependent diabetes mellitus – NIDDM – or adult-onset diabetes) occurs in approximately 90–95% of diabetic people in the Western world, resulting from insulin resistance and insufficient compensatory insulin secretion. The disease has an insidious onset and remains asymptomatic and undiagnosed for a long period, even if the moderate hyperglycemia is able to induce severe diabetic late complications.
Type 2 diabetes is strongly favored by genetic predisposition. However, although it shows familial aggregation as well as a high concordance (80%) in monozygotic twins, its mode of inheritance is not fully understood. It may well be a polygenic disease. In any case, the risk of offspring and siblings of type 2 diabetic patients to develop the disease is relatively elevated.
In addition to the genetic predisposition, favoring environmental factors are involved, such as excessive caloric intake, obesity with increased body fat in the abdominal (visceral) site, sedentary habit, etc. The insulin levels may be normal or even increased (especially in presence of obesity) for a long time, but may decrease in the late stage of the disease. The abnormal carbohydrate metabolism can be early identified measuring fasting glycemia (FPG) or per-forming an oral glucose tolerance test (OGTT). This type of diabetes is nonin-sulin-dependent for survival and is nonketosis prone. Hyperglycemia is usually improved or corrected by diet, weight loss and oral hypoglycemic drugs. In type 2 diabetics an acute life-threatening complication, the nonketotic hyperos-molar coma, can develop whereas ketoacidosis seldom occurs spontaneously, although it may arise during stress, infections or other illnesses.
Pathophysiology of Type 2 Diabetes
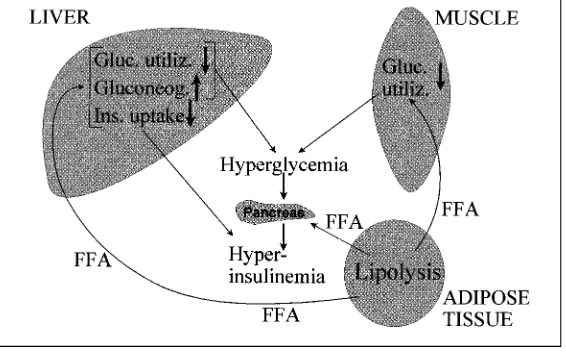
This disease is due to a varying combination of insulin resistance and reduction (especially in the late stage of the disease) in insulin secretion (see chapter II on Insulin Secretion and chapter III on Insulin Resistance). The metabolic alterations are less pronounced than those in type 1 diabetes, out-lined in figure 2 (see also chapter III on Insulin Resistance). Due to insulin resistance (and to enhanced counterregulatory hormones), there is increased HGO (which contributes primarily to fasting hyperglycemia) and reduced peripheral glucose utilization. There is also elevation of plasma FFA (resulting from activation of lipolysis and/or the often enhanced fat mass due to coexisting
obesity), which in turn contributes to insulin resistance through the mechanism of the glucose-FFA cycle. As mentioned above (under Type 1 Diabetes), hyper-glycemia itself favors glucose utilization (glucose effectiveness). This mecha-nism may be impaired in type 2 diabetes, i.e. ‘glucose resistance’ may be present. It has been observed that in obesity and type 2 diabetes (as well as in acromegaly and Cushing’s disease), in the postabsorptive period, noninsulin-mediated glucose uptake is a major determinant of glucose disposal and is similar in the different pathologies studied. On the other hand, although absolute rates of basal mediated glucose uptake are reduced in insulin-resistant states, they do not achieve statistical value compared with control subjects because of compensatory hyperinsulinemia.
Other Specific Types of Diabetes
Various, less common, types of diabetes are known to occur, in which the secretory defect is based upon different mechanisms.
Genetic Defects ofb-Cell Function
The maturity-onset diabetes of the young (MODY) is a genetically hetero-geneous monogenic form of noninsulin-dependent diabetes, characterized by early onset, usually before 25 years of age and often in adolescence or child-hood, and by autosomal dominant inheritance. There is no HLA association nor evidence of cell-mediated autoimmunity. It has been estimated that 2–5% of patients with type 2 diabetes may have this form of diabetes mellitus. However, the frequency of MODY is probably underestimated. Clinical studies have shown that prediabetic MODY subjects have normal insulin sensitivity but suffer from a defect in glucose-stimulated insulin secretion, suggesting that pancreaticb-cell dysfunction, rather than insulin resistance, is the primary defect in this disorder. To date, three MODY genes have been identified.
MODY-1. Studies in an affected family showed that the gene responsible for MODY-1 is tightly linked to the adenosine deaminase gene on chromosome 20q. Further research has shown that responsible for MODY-1 is a mutation in the gene-encoding hepatocyte nuclear factor (HNF)-4a, a member of the steroid/thyroid hormone receptor superfamily and an upstream regulator of HNF-1aexpression.
characterized by mild chronic hyperglycemia. The hyperglycemia due to GK deficiency is often mild (fewer than 50% of subjects have overt diabetes) and is evident during the early years of life. Despite the long duration of hyperglycemia, GK-deficient subjects have a low prevalence of micro- and macrovascular complications of diabetes. Obesity, arterial hypertension and dyslipidemia are also uncommon in this form of diabetes.
MODY-3. In several families, this form of MODY was found to be linked
with microsatellite markers on chromosome 12q. The disease was estimated to be linked to this chromosome region in approximately 50% of families in a heter-ogeneity analysis. It is the most common form of MODY. Affected patients ex-hibit major hyperglycemia with a severe insulin secretory defect, suggesting that the causal gene is implicated in pancreaticb-cell function. MODY-3 was further shown to be due to mutations in the gene-encoding HNF-1a(which is encoded by the gene TCF1). HNF-1ais a transcription factor that helps in the tissue-specific regulation of the expression of several liver genes and also functions as a weak transactivator of the rat insulin-I gene.
Familial Hyperinsulinemia. The high-affinity sulfonylurea receptor, a novel member of the ATP-binding cassette superfamily, is one component of the ATP-sensitive K+
channel. The protein is critical for regulation of insulin secretion from pancreaticb-cells, and mutations in the receptor (or in the KATP channels) have been linked to familial hyperinsulinemia, a disorder character-ized by unregulated insulin release despite severe hypoglycemia. Other forms may be due to mutation in the GK gene, leading to a hyperresponsive enzyme.
Other. In addition, a diabetes type associated with deafness may be linked to point mutations in mitochondrial DNA, and still other forms with less clearly defined defects are known to occur. In about 50% of cases of MODY, the genetic background is uncertain. It should be stressed that the role of the above genes (responsible for b-cell dysfunction) in the susceptibility to the more common late-onset form of type 2 diabetes remains uncertain. Genetic studies seem to exclude any function as major susceptibility genes, although they might play a minor role in a polygenic context or a major role in particular populations.
Rare Genetic Defects of Insulin Action
binding (type B syndrome). Interestingly, some cases have been reported in which antibodies to the receptor exert an agonistic effect, producing hypoglyce-mia. (b) Generalized or partial (face and trunk) lipodystrophies, which may be congenital or acquired, are characterized by fat depletion, and result from decrease in the number or affinity of the receptor for insulin or from postrecep-tor defects. Patients show high insulin levels, hyperglycemia (without ketoac-idosis for the scarcity of fat), hypertriglyceridemia (with eruptive xanthomas), enlargement of liver, spleen, heart, and hypertrophy of external genitalia. Lymphadenopathy and hirsutism may also occur as well as varicose veins, mental retardation and kidney involvement. In the congenital form, there is also muscle hypertrophy. (c) Leprechaunism syndrome, due to mutation in insulin receptors (which may be altered in both the a and b subunits and whose expression in the cell membrane is markedly reduced), and consisting of insulin resistance associated with severe growth retardation, elfin appearance of the face, hirsutism, absence of subcutaneous fat and thickened skin. (d) Other rare conditions such as the Werner’s syndrome, the Alstro¨m syn-drome, the Rabson-Mendenhall syndrome (which may be associated with acanthosis nigricans), the pineal hypertrophy syndrome, and the ataxia telan-giectasia syndrome.
Diseases of the Exocrine Pancreas
Any disease process affecting the pancreas may involve the islets and pro-duce diabetes (table 1). May we recall the fibrocalculous pancreatopathy, that occurs in India, Africa and West Indies with a frequency similar to that of type 2 diabetes. This form involves young people with malnutrition and pancreatic calculi, and is characterized by severe hyperglycemia and insulin dependence but not by proneness to ketosis, as a moderate insulin secretion is retained.
Gestational Diabetes mellitus (GDM)
Table 1.Etiologic classification of diabetes mellitus
1. Type 1 diabetes A. Immune-mediated B. Idiopathic
2. Type 2 diabetes
3. Other specific types
A. Genetic defects ofb-cell function (MODY-1, MODY-2, MODY-3, mitochondrial DNA, and others)
B. Genetic defects in insulin action (type A insulin resistance, leprechaunism, Rabson-Men-denhall syndrome, lipoatrophic diabetes, and others)
C. Diseases of the exocrine pancreas (pancreatitis, pancreatectomy, trauma, neoplasia, cystic fibrosis, hemochromatosis, fibrocalculous pancreatopathy, and others)
D. Endocrinopathies (acromegaly, Cushing’s syndrome, glucagonoma, pheochromocy-toma, hyperthyroidism, somatostatinoma, aldosteronoma, and others)
E. Drug- or chemical-induced diabetes (vacor, pentamidine, nicotinic acid, glucocorticoids, thyroid hormone, diazoxide,b-adrenergic agonists, thiazides, dilantin,a-interferon, and others)
F. Infections (congenital rubella, cytomegalovirus, and others)
G. Uncommon forms of immune-mediated diabetes (‘stiff-man’ syndrome, anti-insulin re-ceptor antibodies, and others)
H. Other genetic syndromes sometimes associated with diabetes (Down’s syndrome, Kline-felter’s syndrome, Turner’s syndrome, Wolfram’s syndrome, Friedreich’s ataxia, Hun-tington’s chorea, Lawrence-Moon-Biedl syndrome, myotonic dystrophy, porphyria, Prader-Willi syndrome, and others)
4. Gestational diabetes mellitus (GDM)
years after parturition. About 6 weeks after the delivery, the GDM woman should be reclassified as diabetic or glucose intolerant or normoglycemic.
Comment
‘NIDDM’ (which are confusing as they classified the patient according to treat-ment rather than etiology). (b) Preservation of the terms ‘type 1’ or ‘type 2’ diabetes (with Arabic numerals) and elimination of the confusing terms ‘type I’ or ‘type II’ diabetes (with Roman numerals); patients with no evidence of autoimmunity are classified as being affected by type 1 idiopathic diabetes. (c) Type 1 diabetes does not include those forms ofb-cell destruction due to nonautoimmune-specific causes. (d) Type 2 diabetes includes the most common form characterized by insulin resistance and insulin secretory defect. (e) The class previously named malnutrition-related diabetes mellitus has been elimi-nated. (f ) The IGT stage has been retained, and the stage of IFG was added. (g) GDM, as defined by WHO and NDDG, was retained.
Diagnostic Criteria for Diabetes mellitus
A precocious diagnosis of diabetes is important to prevent or attenuate late diabetic complication, and depends upon the adequate use and interpreta-tion of laboratory tests (especially in absence of specific symptoms). Many different diagnostic schemes have been in use. Recently, on the basis of the available data, the diagnostic criteria previously recommended by NDDG or WHO were modified. According to the revised criteria by the Expert Commit-tee [1997], the ‘normal values’ and the ‘diagnostic values’ for diabetes (which do not coincide with the goals of therapy) are as follows (values given in the text refer to venous plasma glucose which is the preferred measurement; equivalents for whole blood and capillary glucose estimations, according to the IDF guidelines [1999] to type 2 diabetes, are indicated in footnotes).
Normal Values. The upper limit of normal venous plasma values has been set at 110 mg/dl (6.1 mmol/l) for FPG and at 140 mg/dl (7.8 mmol/l) for the 2-hour value after glucose load (OGTT).
Diagnostic Values. (a) FPGq126 mg/dl (or 7.0 mmol/l)1
after a fasting of at least 8 h, confirmed on a subsequent day, to rule out a labeling or technical error; (b) 2-hour value during OGTT q200 mg/dl (orq11.1 mmol/l)2, con-firmed in a repeated test to make the final diagnosis; (c) symptoms of diabetes and a casual valueq200 mg/dl (or 11.1 mmol/l) at any time of day.
For epidemiological studies, diabetes prevalence and incidence should be estimated by a FPGq126 mg/dl. The value of FPG was changed from the
1Same value for capillary plasma glucose;q110 mg/dl (?6.0 mmol/l) for venous or
capillary whole blood glucose.
2q220 mg/dl (q12.2 mmol/l) for capillary plasma glucose;q180 mg/dl (q10.0 mmol/l)
previous value (q140 mg/dl) to current value (q126 mg/dl), because (1) the cutpoint of FPGq140 mg/dl defines a greater degree of hyperglycemia than did the cutpoint of the 2-hour value q200 mg/dl, and (2) this degree of hyperglycemia usually reflects a serious abnormality associated with serious chronic diabetic complications. The 2-hour value q200 mg/dl has been re-tained for the diagnosis of diabetes because it was well accepted, and enormous clinical and epidemiological data are based on this cutpoint value. The criteria for diagnosis of diabetes in an asymptomatic child should be stricter than those for the adults to avoid overdiagnosis of diabetes, and it should be considered that normal children commonly present OGTT values lower than adults. The diagnostic values for GDM as proposed by O’Sullivan and Mahan [1993], revised by NDDG and adopted by ADA and the American College of Obstetricians and Gynecologists (ACOG), are set lower than those for nonpregnant adults. A screening test is indicated between 24 and 28 weeks of gestation in asymptomatic female patients at risk, and a value 1 h after a 50 g of glucose load q140 mg/dl (or 7.8 mmol/l) can identify the individuals at risk for GDM in whom a full diagnostic 3-hour OGTT with 100 g of glucose should be performed. GDM occurs with an FPGq105 mg/dl (or 5.8 mmol/l) and a 2-hour value during OGTTq165 mg/dl (or 9.2 mmol/l).
An intermediate metabolic state was introduced, which is characterized by glucose levels above those considered as normal but below those accepted for the diagnosis of diabetes mellitus. Referring to the fasting state, this condition was named impaired fasting glycemia or IFG (FPG q110 but p126 mg/dl or q6.0 butp7.0 mmol/l )3
. Referring to the postload state, it was named impaired glucose tolerance or IGT (2-hour postload value in OGTT q140 mg/dl but p200 mg/dl or q7.8 but p11.1 mmol/l)4, without spontaneous hyperglycemia). IFG or IGT are not clinical entities but rather risk factors for future type 2 diabetes and cardiovascular disease, being associated with the metabolic syndrome or insulin resistance syndrome, charac-terized by abdominal or visceral obesity, hypertension, dyslipidemia (hypertri-glyceridemia and low HDL value) and hyperuricemia. Conversion of IGT to type 2 diabetes takes years or decades and occurs in about 10–50% of IGT patients. Thus, IGT may not progress to overt diabetes and may revert to normoglycemia, especially in obese patients after dietary treatment and weight reduction.
3Same value for capillary plasma glucose;q100 butp110 mg/dl (q5.5 butp6.0 mmol/l)
for venous or capillary whole blood glucose.
4q160 butp220 mg/dl (q8.9 butp12.2 mmol/l) for capillary plasma glucose;q120
but p180 mg/dl (q6.7 but p10.0 mmol/l) for venous whole blood glucose; q140 but
Table 2.Subjects in whom OGTT should be performed
First-degree relative of type 2 diabetic patients (especially if monozygotic twin of a diabetic patient or offspring of two diabetic parents)
Subjects with abnormal or borderline glycemic values (FPGq110 mg/dl butp126 mg/dl) during screening test for diabetes
Pregnant women with suspected GDM
Obese subjects (especially when a family history of diabetes is present) Individuals with a family history of MODY
Members of racial or ethnic groups with high prevalence of diabetes (American Indians or Pacific Islanders, African-Americans, Hispanics, etc.)
Patients with unexplained neuropathy or coronary disease or peripheral vascular disease or retinopathy or nephropathy (especially under 50 years of age)
Patients with hyperglycemia or glycosuria found during acute illness, stress situations, surgical procedures, steroid administration, etc.
Oral Glucose Tolerance Test
The OGTT is not recommended for routine clinical use (being a nonspe-cific test) and should be standardized for both procedure and interpretation, while the use of FPG is encouraged as a simple, convenient, accurate, acceptable to patients and low cost test for diagnosing diabetes. FPG and 2-hour OGTT values are equivalent for the diagnosis of diabetes (even if not perfectly corre-lated with each other), and actually the FPG alone is preferable for its better reproducibility (6% variation) whereas OGTT, repeated in adults during a 2-to 6-week interval, presents an intraindividual coefficient of variation of 17% for the 2-hour value. OGTT remains, however, the most sensitive and practical test for the early recognition of asymptomatic diabetes without high FPG value, and it is an invaluable tool in research studies. If the OGTT is used, the test procedures recommended are that of WHO. The indications of OGTT are outlined in table 2.
The following variables may affect the OGTT results:
Technical Variables. Venous versus capillary blood: In adults venous
blood from an antecubital vein is usually employed, obtained with minimum stasis. In the capillary blood, glucose approximates that of arterial blood, and is higher than in venous blood by 2–3 mg/dl in the fasting state and by 20–70 mg/dl during OGTT.
used when glucose is measured by enzymatic methods), which would result in artifactual low glucose values.
Methods for determining glycemia: The most commonly used methods are the glucose-specific enzymatic methods. The use of strips, read with glucose reflectance meter, is not recommended for diagnostic purpose (for its great variability) whereas it is useful for blood glucose self-monitoring during dia-betes treatment.
Glucose dose and concentration: In the past, glucose doses for OGTT varied from 50 to 100 g. To avoid nausea and to achieve a better standardization the use of an oral flavored solution of 75 g glucose dissolved in 300 ml of water for adults is now recommended. In children, 1.75 g/kg ideal body weight (up to a maximum of 75 g) should be used. During pregnancy, the OGTT is performed utilizing 100 g of glucose. The glucose solution should be consumed over 5 min. Timing of samples for OGTT: Blood samples are obtained in the fasting state and after 30, 60, 90, 120 min according to NDDG for testing individual patients. According to WHO, only 0- and 120-min samples should be used, which makes the test more suitable for testing large population groups or for epidemiological studies. During pregnancy, a 180-min sample should also be obtained. For the diagnosis of reactive hypoglycemia, the OGTT should be prolonged to 5 h.
Time of day: There is a diurnal variation in glucose tolerance (which deteriorates in the afternoon); thus, a standard OGTT should be obtained in the morning, after a fasting of 10–14 h.
Host Variables. Preceding diet: A diet containing 250 g of carbohydrate is recommended for at least 3 days before the test. In subjects on reduced diets, a diet containing at least 200 g of carbohydrates should be taken for 1 week before the OGTT. Coffee or smoking are avoided before and during the test.
Physical activity: OGTT should not be performed in patients at bed rest, hospitalized or immobilized (conditions which may reduce glucose tolerance). A moderate walking during the test is permitted, but physical exercise should be avoided.
Acute or chronic illness: OGTT should not be performed in patients affected by acute infections, acute cardiovascular and cerebrovascular diseases, active endocrinopathies, hepatic or renal diseases, or in subjects under stress or treated with some drugs such as glucocorticoids, estrogens, salicylates, thiazides, nicotinic acid, dilantin, etc.
Other Tests
Oral cortisone-glucose tolerance test is not a diagnostic test but is used for research purpose.
The intravenous glucose tolerance test (or IVGTT) should be used as a diagnostic test only for patients with gastrointestinal disorders interfering with absorption of glucose. It is less physiological than OGTT, bypassing the effects of several relevant gastrointestinal hormones active with oral glucose load. Glucose (25 g as 50% solution) is infused over 3 min and samples are obtained every 10 min for 1 h. Through a formula, the K coefficient can be calculated, whose normal value is between 1.2 and 2.2; values=1 indicate diabetes, values between 1 and 1.2 are regarded as borderline.
Determination of insulin during OGTT is not recommended for routine diagnostic purpose (because of extreme variability in fasting state and after glucose load), although it can be of prognostic value. Values are elevated in subjects with insulin resistance.
HbA1cmeasurement is not currently used for diagnosis of diabetes whereas it is useful in monitoring the metabolic control. Normal values of HbA1c range from 4.0–4.5 to 6.0–6.4% of total hemoglobin, although differences exist among values depending on laboratories and/or methods. According to the IDF guidelines [1999] to type 2 diabetes, HbA1ccan be useful for the diagnosis provided that confirmatory venous plasma glucose estimations are obtained, the assay is DCCT standardized, an HPLC chromatogram is reviewed for presence of abnormal hemoglobins, and erythrocyte turnover is not abnormal. Approximately: HbA1c ?7.5% B fasting plasma glucose q7.0 mmol/l (?125 mg/dl), and HbA1c ?6.5% B fasting plasma glucose ?6.0 mmol/l (q110 mg/dl).
Glycosuria is not useful for the diagnosis, being present only when glyce-mia is higher than the renal threshold for glucose. It may be useful for a coarse monitoring of diabetic control. The aged people may have a higher than normal renal threshold for glucose (having glycosuria only at elevated glucose levels), whereas pregnant women often have a lowered glucose threshold (show-ing glycosuria even with normal glycemia).
Testing for Diabetes mellitus
or delay the clinical onset of disease (when a positive autoantibody test is obtained); (c) the cost-effectiveness of the screening is questionable. The au-toantibody tests, however, may be useful to detect which newly diagnosed patients have immune-mediated type 1 diabetes.
Type 2 Diabetes. Type 2 diabetes is commonly undiagnosed in about 50%
of affected subjects. On the other hand, retinopathy may develop early, even 7 years before the diagnosis of overt diabetes. Thus, the unapparent hyperglyce-mia can cause microvascular complications and favor macrovascular disease. Therefore, the undiagnosed diabetes is a serious problem. Early detection and treatment are indispensable to reduce the late complications of type 2 diabetes. Thus, testing for diabetes (especially with FPG) should be recommended in the clinical setting and in high-risk subjects.
In asymptomatic and undiagnosed individuals, testing for type 2 diabetes by FPG should be performed in: (a) all individuals at age 45 and above, repeated at 3-year intervals if results are normal; (b) individuals at younger age if at risk (obese subjects, first-degree relatives of diabetic patients, compo-nents of high-risk ethnic populations, women with GDM, mothers of obese baby?9 lb or 4 kg, etc.); (c) hypertensive subjects with low HDL cholesterol (p35 mg/dl) or high triglycerides (q250 mg/dl); (d) individuals with IGT or IFG on previous testing.
Suggested Reading
Expert Committee on the Diagnosis and Classification of Diabetes mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes mellitus. Diabetes Care 1997;20:1183–1197. Fajans SS: Classification and diagnosis of diabetes; in Rifkin H, Porte D (eds): Diabetes mellitus. Theory
and Practice, ed 4. New York, Elsevier, 1990, pp 346–356.
International Diabetes Federation (IDF), 1998–1999 European Diabetes Police Group: A Desktop Guide to Type 2 (Non-Insulin-Dependent) Diabetes mellitus. Brussels, IDF, 1999.
National Diabetes Data Group: Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057.
O’Sullivan JB: Diabetes mellitus after GDM. Diabetes 1993;40(suppl):131–135.
Velho G, Blanche H, Vaxillaire M, et al: Identification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY-2 families. Diabetologia 1997;40:217–224.
World Health Organization: Diabetes mellitus: Report of a WHO Study Group. Tech Rep Ser No 727. Geneva, WHO, 1985.
Yamagata K, Furuta H, Oda N, et al: Mutations in the hepatocyte nuclear factor-4agene in maturity-onset diabetes of the young (MODY-1). Nature 1996;384:458–460.
Yamagata K, Oda N, Kaisaki PJ, et al: Mutations in the hepatocyte nuclear factor-1agene in maturity-onset diabetes of the young (MODY-3). Nature 1996;384:455–458.
F. Belfiore, Institute of Internal Medicine, University of Catania, Ospedale Garibaldi, I–95123 Catania (Italy)
Chapter II
Belfiore F, Mogensen CE (eds): New Concepts in Diabetes and Its Treatment. Basel, Karger, 2000, pp 20–37
. . . .
Insulin Secretion and Its
Pharmacological Stimulation
F. Belfiore, S. Iannello
Institute of Internal Medicine, University of Catania, Ospedale Garibaldi, Catania, Italy
Insulin Secretion
Introduction
Pancreatic b-cells synthesize a large polypeptide chain, the proinsulin, which is then cleaved into the so-called connecting peptide (C-peptide) and the insulin molecule, composed of two peptide chains containing 51 amino acid residues. Both insulin and C-peptide are packaged in the secretory gran-ules. During the secretory process, the granule content is discharged outside theb-cell through a process of exocytosis, leading to the release of insulin and C-peptide in equimolar amounts, together with small quantities of uncleaved proinsulin. In contrast to insulin, C-peptide is not taken up by the liver (and the other insulin-sensitive tissues), and therefore its plasma level is a good index of insulin secretion.
Regulation of Insulin Secretion by Substrates
Glucose
a period of 60 min of glucose perfusion. A similar biphasic pattern of secretory response to glucose has also been reported in vivo in man with the hyper-glycemic clamp technique. The two secretory phases, however, are not apparent after a carbohydrate-rich meal because the elevation in blood glucose is not rapid enough. Nevertheless, an efficient initial insulin secretory response (dependent upon theb-cell sensitivity to glucose elevation) is required for an optimal glucose control and for avoiding an excessive secretion during the second phase, which entails the risk of late hypoglycemia (reactive hypogly-cemia).
Glucose, besides its direct stimulation of insulin release, also potentiates the secretory response to nonglucose stimuli, which may play a role during the absorption of mixed meals. In addition, glucose exerts a priming effect ofb -cells, as a previous exposure ofb-cells to glucose causes an enhanced secretory response to a subsequent stimulation with glucose (or even with nonglucose stimuli), as if theb-cell has memory of the previous glucose exposure. Chronic exposure to glucose, however, induces desensitization of b-cells, which does not seem to be due to a reduced content or synthesis of insulin. This is relevant to the condition of persistent hyperglycemia occurring in the diabetic state.
With regard to insulin secretion, three concepts should be distinguished: the set point for blood glucose, the b-cell threshold for glucose, and the b -cell glucose sensor. The set point entails the concept that there is a control system that ‘sets’ the level of glucose at a given value, which in man is fixed to about 5 mmol/l glucose. The set point is the result of the activity ofb-cells as well as ofa-cells andd-cells.
period), whereas its presence in the b-cells allows these cells to perceive the increase in blood glucose and to respond with adequate insulin release.
In order to stimulate insulin release, glucose must first be transported into theb-cell by the glucose transporter (GLUT-2 isoform), and then phos-phorylated by GK to produce glucose-6-P. However, glucose transport inb -cells possesses a very high capacity and therefore plays a little regulatory role. Glucose-6-P produced by GK is further metabolized along several pathways, through which ATP is generated. Shortly, glucose metabolism results in eleva-tion of the ATP/MgADP ratio which inhibits ATP-sensitive K+
-channels, thus lowering membrane potential and triggering Ca influx through the voltage-dependent Ca2+-channels, which stimulates insulin secretion (fig. 1). Genetic alterations of key components of the insulin secretory machinery have been described. Mutations of KATP-channels or the associated sulfonylurea receptors may cause hyperinsulinemia and hypoglycemia due to persistent depolarization of theb-cell membrane. Mutations in the GK gene (most of which affect the glucose-binding site) may result in hyporesponsiveness to glucose, as it occurs in MODY-2 patients, or in hyperresponsiveness, as noted in the familial GK-linked hyperinsulinemia and hypoglycemia (FHI-GK).
Oscillations in the glycolytic pathway andb-cell metabolism contribute to the oscillatory nature ofb-cell ionic events and insulin secretion. Insulin release is a complex oscillatory process with rapid pulses (10 min) superimposed on slower circhoral oscillations (50–100 min). Moreover, ultradian oscillations of insulin secretion appear to be an integral part of the feedback loop between glucose and insulin secretion, and are abnormal in states of glucose intolerance.
Other Substrates
Fats also influence insulin release. In man, FFA were shown to enhance the secretory response to glucose, which is in agreement with the demonstration that pancreatic islets are equipped with the enzymes necessary for the utiliza-tion of FFA and ketone bodies. Amino acids such as isoleucine, arginine and lysine, potentiate the secretory effect of glucose, whereas leucine may be regarded as a primary stimulus, active even in the absence of glucose. Amino acids do not seem to act by serving as fuels for b-cells. They might act by contributing to activate Ca channels.
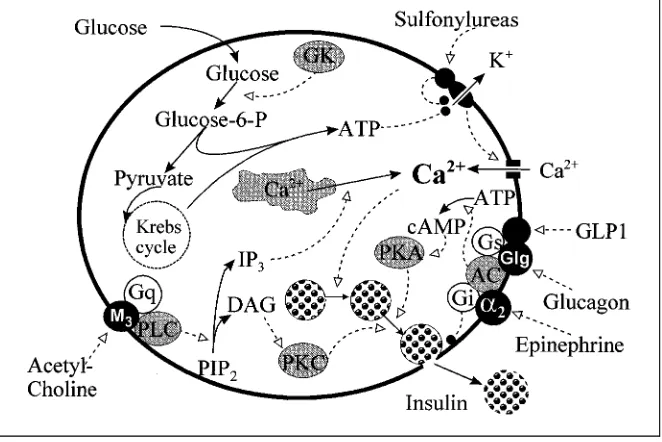
Fig. 1.Regulation of insulin secretion by theb-cell. (Continuous lines ending with black arrows indicate transformation or translocation of substrates or ions; dotted lines ending with white arrows indicate stimulation; dotted lines ending with filled circles indicate inhibition). Glucose metabolism (regulated by GK which acts as ‘glucose sensor’) results in production of ATP which inhibits ATP-sensitive K+
-channels, thus lowering membrane potential and triggering Ca influx through the voltage-dependent Ca2+
-channels. High cytosolic Ca stimu-lates (through complex processes, not shown) insulin secretion. Sulfonylureas stimulate insulin secretion by acting through their receptor, closely associated with the ATP-sensitive K+
-channels. Parasympathetic stimulation (acetylcholine) promotes insulin secretion through activation of PLC, which produces IP3and DAG (from PIP2); IP3causes release of Ca from
the intracellular stores (endoplasmic reticulum) into cytosol; DAG activates PKC which in turn stimulates secretion. Glucagon enhances secretion by activating AC (with the participa-tion of Gs), thus producing cAMP and activaparticipa-tion of PKA. Epinephrine (through thea2
-receptor) inhibits secretion by inhibiting AC (with the participation of Gi), thus exerting effects opposed to those of glucagon.
Abbreviations (alphabetic order):a2>a2-Adrenergic receptor; AC>adenylate cyclase;
cAMP>cyclic AMP; DAG>1,2-diacylglycerol; GK>glucokinase; Glg>glucagon receptor; GLP1>glucagon-like peptide 1; Gq>a further type of G protein; Gs and Gi>stimulatory and inhibitory G proteins; IP3>inositol-1,4,5-trisphosphate; M3>a type of muscarinic
re-ceptor; PIP2>phosphatidylinositol-4,5-P; PKA>protein kinase A; PKC>protein kinase C;
Regulation of Insulin Secretion by Hormones and Neurotransmitters
Acetylcholine, produced by parasympathetic activity, stimulates insulin secretion through muscarinic receptors (which can be blocked by atropine), probably by enhancing DAG (diacylglycerol) and IP3(inositol-3-P) formation (fig. 1). Parasympathetic stimulation may occur during the early (cephalic and intestinal) phase of insulin secretion following a meal as well as during hypoglycemic episodes. In the latter instance, however, hypoglycemia limits the parasympathetic effect on insulin secretion, because this effect is glucose-dependent. The parasympathetic innervation of the pancreas may also trigger the release of vasoactive intestinal polypeptide (VIP), which stimulates the secretion of insulin (and glucagon) while increasing the blood flow to the pancreas and the external pancreatic secretion.
Norepinephrine (released upon sympathetic stimulation) and epinephrine (produced by adrenal medulla) exert both an inhibitory effect, through thea -adrenergic receptors (fig. 1), and a stimulatory effect, through theb-adrenergic receptors, the overall effect being an inhibition of glucose-stimulated insulin release and a little effect in the basal state. Sympathetic nerve activity may also release other neurotransmitters, such as galanin, which would inhibit both basal and stimulated insulin secretion.
Gastrointestinal hormones (or gut hormones) contribute to the overall insulin secretion, as shown by the higher insulin secretion after glucose given per os compared to intravenous glucose. For this action, they are also called incretins. They include: the gastric inhibitory polypeptide (GIP), secreted by the endocrine cells of duodenum and jejunum; cholecystokinin (CCK), both the long (CCK-33) and the short (CCK-8) peptide chain, released by duo-denum and proximal part of jejunum after ingestion of fats and proteins; the glucagon-like peptide-1 (7–36) amide, or GLP-1 (7–36), formed from GLP-1 (the precursor proglucagon, produced by the L-cells in the distal part of small intestine, is processed by tissue-specific proteolysis to produce glucagon in pancreatica-cells and GLP-1 in the intestine), is released after carbohydrate-rich meals (fig. 1); the neuropeptide Y (NPY), a neurotransmitter present in both the central nervous system and the enteric nervous system which produces stimulation of food intake (and of resting metabolic rate), while probably acting as an incretin to enhance insulin release.
because capillaries go from the central part of the islets, where insulin is mainly produced, to the periphery of the islets where glucagon-producinga-cells are mainly located. The other stress hormones affect insulin secretion through a generally inhibitory action. The effect of epinephrine has been mentioned above. Cortisol and growth hormone (GH) are thought to play a role during prolonged stress periods.
Leptin may potentiate glucose-induced insulin secretion by a mechanism involving cAMP or phospholipase C/protein kinase C activation. It also in-hibits NPY release. In contrast to early studies, recent data indicate that amylin is a third active pancreatic islet hormone that works with insulin and glucagon to maintain glucose homeostasis. It would regulate glucose inflow to the circulation by influencing the rate of gastric emptying and would also inhibit hepatic glucose production in the postprandial period.
Assessment of Insulin Secretion
Fasting Insulin Level. The fasting insulin level (normally between 5 and 15lU/ml) may reflect the insulin secretory capacity. It may be very low (=5lU/ml) in subjects with high insulin sensitivity (lean and/or trained sub-jects) and elevated (?15lU/ml) in insulin-resistant subjects. It should be pointed out that an apparent normal insulin level in insulin-resistant diabetic subjects indicates decreased secretory capacity, since an equal level of glucose in a ‘normal’ subject would be associated with a higher insulin level which would promptly normalize glucose.
It should be pointed out that the insulin values usually referred to are those obtained with the commonly used radioimmunoassay method, which yields the total insulin levels, whereas more sophisticated methods are available that allow to distinguish the true insulin from the proinsulin. True insulin may be lower than total insulin by 15–20%.
Acute Insulin Response to Glucose(AIRG). AIRGfollowing glucose given
as intravenous bolus consists of a rapid increase in insulin level which returns towards normal within 10 min. The magnitude of AIRGis not affected by the preexisting glucose level, which makes this test feasible even in diabetic patients. AIRGis often absent in patients with type 2 diabetes whereas it is enhanced in insulin-resistant obese subjects.
Acute Insulin Response to Non-Glucose Stimuli(AIRNG). AIRNGincludes
of this curve, it is possible to deduce the insulin secretory capacity of the pancreas, the maximal acute insulin response to nonglucose stimuli or AIRMAX, and the b-cell sensitivity to the potentiation effect of glucose. The AIRMAX (which indicates the maximal secretory response) is reduced in type 2 diabetes and may increase in insulin-resistant hyperinsulinemic subjects. The b-cell sensitivity to the potentiation effect of glucose is little changed in type 2 patients, suggesting preservedb-cell sensitivity in these patients.
Insulin Secretion in Type 2 Diabetes
Both impaired insulin action (insulin resistance) and reduced insulin secre-tion (insulin deficiency) may contribute to the development of type 2 diabetes. It is now accepted that in type 2 diabetes the situation may range from predominantly insulin resistance with relative insulin deficiency to a predomi-nantly secretory defect with insulin resistance. It is noteworthy that recent data would suggest that the hyperinsulinemia of insulin resistance may result from an increase in insulin secretion secondary to increasedb-cell sensitivity to glucose, as well as a decrease in insulin clearance. In type 2 diabetes the
b-cell mass is reduced by about 50%, which is known from experimental pancreatectomy to be not enough to cause fasting hyperglycemia. Therefore, in most type 2 patients a functional defect in b-cells may occur, leading to insulin secretory defect. This is confirmed by the almost absent acute insulin response to glucose (AIRG), diminished maximal acute insulin response to nonglucose stimuli (AIRMAX), decreased insulin secretory capacity, with nor-malb-cell sensitivity to the potentiation effect of glucose.
from the elevated glucose values. In type 2 diabetes there is also an increased release of proinsulin, which may account for 30% of total insulin compared to 15% in normal subjects.
Concerning the insulin response to intravenous glucose, as occurs during IVGTT, in type 2 diabetes there is a marked reduction in the first phase of insulin release. The second phase may also be reduced in diabetic patients with fasting glycemia?250 mg/dl, but may be normal or increased in ‘compensated’ patients with fasting glycemia=200 mg/dl (even if also in these instances the insulin response should be regarded as diminished, considering the existing hyperglycemia). Reduced insulin response is also recorded during prolonged glucose infusion.
The insulin response to nonglucose stimuli, such as intravenous arginine, secretin, isoproterenol, isoprenaline, tolbutamide, or even a mixed meal, may be normal in type 2 diabetic patients with fasting glycemia=200 mg/dl. This is due to the potentiation of the insulin response to nonglucose stimuli exerted by the hyperglycemia present in the diabetic patients.
Finally, in type 2 diabetic patients the oscillations in insulin secretion, which are significant for glycemic control, cannot be detected, even in the patients with mild form of the disease.
Causes of the Insulin Secretory Defect
A major role is certainly played by genetic predisposition, but several biochemical mechanisms and neurohormonal factors may contribute. Little is known about susceptibility genes to the common polygenic forms of type 2 diabetes. Studies of genes involved in insulin secretion or insulin action have been successful to a certain extent by showing the implication of the insulin-receptor substrate-1 (IRS-1) gene, the ras associated with diabetes (rad) gene, the glucagon receptor gene, or the sulfonylurea receptor (SUR) gene (among others) in a low percentage of cases of type 2 diabetes in particular populations. However, the majority of susceptibility genes are still to be described.
Recently, an inherited or acquired defect of FAD-linked mitochondrial glycerophosphate dehydrogenase in b-cells has been proposed to contribute to the impairment of insulin release in type 2 diabetes.
Intravenous administration ofb-endorphins or naloxone to type 2 diabetic patients enhances both basal and OGTT stimulated insulinemia, which sug-gests a possible pathogenetic role of these compounds in the dysfunction of
b-cells.
glucose potentiation after infusion of sodium salicylate (inhibitor of prosta-glandin synthesis). A similar effect has been observed with the a-adrenergic blocking agent phentolamine, which suggests a role of thea-adrenergic system. It has also been suggested that galanin and pancreostatin, peptides which inhibit insulin secretion, may be increased in the pancreatic islets of type 2 diabetic patients. Finally, hyperglycemia, once established, may contribute to aggravate theb-cell dysfunction, through several mechanisms most of which are included in the concept of ‘glucotoxicity’. The glucotoxicity concept may help to explain the beneficial effect on insulin secretion obtained in type 2 diabetic patients after adequate treatment achieving glycemic control as well as the transient improvement in theb-cell function which may occur in type 1 diabetic patients after therapeutical control of hyperglycemia (‘honeymoon’ phenomenon).
It has been proposed that at least one factor contributing to the pathogen-esis of type 2 diabetes is desensitization of the GLP-1 receptor onb-cells. At pharmacological doses, infusion of GLP-1, but not of GLP, can improve and enhance postprandial insulin response in type 2 patients. Agonists of GLP-1 receptor have been proposed as new potential therapeutic agents in type 2 diabetic patients.
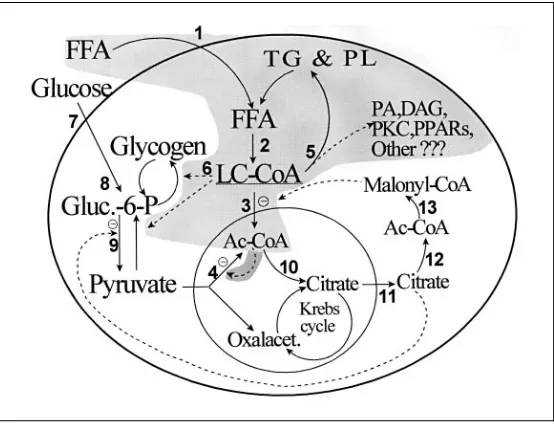
It should also be emphasized that complex alterations of glucidic and lipidic metabolism in theb-cells may play a role. In particular, in obese/diabetic hyperinsulinemic subjects, LC-CoA derived from the enhanced availability of FFA may affect the b-cells’ secretory response according to the following mechanism: as the glycemic level increases, the b-cells utilize more glucose; this leads to enhanced production of malonyl-CoA, which blocks the intrami-tochondrial transport of LC-CoA, which therefore accumulates in the cytosol and (through its complex biological effects) stimulates insulin secretion (see also chapter III and figure 3 for details).
Altered expression of genes encoding enzymes in the pathway of malonyl-CoA formation and FFA oxidation contributes to the b-cell insensitivity to glucose in some patients with type 2 diabetes. Clearly, the detrimental impact of diabetic hyperlipidemia onb-cell function has been a relatively neglected area, but future pharmacological approaches directed at preventing ‘lipotox-icity’ may prove beneficial in the treatment of diabetes.
Insulin Secretion in Other Types of Diabetes
Pharmacological Stimulation of Insulin Secretion
Insulin Secretion as Modified by Sulfonylureas
The main drugs able to stimulate insulin secretion are the sulfonylureas. These compounds have been used in the management of type 2 diabetes since 1955 and, when properly utilized, are easy to use and appear to be effective and safe. It is estimated that 30–40% of diabetic patients are taking oral sulfonylureas. Indications and contraindications for sulfonylureas are shown in tables 1 and 2, respectively.
Table 1.Patients candidate for sulfonylurea treatment
Most patients with type 2 diabetes, not well controlled with dietary restriction and exercise Children and adults with the MODY (maturity-onset diabetes of youth) type of diabetes Obese-diabetic patients with marked insulin resistance
Lean type 2 diabetic patients with preserved insulin secretory capacity
Table 2.Contraindications to sulfonylurea treatment
Patients with type 1 diabetes Patients with pancreatic diabetes
Patients with an acute illness or stress or undergoing surgery Patients with hepatic or liver diseases
Patients predisposed to hypoglycemia: Underweight or malnourished Elderly
Diabetic pregnancy: Potential teratogenicity Perinatal mortality
Severe neonatal hypoglycemia Diabetic female patients during lactation
Patients with a history of severe adverse reactions to sulfonylureas
Different Sulfonylureas
Table 3.Main characteristics of sulfonylureas
Compound Dose, mg/day Doses q.d. Duration of Metabolism/
hypoglycemic excretion effect, h
First generation
Acetohexamide 250–1,500 1–2 12–18 Liver/kidney
Tolbutamide 500–3,000 2–3 6–12 Liver
Chlorpropamide 100–150 1 60 Kidney
Tolazamide 100–1,000 1–2 12–14 Liver
Second generation
Glibenclamide (or glyburide) 1.25–20 1–2 16–24 Liver/kidney
Glyburide, micronized 0.75–12 1–2 12–24 Liver/kidney
Glipizide 2.5–40 1–2 12–24 Liver/kidney
Gliclazide 80–320 1–3 10–20 Liver/kidney
Gliquidone 30–120 1–3 6–12 Liver
Glimepiride 1–8 1 D24 Kidney
Repaglinide1 0.5–16 1–4 4–6 Liver
1Repaglinide is a nonsulfonylurea hypoglycemic agent of the meglitinide family.
have a short duration of action (6 h), others, such as chlorpropamide, have a long duration of action (up to 60 h), several others show an action of inter-mediate duration. Some characteristics of the sulfonylureas which are or have been in clinical use are summarized in table 3.
‘First-Generation’ Sulfonylureas. Tolbutamide has a ‘short’ duration of action (see table 3) and is carboxylated by the liver to a totally inactive derivative. Being metabolized only in the liver, this compound may be useful in nephropathic diabetic patients.
Tolazamide has a more potent hypoglycemic activity than tolbutamide and an ‘intermediate’ duration of action (see table 3). It is metabolized only by the liver with the production of some very little active metabolites excreted in the urine (85%). It is safer in the elderly and in nephropathic diabetic patients. Tolazamide also has a diuretic action.
such as alcohol-induced flushing, occasional hypersensitivity reactions as well as water retention and hyponatremia (due to sensitization of renal tubules to antidiuretic hormone).
Acetohexamide has a more potent hypoglycemic activity than tolbutamide and an intermediate duration of action. It is reduced by the liver to 1-hydroxy-hexamide which is a potent hypoglycemic drug, excreted by 60% in the urine. Thus, it is contraindicated in the elderly and in nephropathic diabetic patients. Acetohexamide also has diuretic and uricosuric actions.
‘Second-Generation’ Sulfonylureas. Glyburide or glibenclamide has been used since 1969. It has a 50–100 times more potent hypoglycemic activity than the ‘first-generation’ drugs and has a relatively long duration of action. It is metabolized by the liver to several both inactive and mildly active metabolites, excreted partially in the urine (50%) and partially in the bile (50%). It may induce severe hypoglycemic episodes and is contraindicated in the elderly and in nephropathic diabetic patients. Glyburide absorption is not affected by food but it takes 30–60 min to achieve adequate plasma levels, so that this drug should be taken before the morning meal.
Glipizide has been used since 1973, has a 50–100 times more potent hypoglycemic activity than the ‘first-generation’ drugs (comparable to that of glyburide) and has an ‘intermediate’ duration of action (see table 3). It is metabolized by the liver to several inactive metabolites, excreted in the urine (by 68%) and in the feces (by 10%). It may induce severe hypoglycemic episodes (similarly to glyburide) and is contraindicated in the elderly and in nephro-pathic diabetic patients. The absorption of glipizide is delayed by about 30 min when it is ingested with a meal, so that it is recommended to take the drug 30 min before meals. Glipizide has a greater effect than glyburide in raising postprandial plasma insulin level and lowering postprandial plasma glucose level while glyburide has a better effect than glipizide in raising fasting insuline-mia and reducing fasting glyceinsuline-mia (probably, reducing fasting hepatic glucose production). For this metabolic difference, a ‘combined’ administration of the two sulfonylureas was suggested.
Gliclazide has a potent hypoglycemic activity (comparable to that of glyburide and glipizide) and has an ‘intermediate’ duration of action. It is metabolized by the liver to several probably inactive metabolites, excreted in the urine (by 60–70%). It has been suggested that gliclazide exerts antiplatelet aggregating activity, with a potential preventing effect on diabetic microangi-opathy, although this effect has not been confirmed.
and in nephropathic diabetic patients. A newly developed sulfonylurea, glimepi-ride, has been reported to have a more potent hypoglycemic action than glibenclamide while its ability to stimulate insulin secretion is much weaker, possibly due to less stimulation of insulin secretion and more pronounced extrapancreatic effects. It is effective at lower dosage, has a more rapid onset of action than glibenclamide and a long duration of action. There is increased plasma elimination of glimepiride with decreasing kidney function, which is explainable on the basis of altered protein binding with an increase in unbound drug.
Efficacy and Interactions. Good response with sulfonylureas will occur in 70–75% of patients during the first years of treatment, provided that the patient selection is appropriate. Primary failure occurs in 15–25% of cases and may depend on a poor selection of the patients (unrecognized type 1 diabetic patients treated with sulfonylureas). Chronic therapy may be associ-ated with progressively less beneficial effects (secondary failure), sometimes as result of intercurrent factors which impair insulin action and secretion (such as stress, infections, dietary disregard, etc.) (see also chapter III on Insulin Resistance and Its Relevance to Treatment). The response to the hypoglycemic drugs may be restored with the disappearance of the intercur-rent event. All sulfonylureas are bound to serum albumin and, since a large number of drugs may compete for ionic binding sites on albumin, sulfonylu-reas can influence the effect of many drugs (and these drugs, conversely, can influence the effect of sulfonylureas). The physician must understand potential interactions with a number of commonly used drugs, that may significantly alter the activity of the sulfonylureas both diminishing (diuretics,
b-blockers, corticosteroids, estrogens, indomethacin, alcohol, rifampicin, etc.) or increasing (sulfonamides, salicylates, clofibrate, chloramphenicol, MAO inhibitors, probenecid, allopurinol, b-blockers, alcohol, etc.) their hypogly-cemic effect. It is noteworthy that some drugs (such as b-blockers and alcohol) can alter sulfonylureas activity in opposite directions. Sulfonylureas of ‘second generation’ may have less interactions than those of the ‘first generation’.
Some data of literature demonstrate that serum levels of sulfonylureas (tolbutamide, chlorpropamide, glyburide and gliquidone) in treated diabetic patients show extremely interindividual variations, with no correlation between the dose and the plasma level.
Mechanism of Sulfonylurea Action
direct effect, as unquestionably proven by studies with perfused pancreases, isolated perifused islets and cultures ofb-cells.
Available data suggest that sulfonylureas bind to a specific receptor (closely associated with the ATP-sensitive K+
-channels) on the outside of plasma membrane of theb-cells. Recent studies with human pancreatic islets showed that3H-glibenclamide binds to saturable sites in islet membrane preparations in a linear fashion. This binding was both temperature- and time-dependent. Scatchard analysis of the equilibrium binding data indicated the presence of a single class of saturable, high-affinity binding sites. The displacement experiments showed the following rank order of potency of the oral hypogly-cemic agents tested: glibenclamide>glimepiride?tolbutamide? chlorprop-amide A metformin. This binding potency order was parallel with the insulinotropic potency of the evaluated compounds. Glimepiride has been reported to bind to a 65-kDa subunit of the sulfonylurea receptor. This charac-teristic may entail a minor effect of the K-channel in other tissues, such as myocardium (where the closure of K-channels may interfere with the repolar-ization process).
Upon binding to their receptors, sulfonylureas inhibit the K+
-channels, diminish K+
efflux and cause depolarization of the plasma membrane. This depolarization induces voltage-dependent Ca2+
-channels to open and extracel-lular Ca2+to enter the cell. Increased cytoplasmic Ca2+stimulates the fusion of the secretory granule membrane with cell membrane, followed by extrusion of insulin outside the cell (exocytosis) (see also fig. 1).
Metabolic studies demonstrate that sulfonylureas stimulate the first phase of insulin release and have little effect on the second phase. They can act in the absence of glucose but also may potentiate glucose-mediated insulin release. As consequence of the stimulation of secretion, sulfonylureas can induce mor-phological alterations of theb-cells such as degranulation, loss of zinc and aspects of emiocytosis.
Table 4.Extrapancreatic effects of sulfonylureas
Hormonal effects
Potentiation of insulin action on skeletal muscle and adipose tissue glucose transport Potentiation of insulin action on hepatic glucose production (activation of glycogen synthase
and glycogen synthesis)
Decrease of hepatic insulin extraction
Decrease of insulin degradation (inhibition of insulinase activity) Stimulating effect on gastrointestinal hormone release
Direct metabolic effects
Insulin receptors (partial restoration of their number in plasma membrane in type 2 obese-diabetic patients)
Liver (increase in fructose 2,6-bisphosphate; increase in glycolysis; decrease in gluconeogen-esis; decrease in long-chain fatty acid oxidation)
Skeletal muscle (increase of glucose and amino acid transport; increase of fructose 2,6-bisphosphate)
Myocardial tissue (increase of contractility; increase of oxygen consumption; increase of glycogenolysis; decrease of Ca2+
-ATPase; increase of glucose transport and glycolysis; increase of phosphofructokinase activity and pyruvate oxidation)
Adipose tissue (increase in glycogen synthase; inhibition of lipolysis, increase in glucose transport)
Platelet arachidonic acid metabolism (inhibition of cycloxygenase and 12-lipoxygenase path-ways)
Other Effects. Sulfonylurea treatment does not appear to stimulate proin-sulin biosynthesis. On the other hand, studies performed with in vivo and in vitro animal perfused pancreases, or with isolated perifused islets and islet-cell cultures, reported an acute and chronic sulfonylurea-induced inhibition of the biosynthesis of proinsulin (through unknown mechanisms). Sulfonylureas, acutely or chronically, do not alter glucagon secretion both in normal subjects and diabetic patients. Sulfonylureas appear to stimulate pancreaticd-cell soma-tostatin release (with unclear physiological effect).
Table 5.Sulfonylurea side or toxic effects
Hematologic reactions (agranulocytosis, bone marrow or red cell aplasia, hemolytic anemia) Skin reactions (rash, pruritus, erythema, purpura, photosensitivity)
Hypersensitivity reaction (rush, fever, arthralgia, angiitis, jaundice, etc.)
Alcohol-induced flushing (most frequently associated with chlorpropamide treatment) Gastrointestinal complaints (nausea, vomiting, jaundice or hepatitis or cholestasis) Antithyroid activity
Diuretic effect or antidiuresis with hyponatremia
Cataract formation (reported in some dogs treated with high doses of glimepiride) Teratogenicity
Side or Adverse Effects of Sulfonylureas. The most important adverse effect of sulfonylureas is hypoglycemia which, although occurring less often than with insulin, when it occurs it tends to be more severe, prolonged and sometimes fatal. The incidence of sulfonylurea-induced hypoglycemia is 0.19–4.2/1,000 treatment years (compared to 100/1,000 patients/year for insulin-induced hy-poglycemia) and is most frequent in patients taking long-acting drugs (such as glyburide and chlorpropramide) which, for this reason, should be avoided in patients with predisposing conditions (the best treatment of hypoglycemia is prevention). The case fatality rate of hypoglycemia induced by sulfonylureas is 4.3% (see also chapter VIII on Clinical Emergencies in Diabetes. 2: Hypogly-cemia). It is noteworthy that sulfonylureas predispose to hypoglycemia during and after exercise. In this regard, it has been claimed that glimepiride maintains a more physiological regulation of insulin secretion during physical exercise, with less risk of hypoglycemia.
Other sulfonylurea side effects or toxic reactions occur at low rate (1.5% for glyburide) (table 5) and appear within the first 2 months of treatment. The chlorpropamide alcohol flushing (CPAF), occurring in 30–40% of type 2 and 10% of type 1 diabetic patients, is linked to a genetic predisposition to diabetes development (autosomic trait) and can be considered a good genetic marker of type 2 diabetes mellitus.
Other Drugs Modifying Insulin Secretion
sites different from those of sulfonylureas (two binding sites have been identi-fied). Repaglinide lowered fasting and postprandial blood glucose levels in animals, healthy volunteers and patients with type 2 diabetes mellitus. Repagli-nide is rapidly absorbed and eliminated, which may allow a relatively fast onset and offset of action. Excretion occurs almost entirely by nonrenal mecha-nisms. In comparative clinical trials in patients with type 2 diabetes mellitus, repaglinide 0.5–4 mg twice or 3 times daily before meals provided similar glycemic control to glibenclamide (glyburide) 2.5–15 mg/day. Addition of repaglinide to existing metformin therapy resulted in improved glycemic con-trol. In contrast with glibenclamide, use of repaglinide allowed patients to miss a meal without apparently increasing the risk of hypoglycemia.
GLP-1 has insulinotropic action, which may explain the increased insulin response after oral compared to intravenous glucose administration, and exerts several other functions such as reduction of glucagon concentration, reduction of gastric emptying, stimulation of proinsulin biosynthesis and reduction of food intake (upon intracerebroventricular administration in animals). On these grounds, GLP-1 seems to offer an interesting perspective in treatment of diabetic patients. The observations that GLP-1 induces both secretion and production of insulin, and that its activities are mainly glucose-dependent, led to the suggestion that GLP-1 may present a unique advantage over sulfonylurea drugs in the treatment of type 2 diabetes. This peptide is able to lower and perhaps normalize fasting hyperglycemia and to reduce postprandial glycemic increments (especially in type 2 diabetic patients) but its usefulness is not completely established. Due to rapid proteolytic cleavage, the half-life of GLP-1 is too short for therapeutical use with subcutaneous injections. GLP-1 analogues with different pharmacokinetic properties (or some preparations that could be orally administered) are in development. Given the large amount of GLP-1 present in L-cells, it appears worthwhile to look for some agents that could ‘mobilize’ this endogenous pool of the ‘antidiabetogenic’ gut hormone GLP-1. Interference with sucrose digestion usinga-glucosidase inhibition moves nutri-ents into distal parts of the gastrointestinal tract and, thereby, prolongs and augments GLP-1 release.
Antiarrhythmic agents with Vaughan Williams class Ia action have been found to induce a sporadic hypoglycemia. Recent investigation has revealed that these drugs induce insulin secretion from pancreaticb-cells by inhibiting ATP-sensitive K+