L
Journal of Experimental Marine Biology and Ecology 247 (2000) 67–83
www.elsevier.nl / locate / jembe
Interactive effects of diet and temperature on the scope for
growth of the scallop Argopecten purpuratus during
reproductive conditioning
a ,* a b b
J.M. Navarro , G.E. Leiva , G. Martinez , C. Aguilera
a
´ ¨
Instituto de Biologıa Marina‘Dr. Jurgen Winter’ Universidad Austral de Chile, Casilla 567, Valdivia, Chile
b
´ ´
Departamento de Biologıa Marina, Universidad Catolica del Norte, Coquimbo, Chile Received 27 January 1999; received in revised form 16 December 1999; accepted 20 December 1999
Abstract
Mature individuals of Argopecten purpuratus from suspended culture in Tongoy Bay, northern Chile were conditioned at two temperatures (16 and 208C) and three different diets (mixture of pure microalgae, microalgae supplemented with lipids and microalgae supplemented with carbohydrates). The food, equivalent to 3% daily of the animal dry weight was supplied continuously by a peristaltic pump. The rates of different physiological processes were measured on 18 scallops (three replicates per temperature / diet combination) during the third week of conditioning. Nine scallops came from the group conditioned at 168C and other nine from the group conditioned at 208C (each scallop from a different tank). As in the conditioning experiment, physiological measurements were made in controlled temperature rooms using the same experimental diets. Argopecten purpuratus did not show significant differences in clearance rate between 16 and 208C when fed with pure microalgae and microalgae1lipids, showing a well known capability of bivalves to acclimate their physiological rates within a certain range of temperature. On the other hand a clear effect of the composition of the diet on the clearance rate was observed. When microalgae were supplemented with a lipid emulsion, clearance rate was highly stimulated, showing values significantly higher in comparison with either pure microalgae, or a mixed diet of microalgae1carbohydrate. These highest feeding rates can be related with the presence of essential fatty acids in the diet, which are considered as very important compounds during the gametogenesis of invertebrates as well as during the development of ova into normal larvae. Thus A. purpuratus can actively regulate clearance rate and does not simply switch between feeding and non-feeding states. The data also suggest the presence of chemical receptors at the level of the gills and / or labial palps, which seem able to detect specific nutritive compounds present in the diet. Absorption efficiency was independent of temperature and was higher with pure microalgae and with microalgae1lipids. The lowest efficiencies were recorded with the diet
*Corresponding author. Fax:156-63-212-953. E-mail address: [email protected] (J.M. Navarro)
supplemented with carbohydrates. The energy expended in oxygen uptake and ammonia excretion was very similar in the different experimental diets and temperatures. Scope for growth in A.
purpuratus appears mainly affected by the diet and not by temperature. While the lower SFG
seems to be associated with diets composed of pure microalgae and microalgae1carbohydrates, the highest values being found for a diet rich in lipids. The data were in agreement with the reproductive conditioning of A. purpuratus, where the highest percentage of ripe scallops occurred in individuals fed with a diet of microalgae supplemented with lipids at both temperatures. Similarly the highest larval survival rate was obtained from gametes released by scallops conditioned with the diet containing lipids. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Scallop; Reproduction; Diet; Temperature; Feeding; Scope for growth
1. Introduction
The scallop Argopecten purpuratus is naturally distributed between central Chile and northern Peru, but the commercial interest in this species has led to its introduction to southern Chile with the objective of developing a major aquaculture industry. Natural production of juvenile A. purpuratus has been highly variable during recent decades in the north of Chile, although seed production has not been successful with the introduced scallops to the south of Chile, mainly due to the low environmental temperatures. These have been the main constraints to a sustained development of A. purpuratus culture in Chile, and the development of fisheries and aquaculture for A. purpuratus will be highly dependent on the hatchery production of juveniles.
Most of the studies on A. purpuratus have been related to its culture (Padilla, 1979; Hojas, 1983; 1984; Illanes, 1987), but only a few have concerned to reproduction and
´ ´ ´
magel-lanicus, species which increases its clearance and ingestion rates in response to specific
metabolites from the diatom Chaetoceros muelleri. This knowledge on the chemorecep-tory capacity of bivalves is important to understand the feeding behaviour of bivalves in nature as well as under laboratory conditions.
The main objective of the present study was to evaluate the interactive effect of diet and temperature by measuring the scope for growth and gonad maturation of the scallop
Argopecten purpuratus during reproductive conditioning, under the hypothesis that both
diet and temperature are key factors influencing potential for production and gameto-genesis in this species.
2. Material and methods
2.1. Experimental design
Mature scallops obtained from an aquaculture site at Tongoy Bay (308 S) were induced to spawn by raising the temperature and adding microalgae. Spent animals were distributed in 18 tanks which were divided into two groups and placed in separate controlled temperature laboratories, one at 168C and the other at 208C. Three groups in each laboratory were fed with a mixture of microalgae (50% Isochrysis galbana150% of Chaetoceros gracilis), three other groups received 70% of the same mixture of microalgae plus 30% by weight of carbohydrates (commercial potato’s starch, particle size: 10.7765.82mm), and the other three tanks received 70% microalgae plus 30% by weight of a lipid emulsion (particle size: 2.1560.54 mm). The experimental ICES (International Council for the Exploration of the Sea) lipid emulsion was provided by Artemia Reference Center in Belgium and correspond to the EmDHA one whose composition is described in Caers et al. (1999). Direct observation on the lipid emulsion showed that this compound is very stable in seawater. It was verified by counting the particles every hour during 5 h, and a very small reduction in particle number was detected (less than 7%). The food, supplied continuously by a peristaltic pump, amounted to 3% daily of the animal dry weight.
The physiological measurements were carried out during the third week of the conditioning of the scallops. The different physiological variables were measured on 18 scallops (three replicates per temperature / diet combination), nine scallops coming from the group conditioned at 168C and the remainder from the group conditioned at 208C (each scallop coming from a different tank). As in the conditioning experiments, the physiological measurements were done in the controlled temperature laboratories with the same three experimental diets described above. Physiological rates were standardised to 3 g dry tissue weight according to the formula given by Bayne and Newell (1983):
b Ys5(W /W )s e ?Ye
2.2. Physiological measurements
2.2.1. Clearance and ingestion rates
These rates were measured using a static system, in which the decrease in particle density in the experimental aquarium was monitored in relation to time (Widdows, 1985). These measurements were carried out using a particle counter (Elzone 180XY, Particle Data), fitted with a 120-mm orifice tube. The experimental medium was maintained homogeneous by aeration in each experimental aquarium. The measurements were carried out over a period of 6 h in 8-l aquaria filled with seawater, each aquarium containing a single scallop. One additional aquarium, but with no scallop, was used as a control. The experiments were carried out at 16 and 208C. The experimental food
6 21
concentration was 30310 particles l at each experimental diet, using the same mixtures of diets as during the conditioning process: (a) mixture of microalgae, (b) mixture of microalgae plus lipids, and (c) mixture of microalgae plus carbohydrates.
Ingestion rate represents the amount of food ingested per unit of time. It was estimated as the product of the clearance rate and the total weight of a given experimental diet. The organic ingestion rate was calculated as the product of clearance rate and the organic fraction of the diets.
2.2.2. Net absorption efficiency
Absorption efficiency was estimated by determining the organic and inorganic content of the food ingested and the faeces, following the ratio method of Conover (1966). Representative samples of each diet mixture were collected during the experiments, as well as faeces from each experimental aquarium. Samples were filtered through pre-ashed, pre-weighed 47-mm glass fiber filters under low vacuum. Filters were rinsed with isotonic ammonium formate (3.4%), dried to a constant weight at 808C, weighed, combusted at 4508C for 3 h and weighed again to estimate the organic and inorganic fraction contained in the food and faeces. Absorption rate was calculated as the product
21
of the organic ingestion rate (mg h ) and absorption efficiency (%).
2.2.3. Oxygen uptake
Rates of oxygen uptake for individual scallops were measured in sealed chambers of 2.5 l volume, using a polarographic analyser (Model YSI 5351). To ensure steady oxygen consumption, the scallops were allowed 30 min of acclimation until their siphons appeared to be open. Two measurements were made on each occasion for each scallop and oxygen concentration was not allowed to fall below 70% of saturation. The water in the respirometer was mixed with a magnetic stirrer and the output signal from the O2
analyser was monitored continuously on a chart recorder. Considering that the experimental scallops were well fed before the experiments, the measured rates of oxygen uptake are assumed to represent the routine metabolism of A. purpuratus. Values
21
2.2.4. Ammonia excretion
Ammonia excretion was determined by the phenol–hypochlorite method of Solorzano (1969). Scallops were well fed and then placed individually in glass beakers containing 0.5 l of filtered (0.45mm) seawater. One additional beaker containing filtered seawater, but with no animals, served as a control. Following an incubation period of 2 h, samples from the water containing the scallops and from the control were analysed. Values for
21
excretion rate were expressed in mg NH –N h4 and transformed to Joules using the conversion factor: 1 mg NH –N4 524.8 J (Elliott and Davison, 1975).
2.2.5. Scope for growth
This physiological index represents the energy available for growth and reproduction after all metabolic demands have been met from the absorbed ration. Scope for growth was calculated by the equation given by Widdows (1985), after converting all the
21
physiological rates to energy equivalents (J h ).
2.2.6. Gonad maturation
Individual scallops from each conditioning tank were sampled every 6–8 days to analyse gonad development and to induce spawning at the end of the conditioning experiment. A visual criterion was used to define ripe scallops, characterised by turgid and highly pigmented gonads. In the present study we consider only gonadal develop-ment and the spawning capacity of scallops conditioned at the different temperatures and diets. Biochemical analyses of the gonad will be described elsewhere.
2.3. Statistical analysis
Physiological rates were related to dry meat weight by linear regression analysis, after log-transformation of all variables. Analysis of variance followed by a Tukey test of significance was carried out to compare the total weight and organic content of the different diets and also the effects of the temperature / diet treatments on each physiological variable. In all statistical tests differences are considered significant when
P,0.05. All analyses were carried out with the statistical package Statistica for Windows, v. 4.2.
3. Results
3.1. Diets
6
The three experimental diets were supplied at a concentration of 30310 particles
21
l . The diet composed of a mixture of microalgae (50% Isochrysis galbana150%
21
Chaetoceros gracilis) corresponded to a total dry weight of 0.98 mg l , with an organic
21
content of 0.88 mg l (89.8%). The diet composed of 70% of the mixture of microalgae
21
plus 30% of lipids represented a total dry weight of 1.89 mg l , with an organic content
21
Fig. 1. Total, organic and inorganic content of the experimental diets. Values are means6S.E.
21
carbohydrates represented a total dry weight of 2.13 mg l , with an organic content of
21
1.87 mg l (87.7%) (Fig. 1). The energetic content of these diets was calculated according to Whyte (1987) and to our own calculation based on the conversion factors
21 21
for lipid (39.5 J mg ) and carbohydrate (17.5 J mg ) given by Gnaiger (1983). The results of energy content for the different diets are presented in Table 1.
3.2. Clearance and ingestion rates
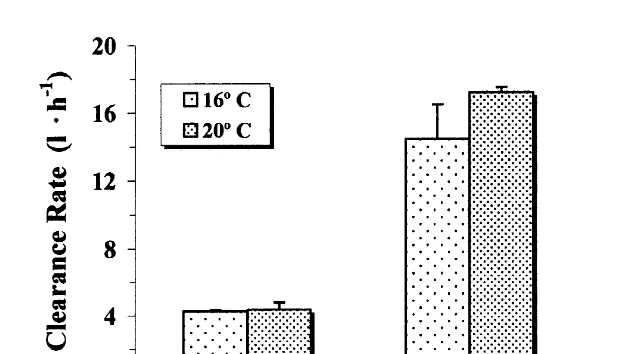
Mean values of clearance rates as well as means of the other physiological variables from each treatment were compared by analysis of variance followed by a Tukey test of significance. Argopecten purpuratus fed with the diet microalgae1lipid showed a significantly higher clearance rate. No significant differences (P.0.05) were found between the groups treated at the two experimental temperatures (Fig. 2). Scallops of
21
standard size (3 g dry tissue wt) showed clearance rates as high as 16 l h when fed with this diet. Significantly (P,0.05) lower values were observed for animals fed with
21
microalgae alone, with clearance rates around 4.5 l h at both experimental tempera-tures (Fig. 2). There were no significant differences (P.0.05) between clearance rates measured at 16 and 208C, when microalgae were used as food. The lowest clearance rate
Table 1
6 21
Organic and energetic content of the experimental diets at a concentration of 30310 particles l
Total Organic Inorganic Energy
21 21 21 21
(mg l ) (mg l ) (mg l ) (J l )
Microalgae 0.98 0.88 0.10 13.73
Micr.1Lip. 1.89 1.46 0.43 33.68
Fig. 2. Argopecten purpuratus. Clearance rate at different temperatures and diets. Values are means6S.E.
values were observed at the combination 168C and microalgae1carbohydrate. However, feeding rate increased significantly when the scallops were exposed to the same diet but at 208C (P,0.05). There were no significant differences (P.0.05) in clearance rate when various treatments involving microalgae and microalgae1carbohydrates were compared (Table 2).
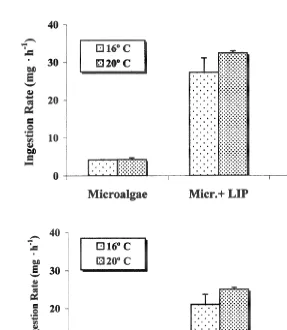
Ingestion rate followed a similar trend to that described for clearance rate, the highest values being recorded for scallops fed the microalgae1lipids diet (Fig. 3a). Total
21
ingestion rate for a scallop of 3 g dry tissue weight was as high as 30 mg h . The lowest values were observed with the diet of microalgae, but slightly higher values were recorded when microalgae1carbohydrates was supplied. The organic ingestion rate represented a large fraction of the total ingestion rate. The highest values were observed
21
for scallops fed with the diet microalgae1lipids, with values as high as 25 mg h and not significantly different (P.0.05) to the condition microalgae1carbohydrates at 208C. The lowest organic ingestion rate was observed with the diet composed of a mixture of
21
microalgae, with values near 3.2 mg h (Fig. 3b).
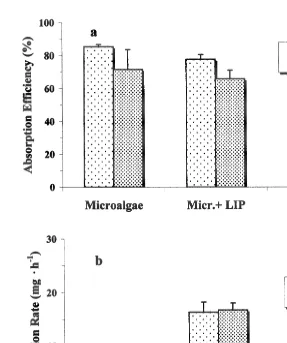
3.3. Absorption efficiency
Table 2
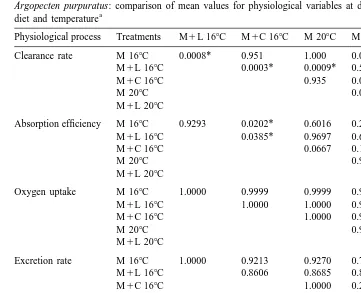
Argopecten purpuratus: comparison of mean values for physiological variables at different combinations of a
diet and temperature
Physiological process Treatments M1L 168C M1C 168C M 208C M1L 208C M1C 208C
Clearance rate M 168C 0.0008* 0.951 1.000 0.0002* 0.067
M1L 168C 0.0003* 0.0009* 0.578 0.251
M1C 168C 0.935 0.0002* 0.0198*
M 208C 0.0002* 0.073
M1L 208C 0.0249*
Absorption efficiency M 168C 0.9293 0.0202* 0.6016 0.2693 0.0632
M1L 168C 0.0385* 0.9697 0.6565 0.1737
M1C 168C 0.0667 0.1251 0.3477
M 208C 0.9560 0.3871
M1L 208C 0.7735
Oxygen uptake M 168C 1.0000 0.9999 0.9999 0.9988 0.9991
M1L 168C 1.0000 1.0000 0.9957 0.9966
M1C 168C 1.0000 0.9911 0.9926
M 208C 0.9878 0.9898
M1L 208C 1.0000
Excretion rate M 168C 1.0000 0.9213 0.9270 0.7936 0.6447
M1L 168C 0.8606 0.8685 0.8693 0.5499
M1C 168C 1.0000 0.2904 0.9904
M 208C 0.2980 0.9889
M1L 208C 0.1213
Scope for growth M 168C 0.0154* 0.9991 1.0000 0.0141* 0.5534
M1L 168C 0.0494* 0.0131* 1.0000 0.0914
M1C 168C 0.9999 0.0461* 0.6411
M 208C 0.012* 0.4704
M1L 208C 0.0825
a
Probability values are derived from an a posteriori multiple range test (Tukey’s honestly significant difference) following ANOVA. * Significant differences at P,0.05 (M, microalgae; M1L, microalgae1lipid; M1C, microalgae1carbohydrate).
values being very similar at 16 and 208C (Fig. 4b). The amount of food material absorbed was greatly reduced for animals fed solely with microalgae, representing only 16% of the amount absorbed from the food containing lipids at the two experimental temperatures. The lowest values for absorbed ration were observed for the diet containing carbohydrates at 168C, where the food material absorbed represented less than 7% compared with the food absorbed with the diet microalgae1lipids. However, absorbed ration increased significantly when scallops were exposed to this same carbohydrate diet at 208C (Fig. 4b).
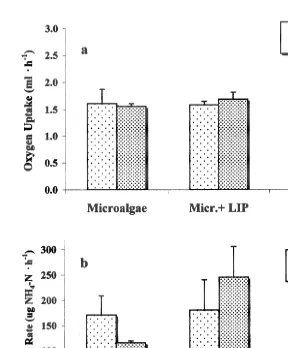
3.4. Oxygen uptake
Oxygen uptake for scallops of standard size (3 g dry tissue wt) showed values around
21
Fig. 3. Argopecten purpuratus. Total ingestion rate (a) and organic ingestion rate (b) at three different diets and two temperatures. Values are means6S.E.
3.5. Ammonia excretion
There were no significant differences (P.0.05) between treatments (Table 2). However, the excretion rate for scallops of 3 g dry tissue weight was higher at the combination 208C microalgae1lipids and lower when the scallops were fed with microalgae1carbohydrates (Fig. 5b).
3.6. Scope for growth
This physiological index was significantly higher when scallops were fed with the diet microalgae1lipids, at both experimental temperatures (Fig. 6). At 168C the scope for
21
growth reached a mean value of 337657.4 J h , which was not significant different
21
Fig. 4. Argopecten purpuratus. Absorption efficiency (a) and absorption rate (b) at three different diets and two temperatures. Values are means6S.E.
2, Fig. 6). When microalgae were used as diet, scope for growth was much lower, with
21 21
values of 15.563.8 J h at 168C and 4.668.4 J h at 208C. These values represented only 1.5–4.5% of the energy gain in the diet containing lipids. A negative scope for
21
growth (214.2 J h ) was observed at 168C when the scallops were fed with the diet containing carbohydrates, although at 208C and the same diet, scope for growth
21
increased to 124616.6 J h .
3.7. Gonad maturation
Fig. 5. Argopecten purpuratus. Oxygen uptake (a) and ammonia excretion (b) at three different diets and two temperatures. Values are means6S.E.
Fig. 6. Argopecten purpuratus. Scope for growth estimated at three different diets and two temperatures. Values are means6S.E.
4. Discussion
The three diets supplied to the experimental scallops were significantly different in total and organic weight. However, the relative organic content was very similar, with
6 21
values around 88%. As the same particle concentration (30310 cells l ) was used in all the experiments, it was expected that the significantly different weights of the particles of each diet could have different effects on some physiological processes, mainly on those expressed by weight (e.g. ingestion and absorption rates).
The capability of marine bivalves to acclimate to a wide range of temperatures (e.g. 10–208C) has been reported by some authors (Schulte, 1975; Widdows et al., 1979; Bayne and Newell, 1983). In the present study Argopecten purpuratus was no exception, showing no significant differences in clearance rate between 16 and 208C
Table 3
Argopecten purpuratus: quantification of ripe and spawned scallops after 72 days of reproductive conditioning a
at different diets and temperature Number of scallops
Total Ripe % Not ripe % Induced Spawned %
Diet (168C)
M 37 10 27.03 27 69.23 8 6 75
M1L 38 26 68.42 12 31.58 24 11 45.83
M1C 39 12 30.77 27 72.97 10 1 10
Diet (208C)
M 37 9 24.32 28 75.68 6 6 100
M1L 38 16 42.11 22 57.89 11 11 100
M1C 39 6 15.38 33 84.62 4 4 100
a
when fed with the diets microalgae and microalgae1lipid. Significant differences in clearance rate between 16 and 208C were only observed with the diet microalgae1
carbohydrate. The composition of the diet had a clear effect on the clearance rate of A.
purpuratus. When microalgae were combined with a lipid emulsion (70% microalgae1
30% lipid), clearance rate was strongly stimulated, with values significantly higher than those from either pure microalgae or a mixed diet of microalgae1carbohydrate. These higher feeding rates can be related with the essential fatty acids contained in the diet (Caers et al., 1999), which is known they are required by marine bivalves. Invertebrates also require lipids by its role in the development of ova into normal larvae (Thompson, 1977; Gallager and Mann, 1981). Thus it is considered that quality of eggs may be improved by feeding broodstock bivalves with rich lipid diets or lipid supplements. These results suggest that A. purpuratus can actively regulate clearance rate, and do not simply switch between feeding and non-feeding states. The data also suggest the presence of chemical receptors at the level of the gills and / or labial palps, which seem able to detect specific nutritive compounds present in the diet. These conclusions are in agreement with Ward et al. (1992), who demonstrated the capacity of chemoreception in the sea scallop Placopecten magellanicus, which increases its clearance and ingestion rates in response to metabolites from the diatom Chaetoceros muelleri. Nevertheless, this is a fundamental question about the mechanisms by which feeding rate can be controlled in filter feeding bivalves.
21
Cranford and Grant (1990) described higher clearance rates (¯16 l h ) in the giant
21
scallop Placopecten magellanicus fed with pure microalgae and lower values (¯4 l h )
when sediment or kelp detritus were used as less nutritional diets. Their values obtained with microalgae were significantly higher than those we recorded in A. purpuratus with a similar diet. This can be explained in part by the fact that they used larger individuals (110 mm shell height) than those used in the present study (80 mm shell height), which can represent twice the tissue biomass in the larger individuals of P. magellanicus (Grant and Cranford, 1989). Clearance rate for A. purpuratus of 80 mm shell height fed with a diet of microalgae supplemented with lipids was similar to that described by Cranford and Grant (1990) for P. magellanicus of 110 mm shell height fed with pure microalgae. This suggests that when similar sizes of individual are compared, A. purpuratus shows higher feeding rates with a diet of microalgae1lipids than P. magellanicus fed with pure microalgae.
The highest total and organic ingestion rates were obtained with the diet of microalgae supplemented with lipids. Total ingestion rate was similar to the values described by Cranford and Grant (1990), even when these authors used larger individuals, with significantly larger tissue weights. The lower ingestion rates obtained with pure microalgae were attributable to the reduced clearance rate and also because this diet is composed by the lightest particles.
digesting carbohydrate by marine bivalves. Other authors (Epifanio, 1979; Romberger and Epifanio, 1981) have reported similar results with juveniles of V. pullastra and M.
mercenaria fed with carbohydrate-rich algae. This diet was also low in protein and
lipids, which are considered very important components for producing high growth rates in bivalves. The food absorbed with microalgae1lipids was significantly greater than at the other two experimental diets, owing to high clearance rate and absorption efficiency (¯70%). Our results show that clearance rate and the amount of food ingested are
regulated in such a way that absorption efficiency is maintained independent of temperature with two of the experimental diets. However, with the diet containing carbohydrates absorption efficiency was significantly higher at 208C that at 168C. Similar results for the effects of the high temperature on absorption efficiency have been reported by Winter (1969) for other species of bivalves.
Oxygen uptake and ammonia excretion in A. purpuratus were not affected by the different experimental conditions, which can be explained by the capacity of this species to acclimate its metabolism to the different experimental diets and temperatures. Widdows et al. (1979) and Bayne and Newell (1983) reported similar results for other species of bivalves within a temperature range of 10–208C.
Scope for growth (SFG) in Argopecten purpuratus is mainly affected by the diet composition, not by temperature. While the lower values of SFG seem to be associated with the diets composed of pure microalgae and by microalgae1carbohydrates, the highest values are associated with a diet rich in lipids at both temperatures. The negative SFG observed at the combination 168C and microalgae1carbohydrates is the result of reduced clearance rates and absorption efficiency. On the other hand, the higher value of SFG obtained at 208C with the diet containing carbohydrates, is the result of increased clearance rate and absorption efficiency. There is no increase in the energy expended in metabolic activity as could be expected at the higher temperature, due to the capacity of this species to show thermal compensation of physiological rates within the experimental range of temperature. Such physiological compensations are not apparent if one relies exclusively on the measurement of individual physiological processes, and it is important to estimate the SFG, which represents an integrated response of the whole organism. The results obtained show that clearance rate is an important physiological process, maximising SFG when A. purpuratus is provided with suitable diets. Similar results were obtained by Navarro and Ulloa (1995), who studied the effects of a natural food supply on the SFG of the bivalve Aulacomya ater. Navarro et al. (1992) also described feeding rate as the main limiting factor for the growth in the cockle
Cerastoderma edule. The present study should encourage further research to determine
the mechanisms used by filter feeding bivalves to control feeding rate by detecting the presence of specific chemical compounds in the diet.
agreement with the present study, where the highest percentage of ripe scallops occurred in individuals fed with an algal diet supplemented with a lipid emulsion at both temperatures (68% at 168C and 42% at 208C). The lower percentages of ripe scallops were obtained with microalgae supplemented with carbohydrate and with pure mi-croalgae, which is also in agreement with the results reported by Coutteau et al. (1996) for broodstocks of A. purpuratus fed with pure microalgae. Our results are also in agreement with larval survival, where the highest survival rate (70.4% at 168C and 81.6% at 208C) of larvae 48 h after fertilisation was obtained from those gametes from
´
scallops conditioned with the diet containing lipids (Martınez et al., 2000). Significantly lower larval survival rates were observed when pure microalgae or microalgae1
carbohydrate were used as diets. The high SFG obtained at 208C with microalgae1
carbohydrates was not reflected in reproductive success (only 15.4% of ripe scallops, see Table 3), suggesting that under these conditions more energy is allocated to tissues other than the gonad.
The findings of the present study can be considered highly relevant from a physiological point of view, mainly in relation to feeding physiology, but great importance also can be attributed to aquaculture activities, especially when hatchery operations (i.e. reproductive conditioning) are involved.
Acknowledgements
The study was carried out with financial support from the Programa Acuicultura y ´
Biotecnologıa Marina, 1 (97), Fondap, Chile (Subprograma Invertebrados). We thanks Dr Gonzalo Gajardo from Universidad de Los Lagos and Dr Patrick Sorgeloos, from the Artemia Reference Center, University of Gent, Belgium providing the lipid supple-mentation. Thanks are also due to Maritza Araneda and Miguel Rivera for their assistance in the laboratory. [RW]
References
Bayne, B.L., Newell, C., 1983. Physiological energetics of marine molluscs. In: Saleuddin, A.S.M., Wilbur, K.M. (Eds.), The Mollusca., pp. 407–515.
Bayne, B.L., Hawkins, A.J.S., 1990. Filter feeding in bivalve molluscs: controls on energy balance. In: Mellinger, J., Truchot, J.P., Lahlou, B. (Eds.), Comparative Physiology, Animal Nutrition and Transport Processes. I. Nutrition in Wild and Domestic Animals, Vol. 5, Karger, Basel, pp. 70–83.
Caers, M., Coutteau, P., Cure, K., Morales, V., Gajardo, G., Sorgeloos, P., 1999. The Chilean scallop Argopecten purpuratus (Lamarck, 1819): II. Manipulation of the fatty acid composition and lipid content of the eggs via lipid supplementation of the broodstock diet. Comp. Biochem. Physiol. (B) 123, 97–103. Conover, R.J., 1966. Assimilation of organic matter by zooplankton. Limnol. Oceanogr. 11, 338–354.
´
Coutteau, P., Caers, M., Cure, K., Gajardo, G., 1996. Supplementation of lipid emulsions to algal diets in the hatchery rearing of bivalves. In: Gajardo, G., Coutteau), P. (Eds.), Proc. of a Workshop on Fish and Mollusc Larviculture, Impresora Creces, Santiago, Chile, pp. 145–154.
´ ´ ´
Dıaz, M., Martınez, G., 1992. Efecto de diferentes dietas sobre el balance energetico en juveniles de ´
Argopecten purpuratus L. Rev. Biol. Mar. Valparaıso 27, 163–173.
Elliott, J.M., Davison, W., 1975. Energy equivalents of oxygen consumption in animal energetics. Oecologia (Berl.) 19, 195–201.
Epifanio, C.E., 1979. Growth in bivalve molluscs: nutritional effects of two or more species of algae in diets fed to the American oyster, Crassostrea virginica (Gmelin) and the hard clam, Mercenaria mercenaria (L.). Aquaculture 18, 1–12.
Epifanio, C.E., 1983. Phytoplankton and yeast as food for juvenile bivalves: a review of research at the University of Delaware. In: Pruder, G.D., Langdon, C., Conclin, D. (Eds.), Proc. of the 2nd Int. Conf. of Aquaculture Nutrition, Biochemical and Physiological Approaches To Shellfish Nutrition, Louisiana State University Press, Baton Rouge, LA, pp. 292–304.
Gabbott, P.A., 1975. Storage cycles in marine bivalve molluscs: a hypothesis concerning the relationship between glycogen metabolism and gametogenesis. In: Barnes, H. (Ed.), Proc. of the 9th Eur. Mar. Biol. Symp, Aberdeen University Press, Aberdeen, pp. 191–211.
Gallager, S.M., Mann, R., 1981. Use of lipid-specific staining techniques for assaying condition in cultured bivalve larvae. J. Shellfish. Res. 1, 69–73.
Gallager, S.M., Mann, R., 1986. Growth and survival of larvae of Mercenaria mercenaria (L.) and Crassostrea virginica (Gmelin) relative to broodstock conditioning and lipid content of eggs. Aquaculture 56, 105–121. Gnaiger, E., 1983. Calculation of energetic and biochemical equivalents of respiratory oxygen consumption. In: Gnaiger, E., Forstner, H. (Eds.), Polarographic Oxygen Sensors, Springer, Berlin, pp. 337–345, Appendix C.
Grant, J., Cranford, P.J., 1989. The effect of laboratory diet conditioning on tissue and gonad growth in the sea scallop Placopecten magellanicus. In: Ryland, F.J., Tyler, P.A. (Eds.), Proc. 23rd Eur. Mar. Biol. Symp, Reproduction, Genetics and Distribution of Marine Organisms, Olsen and Olsen, Denmark, pp. 95–105. Helm, M.M., Holland, D.L., Utting, S.D., East, J., 1991. Fatty acid composition of early non-feeding larvae of
the European flat oyster, Ostrea edulis. J. Mar. Biol. Assoc. UK 71, 691–705.
´ ´
Hojas, F., 1983. Informe Proyecto Recuperacion del Ostion del Norte Chlamys(Argopecten) purpurata en ´
Bahıa Mejillones del Sur, Instituto de Fomento Pesquero, Stgo, Chile.
Illanes, J.E., 1987. Cultivation of the Northern Scallop of Chile Chlamys(Argopecten) purpurata in controlled and natural environment. In: Proc. 6th Pectinid Workshop, Menai Bridge, Wales.
Laing, I., Utting, S.D., Kilada, R.W.S., 1987. Interactive effect of diet and temperature on the growth of juvenile clams. J. Exp. Mar. Biol. Ecol. 113, 23–38.
Langdon, C.J., Waldock, M.J., 1981. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. UK 61, 431–448.
MacDonald, B.A., Thompson, R.J., 1986. Influence of temperature and food availability on the ecological energetics of the giant scallop Placopecten magellanicus. III. Physiological ecology, the gametogenic cycle and scope for growth. Mar. Biol. 93, 37–48.
´ ´
Martınez, G., Torres, M., Uribe, E., Dıaz, M.A., Perez, H., 1992. Biochemical composition of broodstock and early juvenile chilean scallops, Argopecten purpuratus Lamarck, held in two different environments. J. Shellfish Res. 11, 307–313.
´ ´ ´
Martınez, G., Caceres, L.A., Uribe, E., Dıaz, M.A., 1995. Effects of different feeding regimes on larval growth and the energy budget of juvenile Chilean scallops, Argopecten purpuratus Lamarck. Aquaculture 132, 313–323.
´
Martınez, G., Aguilera, C., Mettifogo, L., 2000. Interactive effects of diet and temperature on reproductive conditioning of Argopecten purpuratus broodstock. Aquaculture 183, 149–159.
Navarro, J.M., Ulloa, L., 1995. Effects of environmental factors on physiological responses and fluctuations in the soft tissue weight of the bivalve Aulacomya ater in a bay of southern Chile. In: Hawkins, L.E., Hutchinson, S. (Eds.), Proc. 30th Eur. Mar. Biol. Symp., Southampton, UK, September 1995, The Responses of Marine Organisms To Their Environments, University of Southampton Press, UK. Navarro, E., Iglesias, J.I.P., Ortega, M.M., 1992. Natural sediment as a food source for the cockle
Cerastoderma edule (L.): effect of variable particle concentration on feeding,digestion and the scope for growth. J. Exp. Mar. Biol. Ecol. 156, 69–87.
´
Padilla, M., 1979. Desarrollo larval del ostion Chlamys (Argopecten) purpurata Lamarck (1819) en ´
condiciones de laboratorio, Ciencia y Tecnologıa del Mar, Stgo, Chile, CONA 4 1979.
Robinson, A., 1992. Dietary supplement for the reproductive conditioning of Crassostrea gigas Kumamoto (Thunberg). II. Effects on glycogen, lipid and fatty acid content of broodstock oysters and eggs. J. Shellfish Res. 11, 443–447.
Romberger, H.P., Epifanio, C.E., 1981. Comparative effects of diets consisting of one or two algal species upon assimilation efficiencies and growth of juvenile oysters, Crassostrea virginica (Gmelin). Aquaculture 25, 77–87.
Sastry, A.N., 1966. Temperature effects in the reproduction of the bay scallop, Aequipecten irradians Lamarck. Biol. Bull. 130, 118–143.
Sastry, A.N., 1968. The relationships among food, temperature, and gonad development of the bay scallop Aequipecten irradians. Physiol. Zool. 41, 44–53.
Schulte, E.H., 1975. Influence of algal concentration and temperature on the filtration rate of Mytilus edulis. Mar. Biol. 30, 331–341.
Thompson, R.J., 1977. Blood chemistry, biochemical composition, and the annual reproductive cycle in the giant scallop, Placopecten magellanicus, from Southeast Newfoundland. J. Fish. Res. Board Can. 34, 2104–2116.
Uki, N., Kikuchi, S., 1984. Regulation of maturation and spawning of an abalone Haliotis (Gastropoda) by external environmental factors. Aquaculture 39, 247–261.
Ward, J.E., Cassel, H.K., MacDonald, B.A., 1992. Chemoreception in the sea scallop Placopecten magellanicus (Gmelin). I. Stimulatory effects of phytoplankton metabolites on clearance and ingestion rates. J. Exp. Mar. Biol. Ecol. 163, 235–250.
Whyte, J., 1987. Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture 60, 231–241.
Widdows, J., 1985. Physiological procedures. In: Bayne, B.L. (Ed.), The Effects of Stress and Pollution On Marine Animals, Praeger, New York, pp. 161–178.
Widdows, J., Fieth, P., Worrall, C.M., 1979. Relationships between seston, available food and feeding activity in the common mussel Mytilus edulis. Mar. Biol. 50, 195–207.
¨