Changes in wheat germination following
g
-ray irradiation:
an in vivo electronic paramagnetic resonance spin-probe
study
Ze-neng Wang, Rui-lin You *
College of Life Sciences,Peking Uni6ersity,Beijing100871,PR China
Received 5 June 1999; received in revised form 8 November 1999; accepted 11 November 1999
Abstract
Embryos excised from wheat (Triticum aesti6um) grains followingg-ray irradiation at different doses were analyzed
on membrane permeability by electron paramagnetic resonance (EPR) technique with 4-oxo-2, 2, 6, 6-tetramethyl-1-piperidinyloxy (TEMPONE) as spin probe to acquire an EPR spectrum. The broadening agent ferricyanide added leads to changes in the high-field region of the EPR spectrum, which reflects differences in membrane permeability.
R-value, defined as the ratio of water (W) to lipid (L) component in height in the high-field region of the EPR spectrum, symbolizes membrane permeability for a given sample. The R-values corresponding to a certain dose treatment of grains displayed a definitive distribution pattern. A unit row vector with 20 components was used to describe theR-value distribution pattern for a given treatment. The transaction angle between vectors corresponding to grains irradiated and unirradiated,u, was used as quantitative index for membrane permeability changes following
g-ray irradiation.g-Ray irradiated grains germinated at low rates, and the regression equation of germination rate as
a function of the irradiation dose is: Germination Rate (%)=94.8 exp[−0.264×Irradiation Dose (kGy)] (r2=0.991, PB0.001). Embryos excised from grains followingg-ray irradiation show increases inuvalues with irradiation dose.
Theu value is negatively linearly correlated with the germination rate. It suggests thatg-ray irradiation leading to
increases in membrane permeability is consistent with that leading to low germination rate of grains. The introduction to vector analysis method on membrane permeability changes in this study is very practical. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Membrane permeability; Potassium ferricyanide;g-Ray; TEMPONE;Triticum aesti6um; Vector; Wheat
www.elsevier.com/locate/envexpbot
1. Introduction
The plasma membrane is a highly selective filter and a device for active transport, through which
essential nutrients for cellular life are transported inside cells (Alberts et al., 1989). Membrane per-meability is defined as the capacity of solute leak-age out of cells or penetrating into cells not through active transport. There are a lot of fac-tors, physical, osmotic, chemical stress or
physio-logical conditions, to influence membrane
* Corresponding author. Fax: +86-10-6275-1526. E-mail address:[email protected] (R.-l. You)
permeability (Miller, 1978; Hoekstra and Roekel, 1988; Tetteroo et al., 1996; Sinha et al., 1997), and the methods applied in the research on membrane permeability include ultrastructural observations, conductivity measurements, ultraviolet light ab-sorbance at various wavelengths and electron paramagnetic resonance (EPR) spin-probe tech-nique (Duke et al., 1983; Shirazi et al., 1996; Golovina et al., 1997). Conductivity measure-ments and ultraviolet light absorbance are based on solute leakage out of cells. During imbibition of seeds, solutes leakage usually can be detected by these methods, but the leaked solutes were supposed to exist in cell surface before imbibition to a great extent (Tetteroo et al., 1996). Some of the disadvantages for ultrastructural observations and conductivity measurements to measure mem-brane permeability were summarized, and EPR spectroscopy of spin probes was regarded as an ideal method in studying membrane permeability (Smirnov et al., 1992; Golovina and Tikhonov, 1994; Golovina et al., 1997).
Amphipathic nitroxide spin probe can be used to measure membrane permeability. It can be dissolved both in water and in lipid, showing differences in the high-field region of the EPR spectrum (Golovina et al., 1997). Differences in membrane permeability can be characterized by differences in penetration of broadening agent, which can dissolve in the water phase of the cytosol and decreased the line height of the water component; the ratio between the line heights of the water (right) and the lipid component (left) in
the high-field region of the EPR spectrum, R
-value, can quantitatively characterize membrane permeability in different tissues (Golovina et al., 1997). There are several amphipathic probes.
4-Oxo-2, 2, 6, 6-tetramethyl-1-piperidinyloxy
(TEMPONE) has a relatively great polarity com-pared with other amphipathic spin labels such as di-tertiary butyl nitroxide (DTBN) and 2, 2, 6, 6-tetramethyl-1-piperidinyloxy (TEMPO). So it has been widely used (Boggs and Rangaraj, 1997; Golovina et al., 1997; Wolkers et al., 1998). The optimum broadening agent should have charac-teristics of impermeability to cell membrane and non-toxicity to cells (Zaplatin et al., 1996), in this EPR study potassium ferricyanide was applied.
However, no report on the quantification of membrane permeability changes has been seen yet. Golovina et al. (1997) stated that different
R-value distribution patterns correspond to
dif-ferent ages of seed lots, but the functional rela-tionship between them was not elaborated. This paper introduced a unit vector expressing in a
one-row matrix to symbolize certainR-value
dis-tribution patterns for a given dose ofg-ray
irradi-ation. The transaction angle,u, between two unit
vectors corresponding to non-irradiated and irra-diated grains was defined as quantitative index of a membrane permeability change following a cer-tain dose of g-ray irradiation.
g-Ray irradiation plays an important role in
clinic and plant improvement, and the effects on membrane permeability have been summarized (Xia, 1992). The aim of the introduction to vector analysis on membrane permeability changes is to establish a functional relationship to quantify membrane permeability change with irradiation dose and test the hypothesis that the lipid mem-brane system of an embryo in a grain can be regarded as single target, where irradiation effects occur through single hit.
2. Material and methods
2.1. Plant material and germination test
Wheat (Triticum aesti6um L.) grains, No. 9503,
acquired from the Chinese Academy of Agricul-ture Sciences after 2 years of storage, were
irradi-ated with 60Co
g-rays at doses of 0, 0.41, 0.83,
1.65, 3.30, 6.60, 13.2 kGy, respectively. The irradi-ated grains were sealed and stored at 4°C in a refrigerator for 2 – 3 months for the following experiments.
Wheat grains were soaked in distilled water at 25°C in the dark for 8 h. After that period embryos were carefully excised from wheat grains with a pair of tweezers and a probe. The excised embryo with scutellum removed in this study is used for EPR spectra measurement.
2.2. Sample preparation for EPR experiments
The excised embryos were immediately incu-bated for 15 min in a solution of 1 mM TEM-PONE (Sigma), completely broadened by 120 mM potassium ferricyanide (Golovina et al., 1997). Three embryos were placed into a capillary with an outer diameter of 2 mm, and a very small amount of solution was injected on the top of the sample to prevent drying. Fifty independent repli-cates for a given treatment were conducted to
draw anR-value distribution histogram.
2.3. EPR methods
The EPR spectra were recorded at 25°C with an ER 200 D-SRC EPR spectrometer (X-band) using
the following settings: scan range 950 G, field
setting 3466 G, time constant 0.2 s, modulation amplitude 0.5 G, microwave power 1 mW.
2.4. Statistical analysis of data
The regression equations were acquired using linear (y=a+bx), mutiplicative (y=axb),
expo-nential [y=exp(a+bx)] models with an aid of
Microsoft Excel 97 software, from which the best-fitted model was chosen. The significance of corre-lation coefficient was tested through one-sample t-test (Rosner, 1995).
3. Results
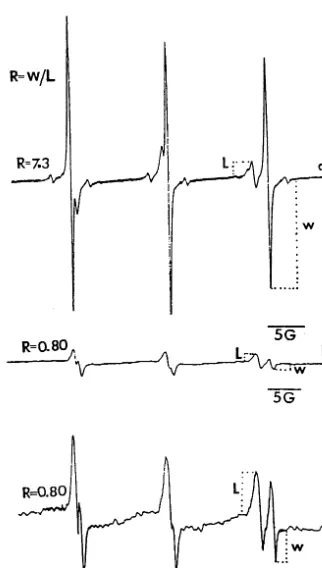
Fig. 1 shows the EPR spectra of TEMPONE in excised embryos in vivo. From the representative EPR spectrum of TEMPONE in embryos excised
from wheat grains without g-ray irradiation (Fig.
1(a)), a triplet signal with narrow, equidistant lines having an isotropic hyperfine splitting con-stant of about 16 G was reflected. Fig. 1(b) was the spectrum of TEMPONE in embryos excised
from 13.2 kGyg-ray irradiated wheat grains, Fig.
1(c) was Fig. 1(b) amplified five times in signal intensity, and the gain amplitudes in Fig. 1(a, b, c) were 8.0×104, 8.0
×104, and 4.0
×105, respec-tively. Signal intensity in Fig. 1(b) is far lower than that in Fig. 1(a), which is broadened by the penetration of potassium ferricyanide into cytosol through the membrane via spin – spin interaction (Eaton and Eaton, 1978). The ratio between the
line heights of the water (right), designated W,
and the lipid component (left), designatedLin the
high-field region of the spectrum (Fig. 1(a)) was
defined as R-value, which symbolizes membrane
permeability (Golovina et al., 1997).
R-values in the EPR spectra of TEMPONE in
embryos excised from wheat grains with different
doses of g-ray irradiation were characterized by
different distribution patterns (Fig. 2), the higher
the dose, the closer the R-value is to zero. In
order to determine the differences in R-value
dis-tribution pattern among different treatment doses, we introduce a row unit vector with 20
compo-nents to describe a given R-value distribution
pattern. Every component corresponds to the fre-Fig. 1. EPR spectra of TEMPONE in embryos excised from
Fig. 2. Distribution of theR-values calculated from the EPR spectra of TEMPONE in embryos excised from wheat grains with g-ray irradiation at different doses. A, 0 kGy; B, 0.41 kGy; C, 0.83 kGy; D, 1.65 kGy; E, 3.30 kGy; F, 6.60 kGy; G, 13.2 kGy.
quency for each unitR-value space shown in Fig.
2 and equals the square root of the ratio of the frequency to 100. The unit row vectors
corre-sponding to grains irradiated withg-rays at doses
of 0, 0.41, 0.83, 1.65, 3.30, 6.60, 13.2 kGy were designated asa0,a1,a2,a3,a4,a5,a6, respectively.
u, the angle between two vectors, is used as
quantitative index to determine the difference in
R-value distribution between two corresponding
treatments, and cosu01=a0a1%, cosu02=a0a%2, cosu03=a0a3%, cosu04=a0a4%, cosu05=a0a%5, cosu06=a0a6%, wherea0%,a1%, a%2,a3%,a4%, a5%,a6%, are the transposes of the row vectors,a0,a1,a2,a3,a4,
a5, a6, respectively.
Theuvalues calculated between different doses
of g-ray irradiated and non-irradiated grains are
given in Table 1.
Some grains irradiated with g-rays could not
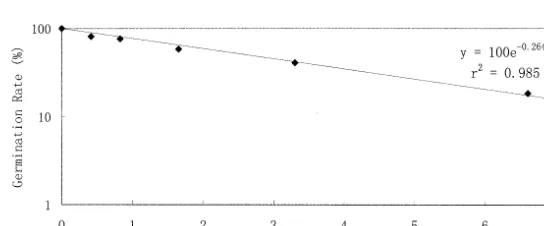
germinate. The relationship of germination rate as a function of irradiation dose (Fig. 3) is linear:
Germination Rate (%)
=100 exp[−0.264×Irradiation Dose (kGy)],
r2
=0.985 (PB0.001)
In Fig. 3, the point (13.2, 0) cannot be plotted. In order to determine the regression relationship between germination rate and irradiation dose
including the point (13.2, 0), we regarded exp[−
0.264×Irradiation Dose (kGy)] as independent
variable and germination rate as dependent vari-Table 1
Changes in u value after grains of T. aesti6um had been irradiated with different doses ofg-rays
1.65 13.2
0.83 0.41 0
Dose (kGy) 3.30 6.60
46.6 60.9
u(degree) 0 33.9 34.5 77.0 90
Fig. 4. Plot of data between seed germination rate following different doses of g-ray irradiation and u corresponding to each dose as given in Table 1. The intercept and the regression coefficient in the linear equation were both calculated through least square method.
4. Discussion
The integrity of biomembranes has to be pre-served for embryos to survive desiccation. Phase transitions in membranes occurring in desicca-tion-intolerant embryos lead to loss of seed viabil-ity (Tetteroo et al., 1996). There are several ways for embryos to avoid phase transitions in mem-branes in order to survive dry conditions, such as synthesis of more unsaturated acyl chains and accumulation of soluble sugars, sucrose and oligosaccharides to form hydrogen bonds with the phospholipid polar headgroup (Crowe et al., 1984, 1987; Lynch and Steponkus, 1989; Crowe et al., 1992; Tetteroo et al., 1996; Obendorf, 1997;
Sun and Leopold, 1997). Duringg-ray irradiation,
large quantities of free radicals arise (Basly et al., 1996; Badr et al., 1998). The more unsaturated acyl chains make biomembranes more susceptible to free radicals’ attack. Therefore membrane meability increased through membrane lipid per-oxidation (Della Rovere et al., 1995).
There are several reasons leading to seed vigor loss. The increased membrane permeability is one of them (Parera et al., 1996; Golovina et al., 1997). Seed vigor loss is characterized by low germination rate. The functional relationship
be-tween wheat grain germination rate and g-ray
irradiation dose is in agreement with the one described as single hit model in target theory on the biological effects of irradiation (Xia, 1992), and the grains can be regarded as a unit structure sensitive to g-rays, i.e. single target. The
relation-ship between u value and germination rate
sug-gests that increases in membrane permeability
with g-ray irradiation dose is consistent with
de-creases in germination rate. According to the two functional relationships, we can suppose that the lipid membrane system of an embryo in a grain can be regarded as single target, where irradiation effects occur through single hit.
Meanwhile, the negative linear correlation
be-tween vector transaction angel, u, and
germina-tion rate indicates that the unit vector introduced
to describe R distribution and the transaction
angle between two vectors corresponding to
non-irradiated grains and g-ray irradiated grains are
very reasonable. able, and found a linear function given below best
fitted for the relationship.
Germination Rate (%)
=94.8 exp[−0.264×Irradiation Dose (kGy)],
r2=0.991 (PB0.001)
From Table 1, it can be concluded that with
increasing dose,u increased. The relationship
be-tween grain germination rate following different
doses of g-ray irradiation and u is a negative
linear function (Fig. 4):
u (degree)
= −0.825×Germination Rate (%)+93.1,r2
=0.963 (PB0.001)
From the regression equations plotted in Figs. 3
and 4, a function relationship between u value,
symbolizing membrane permeability change, and irradiation dose can be established as:
u (degree)
= −99.5 exp[−0.264×Irradiation Dose (kGy)]
+99.5,r2
=0.956 (PB0.001)
where Irradiation Dose=0 and u=0 were
sup-posed to be a set of mathematical solution to this equation beforehand, the slope of the equation was calculated through least square method,
where exp[−0.264×Irradiation Dose (kGy)] was
The establishment of a relationship between
vector transaction angel, u, and irradiation dose
indicates that there is really a definitive functional
relationship, so the membrane permeability
change described in vector transaction angel can be used to compare the relative biological effects among different doses of irradiation and different kinds of irradiation.
Normal distribution and Poisson distribution have been widely used to fit
frequency-distribu-tion diagrams, which were characterized by m(m)
andm(s) (Wardlaw, 1985). In this paper, we
intro-duced a vector to describe R-value frequency
distribution, which made it easy to compare the
effects of g-ray irradiation on the changes in
membrane permeability, quite different from nor-mal distribution and Poisson distribution in ex-plaining experimental results, which need complex data analysis. Some physical and chemical factors induced changes in biological parameters usually display a certain frequency distribution. We be-lieve that the introduction to vector analysis method on studies of these quantity relationships will bring about more ideal conclusions. In this study, we established a functional relationship to quantify membrane permeability change with irra-diation dose by the introduction to vector analysis method on wheat embryo membrane permeability
changes followingg-ray irradiation and tested the
hypothesis that the lipid membrane system of an embryo in a grain can be regarded as single target, where irradiation effects occur through single hit.
Acknowledgements
We thank the Chinese Academy of Agricultural Sciences for the gift of the wheat grains. We would also like to thank Professor En Wu, EPR Laboratory, Physical Department of Peking Uni-versity, for his tolerant direction in EPR spec-trometer manipulation and manuscript revision.
References
Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K.,
Wat-son, J.D., 1989. Molecular Biology of the Cell, 2nd ed. Garland Publishing, New York, pp. 275 – 340.
Badr, F.M., El-Habit, O.H.M., Hamdy, M., Hassan, G.A.R., 1998. The mutagenic versus protective role of vitamin A on the induction of chromosomal aberration in human lymphocyte cultures. Mut. Res. 414, 157 – 163.
Basly, J.P., Duroux, J.L., Bernard, M., 1996. Gamma irradia-tion sterilisairradia-tion of orciprenaline and fenoterol. Int. J. Pharm. 142, 125 – 128.
Boggs, J.M., Rangaraj, G., 1997. Greater partitioning of small spin labels into interdigitated than into non-interdigitated gel phase bilayers. Chem. Phys. Lipids 87, 1 – 15. Crowe, J.H., Crowe, L.M., Chapman, D., 1984. Preservation
of membranes in anhydrobiotic organisms: the role of trehalose. Science 223, 701 – 703.
Crowe, J.H., Crowe, L.M., Carpenter, J.F., Aurell Wisrom, C., 1987. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 242, 1 – 10.
Crowe, J.H., Hoekstra, F.A., Crowe, L.M., 1992. Anhydro-biosis. Annu. Rev. Physiol. 54, 579 – 599.
Della Rovere, F., Gamata, A., Broccio, M., Zirilli, A., Broc-cio, G., 1995. Haemoglobin oxidative stress in cancer. Anticancer Res. 15, 2089 – 2095.
Duke, S.H., Kakefuda, G., Harvey, T.M., 1983. Differential leakage of intracellular substances from imbibing soybean seeds. Plant Physiol. 72, 919 – 924.
Eaton, S.S., Eaton, G.R., 1978. Interactions of spin labels with transient metals. Coord. Chem. Rev. 26, 207 – 262. Golovina, E.A., Tikhonov, A.N., 1994. The structural
differ-ences between the embryos of viable and non-viable wheat seeds as studied with the EPR spectroscopy of lipid-soluble spin labels. Biochim. Biophys. Acta 1190, 385 – 392. Golovina, E.A., Tikhonov, A.N., Hoekstra, F.A., 1997. An
electron paramagnetic resonance spin-probe study of mem-brane-permeability changes with seed aging. Plant Physiol. 114, 383 – 389.
Hoekstra, F.A., Roekel, T.V., 1988. Desiccation tolerance of Papa6er dubium L. Pollen during its development in the anther: possible role of phospholipid composition and sucrose content. Plant Physiol. 88, 626 – 632.
Lynch, D.V., Steponkus, P.L., 1989. Lyotropic phase behavior of unsaturated phosphatidylcholine species relevance to the mechanism of plasma membrane destabilization and freez-ing injury. Biochim. Biophys. Acta 984, 267 – 272. Miller, R.W., 1978. Osmotically induced removal of water
from fungal cells as determined by a spin probe technique. Plant Physiol. 62, 741 – 745.
Obendorf, R.L., 1997. Oligosaccharides and galactosyl cycli-tols in seed desiccation tolerance. Seed Sci. Res. 7, 63 – 74. Parera, C.A., Cantliffe, D.T., McCarty, D.R., Hannah, L.C., 1996. Improving vigor in shrunken-2 corn seedlings. J. Am. Soc. Hort. Sci. 121, 1069 – 1075.
Rosner, B., 1995. Fundamentals of Biostatistics. Duxbury, New York, pp. 506 – 507.
Sinha, R.P., Singh, N., Kumar, A., Kumar, H.D., Haeder, D.P., 1997. Impact of ultraviolet-B irradiation on nitrogen-fixing cyanobacteria of rice paddy fields. J. Plant Physiol. 150, 188 – 193.
Smirnov, A.I., Golovina, E.A., Yakimchenko, O.E., Aksy-onov, S.I., Lebedev, Y.S., 1992. In vivo seed investigation by ESR spin probe technique. J. Plant Physiol. 140, 447 – 452.
Sun, W.Q., Leopold, A.C., 1997. Cytoplasmic vitrification and survival of anhydrobiotic organisms. Comp. Biochim. Physiol. A 117, 327 – 333.
Tetteroo, F.A.A., de Bruijn, A.Y., Henselman, R.N.N., Wolk-ers, W.F., van Aelst, A.C., Hoekstra, F.A., 1996. Charac-terization of membrane properties in desiccation-tolerant
and -intolerant carrot somatic embryos. Plant Physiol. 111, 403 – 412.
Wardlaw, A.C., 1985. Practical Statistics for Experimental Biologists. Wiley, New York, pp. 16 – 41.
Wolkers, W.F., Bochicchio, A., Selvaggi, G., Hoekstra, F.A., 1998. Fourier transform infrared microspectroscopy de-tects changes in protein secondary structure associated with desiccation tolerance in developing maize embryos. Plant Physiol. 116, 1169 – 1177.
Xia, S.X., 1992. Molecular Radiobiology (in Chinese). Atomic Energy Press, Beijing, pp. 54 – 86.
Zaplatin, A.N., Baker, K.A., Kleinhans, F.W., 1996. Effective-ness and toxicity of several DTPA broadening agents for biological ESR spectroscopy. J. Mag. Resonance Ser. B 110, 249 – 254.