www.elsevier.com / locate / bres
Short communication
Co-induction of HSP70 and heme oxygenase-1 in macrophages and
glia after spinal cord contusion in the rat
a b ,
*
Angelika E.M. Mautes , L.J. Noble
a
Neurosurgical Research Laboratory, Saarland University Medical School, Saarland, Germany
b
Department of Neurological Surgery, University of California, C224, 521 Parnassus Avenue, San Francisco, CA 94143-0520, USA
Accepted 15 August 2000
Abstract
HSP70 and heme oxygenase-1 (HO-1) are thought to be markers of cell injury and oxidative stress, respectively. We have immunolocalized these proteins in the spinal cord at 1–14 days after contusion. HSP70 and HO-1 were co-induced in glia and macrophages within the injured segment at all time points. This co-induction may reflect complementary functions that serve to protect these cells as they respond to the postcontusional environment. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Trauma
Keywords: Heat shock protein; Spinal cord contusion; Microglia / macrophages; Astrocyte
Heme oxygenase (HO), an essential enzyme in heme pathological conditions including ischemia [7,24], catabolism, catalyzes heme to iron, biliverdin, and carbon traumatic brain injury [5,23], exposure to lysed blood monoxide [14]. We have previously demonstrated expres- [16,30], and opening of the blood–brain barrier [28]. sion of heme oxygenase-1 (HO-1), the inducible HO, in HSP70 may likewise be induced in glia and macrophages microglia / macrophages and astrocytes in the injured spinal since the contused spinal cord incorporates many of these cord [18]. This induction was most pronounced within the pathologic features.
injured segment within the first week post injury. Although Male, Sprague–Dawley rats were anesthetized with 4% the nature of this induction is unclear, likely candidates chloral hydrate (0.9 cc / 100 g body weight) intraperitoneal-include heme, denatured proteins, and oxidative stress ly and subjected to a moderate spinal cord contusion injury [25]. as previously described [10]. Animals were re-anesthetized In the present study, we determine if induction of HO-1 and perfused with fixative (4% paraformaldehyde in 0.1 M in glia and macrophages coincides with the induction of phosphate buffer, pH 7.4) at 1 day (N53), 3days (N53), 7 HSP70. HSP70 has been shown to be a suitable molecular days (N53) and 14 days (N53) after injury. Surgical marker of reversible cell injury and thus serves as a useful controls consisted of uninjured animals (N52) and animals tool for characterizing cell responses [8,16]. In addition, that were subjected to laminectomy and euthanized at 1 HSP70 protects cells by binding to denaturing proteins and day post laminectomy (N52).
preventing their further denaturation [2,25]. This function A 12 mm length of cord, centered over the contused site, may be critical to those cells that survive the initial was postfixed for 4 h, cryoprotected in sucrose (30%), and traumatic insult and are actively responding to dying cells frozen. Parasagittal sections, 14mm in thickness, were cut and intraparenchymal hemorrhage. Induction of HSP70 has in a cryostat, mounted on slides, and dried overnight. been reported in glia and / or macrophages after various Alternate sections were incubated in either normal goat
or sheep and then sequentially in the following; rabbit anti-HO-1 for 12–24 h (Stress Gen, Victoria, BC, Canada,
*Corresponding author. Tel.: 11-415-476-4850; fax: 1
1-415-476-1:40,000 in goat serum / Triton X-100 / bovine serum
al-5634.
E-mail address: [email protected] (L.J. Noble). bumin, GS / TX / BSA), biotinylated goat anti-rabbit IgG
234 A.E.M. Mautes, L.J. Noble / Brain Research 883 (2000) 233 –237
(1:200 in GS / TX / BSA, Vector Laboratories, Burlingame, fiber tracts after spinal cord hemisection [19]. Both HSP70 CA), Texas Red avidin D (1:100 in BBS, Vector Labs), and HO-1, also known as HSP32, are members of the mouse antiHSP (1:3000 in sheep serum / Triton X-100 / stress protein superfamily of multifunctional proteins that bovine serum albumin, SS / TX / BSA, Amersham, Life are induced by a variety of stresses and injuries that Science Inc. Arlington Heights IL) for 36 h, biotinylated denature proteins [4,11,21]. Induction of these stress sheep anti-mouse IgG (1:200 in SS / TX / BSA; Amersham), proteins may be a critical feature for the proper functioning fluorescein isothiocyanate (1:100). Sections were mounted, of these cells in a hostile microenvironment, by acting as coverslipped with mounting medium, and viewed with a ‘molecular chaperones’ or ‘polypeptide binding proteins’. Nikon Optiphot microscope equipped with epi-fluores- They protect proteins from improper folding and / or im-cence. The HSP antibody recognizes only HSP70 and not proper interactions with each other before their synthesis is HSC70, the heat shock cognate [12]. The HO-1 antibody completed and thereby maintain their translocation compe-was originally prepared using purified rat liver HO-1 as the tence [2].
immunogen and characterized by Western immunoblot Heme may be an important inducer of both HSP70 and [15]. HO-1. Heme is not only present in the injured cord as a HO-1 was co-localized with either the complement 3 consequence of intraparenchymal hemorrhage but also receptor type 3 or glial fibrillary acidic protein (GFAP) in accumulates when dying cells release their hemoproteins alternate sections using procedures described in the preced- [25]. Heme can induce HSP70 via the heat shock element ing paragraph and mouse anti-OX42 (1:3000 in SS / TX / as well as HO-1 via the metal regulatory element [29]. BSA; Serotec, Kidlington, OX, UK) or mouse anti-GFAP Induction of HSP70 in macrophages may be integral to (1:1000 in SS / TX / BSA, ICN, Costa Mesa, CA). their role as phagocytes. HSP70 is synthesized by macro-Immunocytochemical controls consisted of the same phages in response to the challenge of an oxidative burst reaction procedures described above, in the absence of [9]. After spinal cord contusion there is a massive accumu-primary antibody and preabsorption controls. The accumu-primary lation of macrophages at the lesion site [18,22]. A primary antibody was co-incubated with the purified HO-1 (0.005– role of these macrophages is to phagocytose the cellular 0.5 mg / ml, Stress Gen) and HSP70 (0.02 mg / 1 ml), debris. During this process oxidative metabolism is rapidly respectively, 30 min prior to addition to the tissue section. increased. As a result, macrophages generate H 0 through2 2 No staining was observed in sections when the primary the respiratory burst enzyme nicotinamide adenosine di-antibody was either omitted or incubated with purified nucleotide phosphate dehydrogenase. This phagocytosis is protein. There was limited expression of HSP70 in neuro- accompanied by induction of HSP70 [9], and can be nal populations, particularly ventral horn motoneurons, in further enhanced by additional iron. Iron is a crucial factor uninjured animals and animals that were subjected to in oxidative injury because it catalyzes the Fenton reaction. laminectomy only. No glial expression, however, was In this scenario HSP70 may serve to protect the macro-noted. phage against autoxidative damage associated with the
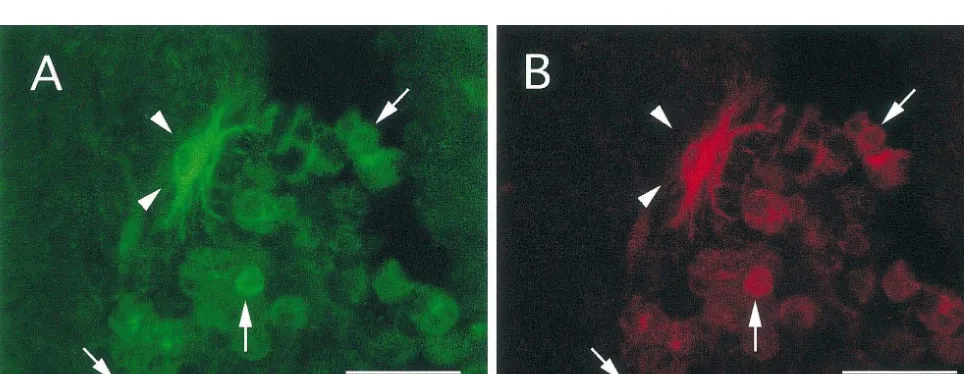
There was consistent co-induction of HSP70 and HO-1 respiratory burst.
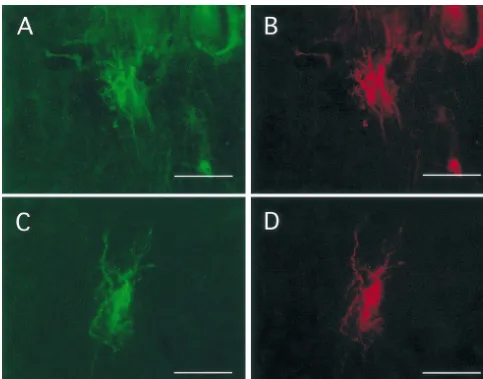
after spinal cord injury. All cells that expressed HSP70 The induction of HSP70 in macrophages may also be a also expressed HO-1. Similarly, all cells that expressed consequence of their involvement in the inflammatory HO-1 likewise expressed HSP70. Based upon double response [1,32]. There are markedly elevated levels of immunolabeling, this co-induction was limited to micro- endogenous proinflammatory mediators including TNFal-glia / macrophages and astrocytes. Co-induction in micro- pha and interleukines in the traumatized spinal cord glia / macrophages was identified at all time points and was [27,34]. Macrophages are one source of these cytokines in most prominent within the lesioned site (Fig. 1). This the injured cord. Cytokines are known to induce HSP70 in co-induction dominated in cells with a macrophage pheno- monocytes and thus may function in a similar manner in type, namely, large round cells with no processes. the macrophage [6]. HSP70 serves as a negative feed back Few to no astrocytes were identified within the lesioned loop in cytokine production. It activates heat shock tran-site at all time points. Co-induction in astrocytes was most scription factor which results in the inhibition of NfkappaB apparent from 3 to 14 days post injury and was most [31,33], a transcription factor that is central to cytokine prominent in a penumbral zone, surrounding the lesioned production. NfkappaB has been immunolocalized in acti-site (Fig. 2). In addition, there were limited numbers of vated microglia / macrophages after spinal cord contusion astrocytes in both grey and white matter that expressed [3]. Thus, induction of HSP70 may lead to the downregu-both proteins along the axis of the cord (Fig. 3). lation of cytokines [26].
Fig. 1. Co-induction of HSP70 (A, C) and HO-1 (B, D) within the lesioned segment. HSP70 is co-localized with HO-1 in macrophages at 3 (A, B) and 14 days (C, D) post injury. A,C, Fluorescein label, B,D Texas Red label. Scale bar50.02 mm.
236 A.E.M. Mautes, L.J. Noble / Brain Research 883 (2000) 233 –237
Fig. 3. Co-induction of HSP70 (A, C) and HO-1 in segments remote from the injured site at 3 days post injury. HO-1 and HSP70 are co-expressed in astrocytes in white matter at approximately 5 mm rostal (A, B) and 5 mm caudal (C, D) to the injured site. A, B, Fluorescein label, B, D Texas Red label. Scale bar50.02 mm.
cord barrier, and metabolic alterations [17,20,22]. As- cells that actively participate in phagocytosis, breakdown trocytes play a central role in the resolution of edema and of heme, and resolution of edema.
function in the removal of denatured proteins [13]. Induc-tion of HSP70 in these cells may occur via the heat shock factor or the serum responsive element in response to
Acknowledgements
extravasated, denatured proteins or serum, respectively. Metabolic stress may also induce HSP70 in astrocytes.
This research was supported by NS23324 and NS39278. In vitro studies have demonstrated that HSP70 is induced
in astrocytes when challenged by metabolic stress pro-duced by oxygen–glucose deprivation. Metabolic stress
likewise exists in the injured spinal cord where ATP levels References significantly decline in segments adjacent to a contusion
[17]. [1] M. Bachelet, C. Adrie, B.S. Polla, Macrophages and heat shock proteins, Res. Immun. 149 (1998) 727–732.
In summary, we demonstrate the co-induction of HSP70
[2] R.P. Beckmann, L.E. Mizzen, W.J. Welch, Interaction of Hsp 70 with
and HO-1 in macrophages and glia after spinal cord injury.
newly synthesized proteins: implications for protein folding and
This co-induction may be a consequence of a number of
assembly, Science 248 (1990) 850–854.
factors including denatured proteins and heme which [3] J.R. Bethea, M. Castro, R.W. Keane, T.T. Lee, W.D. Dietrich, R.P. accumulate in the injured cord. Together, HSP70 and Yezierski, Traumatic spinal cord injury induces nuclear
[4] I.R. Brown, S. Rush, G.O. Ivy, Induction of a heat shock gene at the [20] L.J. Noble, J.R. Wrathall, Distribution and time course of protein site of tissue injury in the rat brain, Neuron 2 (1989) 1559–1564. extravasation in the rat spinal cord after contusive injury, Brain Res. [5] S.A. Dutcher, B.D. Underwood, P.D. Walker, F.G. Diaz, D.B. 482 (1989) 57–66.
Michael, Patterns of heat-shock protein 70 biosynthesis following [21] H.R. Pelham, Speculations on the functions of the major heat shock human traumatic brain injury, J. Neurotrauma 15 (1998) 411–420. and glucose-regulated proteins, Cell 46 (1986) 959–961.
[6] G. Fincato, N. Polentarutti, A. Sica, A. Mantovani, F. Colotta, [22] P.G. Popovich, P. Wei, B.T. Stokes, Cellular inflammatory response Expression of a heat-inducible gene of the HSP70 family in human after spinal cord injury in Sprague–Dawley and Lewis rats, J. Comp. myelomonocytic cells: regulation by bacterial products and cyto- Neurol. 377 (1997) 443–464.
kines, Blood 77 (1991) 579–586. [23] R. Raghupathi, F.A. Welsh, D.H. Lowenstein, T.A. Gennarelli, T.K. [7] H. Gaspary, S.H. Graham, S.M. Sagar, F.R. Sharp, HSP70 heat McIntosh, Regional induction of c-fos and heat shock protein-72 shock protein induction following global ischemia in the rat, Brain mRNA following fluid-percussion brain injury in the rat, J. Cereb. Res. Mol. Brain Res. 34 (1995) 327–332. Blood Flow Met. 15 (1995) 467–473.
[8] M.F. Gonzalez, D. Lowenstein, S. Fernyak, K. Hisanaga, R. Simon, [24] F.R. Sharp, D. Lowenstein, R. Simon, K. Hisanaga, Heat shock F.R. Sharp, Induction of heat shock protein 72-like immunoreactivi- protein hsp72 induction in cortical and striatal astrocytes and ty in the hippocampal formation following transient global ischemia, neurons following infarction, J. Cereb. Blood Flow Metab. 11 Brain Res. Bull. 26 (1991) 241–250. (1991) 621–627.
[9] S. Kantengwa, B.S. Polla, Phagocytosis of Staphylococcus aureus [25] F.R. Sharp, S.M. Massa, R.A. Swanson, Heat-shock protein protec-induces a selective stress response in human monocytes-macro- tion, Trends Neurosci. 22 (1999) 97–99.
phages (M phi): modulation by M phi differentiation and by iron,
[26] Y.M. Snyder, L. Guthrie, G.F. Evans, S.H. Zuckerman, Transcrip-Infect. Immun. 61 (1993) 1281–1287.
tional inhibition of endotoxin-induced monokine synthesis following [10] D.H. Kim, P.H. Gutin, L.J. Noble, D. Nathan, J.S. Yu, R.P. Nockels,
heat shock in murine peritoneal macrophages, J. Leukocyte Biol. 51 Treatment with genetically engineered fibroblasts producing NGF or
(1992) 181–187. BDNF can accelerate recovery from traumatic spinal cord injury in
[27] W.J. Streit, S.L. Semple-Rowland, S.D. Hurley, R.C. Miller, P.G. the adult rat, Neuroreport 7 (1996) 2221–2225.
Popovich, B.T. Stokes, Cytokine mRNA profiles in contused spinal [11] S. Lindquist, The heat-shock response, Ann. Rev. Biochem. 55
cord and axotomized facial nucleus suggest a beneficial role for (1986) 1151–1191.
inflammation and gliosis, Exp. Neurol. 152 (1998) 74–87. [12] F.M. Longo, S. Wang, P. Narasimhan, J.S. Zhang, J. Chen, S.M.
[28] H. Tanno, R.P. Nockels, L.H. Pitts, L.J. Noble, Immunolocalization Massa, F.R. Sharp, cDNA cloning and expression of stress-inducible
of heat shock protein after fluid percussive brain injury and rat hsp70 in normal and injured rat brain, J. Neurosci. Res. 36
relationship to breakdown of the blood–brain barrier, J. Cereb. (1993) 325–335.
Blood Flow Metab. 13 (1993) 116–124. [13] M. Maeda, F. Akai, T. Yanagihara, Neuronal integrity and astrocytic
[29] N.G. Theodorakis, D.J. Zand, P.T. Kotzbauer, G.T. Williams, R.I. reaction in cold injury: an immunohistochemical investigation, Acta
Morimoto, Hemin-induced transcriptional activation of the HSP70 Neuropathol. 94 (1997) 116–123.
gene during erythroid maturation in K562 cells is due to a heat [14] M.D. Maines, Heme oxygenase: function, multiplicity, regulatory
shock factor-mediated stress response, Mol. Cell. Biol. 9 (1989) mechanisms, and clinical applications, FASEB J. 2 (1988) 2557–
3166–3173. 2568.
[30] C.P. Turner, S.S. Panter, F.R. Sharp, Anti-oxidants prevent focal rat [15] M.D. Maines, G.M. Trakshel, R.K. Kutty, Characterization of two
brain injury as assessed by induction of heat shock proteins (HSP70, constitutive forms of rat liver microsomal heme oxygenase. Only
HO-1 / HSP32, HSP47) following subarachnoid injections of lysed one molecular species of the enzyme is inducible, J. Biol. Chem.
blood, Brain Res. Mol. Brain Res. 65 (1999) 87–102. 261 (1986) 411–419.
[31] M. Vayssier, F. Favatier, F. Pinot, M. Bachelet, B.S. Polla, Tobacco [16] P. Matz, P. Weinstein, B. States, J. Honkaniemi, F.R. Sharp,
smoke induces coordinate activation of HSF and inhibition of Subarachnoid injections of lysed blood induce the hsp70 stress gene
NFkappaB in human monocytes: effects on TNFalpha release, and produce DNA fragmentation in focal areas of the rat brain,
Biochem. Biophys. Res. Comm. 252 (1998) 249–256. Stroke 27 (1996) 504–512, discussion 513.
[32] W.J. Welch, Mammalian stress response: cell physiology, structure / [17] A. Mautes, H. Schroeck, A.C. Nacimiento, W. Paschen, Regional
function of stress proteins, and implications for medicine and spinal cord blood flow and energy metabolism in rtas after
laminec-disease, Physiol. Rev. 72 (1992) 1063–1081. tomy and acute compression injury, Euro. J. Trauma (2000) in press.
[33] H.R. Wong, M. Ryan, J.R. Wispae, Stress response decreases NF-[18] A.E. Mautes, D.H. Kim, F.R. Sharp, S. Panter, M. Sato, N. Maida,
kappaB nuclear translocation and increases I-kappaBalpha expres-M. Bergeron, K. Guenther, L.J. Noble, Induction of heme
sion in A549 cells, J. Clin. Invest. 99 (1997) 2423–2428. oxygenase-1 (HO-1) in the contused spinal cord of the rat, Brain