255 (2000) 261–274
www.elsevier.nl / locate / jembe
Fatty acid compositions of gonadal material and diets of the
sea urchin, Psammechinus miliaris: trophic and nutritional
implications
a ,* b c a
Elizabeth J. Cook , Michael V. Bell , Kenneth D. Black , Maeve S. Kelly
a
Scottish Association of Marine Science, Dunstaffnage Marine Laboratory, Oban, Argyll,
Scotland PA37 4AD, UK
b
Institute of Aquaculture, Stirling University, Stirling, Scotland FK9 4LA, UK
c
Centre for Coastal Marine Science, Dunstaffnage Marine Laboratory, Oban, Argyll, Scotland PA37 4AD,
UK
Received 29 May 2000; received in revised form 9 September 2000; accepted 26 September 2000
Abstract
The fatty acid compositions of gonadal material was examined for the sea urchin Psammechinus
miliaris (Gmelin) held in aquaria and fed either salmon feed pellets or the macroalga, Laminaria saccharina for 18 months. Gonadal material was also examined from P. miliaris collected from four field sites, including commercial scallop lines encrusted with the mussel, Mytilus edulis, sea cages stocked with Atlantic salmon Salmo salar and two intertidal sea-loch sites, characterised by either a fine mud or a macroalgal substratum. The fatty acid compositions of known and potential dietary material was examined. The proportions of certain fatty acids in the gonads of P. miliaris were significantly affected by diet type and location. Docosahexaenoic acid (DHA) 22:6 n23 was significantly higher in the gonads of the sea urchins fed salmon feed in aquaria and collected from the salmon cages and scallop lines than in the gonads of the sea urchins fed L. saccharina in aquaria and collected from the intertidal sea loch sites. The salmon feed and the mussel tissue also contained a high proportion of this fatty acid. Stearidonic acid 18:4 n23 and arachidonic acid 20:4 n26, however, were found in significantly higher proportions than DHA in the gonads of the sea urchins fed L. saccharina and collected from the two intertidal sea-loch sites. L. saccharina was also found to contain high proportions of stearidonic and arachidonic acid. The gonads of the sea urchins collected from the intertidal site, characterised by a mud substratum, and from the scallop lines were found to contain a lower 18:1 n29 / 18:1 n27 ratio and a higher proportion of branched and odd-chained fatty acids, signifying a high dietary bacterial input, than the sea urchins held in the aquaria and collected from the salmon cage. 20:2 and 22:2 non-methylene-interrupted dienoic fatty acids (NMIDs) were found in P. miliaris fed diets lacking these fatty acids suggesting de novo biosynthesis. These results, therefore, suggest that the proportions / ratios
*Corresponding author. Tel.: 144-1631-562-244; fax: 144-1631-559-000.
E-mail address: [email protected] (E.J. Cook).
of certain fatty acids in the gonads of P. miliaris could be used to give an indication of the predominant diet type of this species in the wild. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Fatty acid composition; Nutritional implications; Psammechinus miliaris; Sea urchin
1. Introduction
The gonads of Psammechinus miliaris have been found to contain between 14 and 21% (dry matter) total lipid, with the highest values typically found in the period prior to spawning (Comely, 1979). Walker et al. (1998) found that the gonads of S. droebach-iensis are used as a site for lipid storage during the remainder of the year. Variations in fatty acid composition, particularly in the proportions of 20:4 n26, 20:5 n23 and 22:6 n23 in the gonads of 12 different species of sea urchin collected from various locations in Japan have been attributed to differences in their respective habitats, particularly the type of food available (Tagaki et al., 1986). This assumption was based, however, on observational studies of feeding habits in situ and unfortunately there have been no studies involving the experimental manipulation of sea urchin diet to support this claim. The aim of this study, therefore, was to determine the fatty acid profiles of the gonads of P. miliaris fed either (i) a commercially manufactured salmon feed, or (ii) the macroalga, Laminaria saccharina for a period of 18 months and to compare the results with fatty acid profiles of sea urchins collected from four sites on the west coast of Scotland. The fatty acid profiles of their known (i.e., salmon feed and the macroalga, L. saccharina) and potential food sources (i.e., mussels) were also obtained, to increase our understanding of the feeding habits of P. miliaris.
2. Methods
2.1. Sample collection
Sea urchins were collected using SCUBA from a sublittoral population (depth 3–6 m) in Columbas’ Bay, Loch Creran (56832.29N, 05817.09W) in February 1996 and maintained in aquaria at Dunstaffnage Marine Laboratory (DML). The sea urchins were held without additional food for 2 weeks to minimise any variations in nutritional status prior to the start of the experiment (Leighton, 1966; Vadas, 1977). Twenty-five sea urchins (test diameter, 21.3–21.7 mm) were placed into each of six compartments, within two rectangular tanks (0.730.630.3 m) which were supplied by a continuous
21
flow-through system with a flow rate of 1.5 ml min . The groups were either fed the macroalga, L. saccharina (without epiphytes) or salmon feed (TROUW Supreme 6-mm pellets). There were three replicates of each treatment. The salmon pellets were replaced 3 times per week and the macroalgae was replaced as required. The water temperature ranged from 4 to 168C. The feeding period lasted for 18 months from July 1997 and 10 sea urchins were selected at random for analysis from the each of the dietary treatments in January 1999.
for 12 months. These sea urchins had been held in nets (mesh size 15 mm) a period of 10 months, at a depth of 9 m within the salmon cage. In Loch Glencoul, the sea urchins were collected from two sites, 100 m apart, which were exposed at mean low water spring tides and were divided by a shallow spit that protruded into the bay. These sites were characterised by either a fine mud substratum with no macroalgae present or by a boulder substratum, with a dense coverage of the macroalga, L. saccharina. The sea urchins were held for a maximum of 7 days before dissection in aquaria at DML,
21
supplied by a continuous flow-through system with a seawater flow rate of 1.5 l min . Samples of known and potential food items were also collected for analysis. These included; commercial salmon feed (TROUW Supreme 6-mm pellets), which was supplied by Joseph Johnston and Sons in December 1998 and stored at 2208C to prevent any deterioration in the quality of the feed. The macroalga, L. saccharina, which was collected from Loch Creran (56832.29N, 05817.09W) and the mussel, Mytilus edulis collected from commercial scallop lines in Loch Fyne in February 1999. The macroalgae and the mussels were held separately for a maximum of 4 days in aquaria supplied by a
21
continuous flow-through system with a flow rate of 1.5 l min . All the lipid extraction and analysis was undertaken on fresh material, with the exception of the salmon feed.
2.2. Tissue preparation
Ten sea urchins, randomly chosen from each dietary treatment group / location and 10 replicates of each dietary source were analysed. The sea urchins were dissected and the gonads removed. The gonads were weighed and between 0.12 and 1.18 g of gonadal material were used for the extraction procedure depending on the quantity of gonad contained within the sea urchin. The mussels were also dissected and the internal organs and viscera were finely chopped, the excess water drained and between 0.99 and 1.43 g of tissue (wet weight) was used. The L. saccharina was cleaned thoroughly in filtered seawater, chopped coarsely and between 4.37 and 6.76 g of tissue (wet weight) was used for lipid extraction. Both central and peripheral regions of the frond were used in the analysis. The salmon feed pellets were coarsely ground using a pestle and mortar and between 0.18 and 0.24 g of pellet was used for lipid extraction.
2.3. Total lipid extraction
pre-weighed boiling tube at 358C. The boiling tube was re-weighed and the amount of crude lipid calculated.
For analysis of the fatty acid composition, the total lipid extracts from the gonads and diets were subjected to acid-catalysed transesterification. One ml toluene and 2 ml 1% sulphuric acid in methanol were added to the lipid extract and left sealed under nitrogen at 508C overnight. On cooling, the esters were extracted with hexane containing butylated hydroxy toluene (BHT, 0.01%) and purified using thin-layer chromatography. Fatty acid methyl ester (FAME) extracts were spotted onto silica gel 60 plates (20320 cm) and developed with a heptane–diethyl ether–acetic acid (90:10:1, v / v) neutral lipid solvent (Christie, 1992). FAME’s were eluted from the adsorbent with hexane (containing BHT, 0.01%, w / v) to a concentration of 1 mg lipid / 100 ml hexane and analysed using a Carlo Erba 4160 gas chromatograph. This was equipped with a split column injector (100:1), flame ionization detector and a Chrompak CP Wax 58 CB fused-silica capillary column (25 m30.25 mm, i.d.) with hydrogen as the carrier gas.
– 1
The oven temperature was programmed to rise from 160 to 2408C at 48C min . The detector output was coupled to a computerised data system (Varian StarE) for storage and integration of the chromatograms. FAME assignments were made by comparison of retention times with authentic standards (Supelco, Sigma) and by gas chromatography– mass spectrometry (GC–MS) using a Fisons MD 800 fitted with a DB-5MS column (15 m30.25 mm i.d.; J&W Scientific) with helium as the carrier gas. Dimethyl disulphide adducts of monosaturated and non-methylene interrupted diene fatty acids were prepared to assign double bond positions (Nichols et al., 1986).
2.4. Statistical analysis
The total lipid and fatty acid data were presented as percentages, which required transforming using the arcsine transformation to produce a normally distributed data set (Shapiro and Wilk, 1965) with homoscedastic variances (Bartlett’s Test, Zar, 1996). Treatment or diet was used as the fixed factor in a one-way ANOVA to test for differences in total crude lipid or in specific fatty acids (MINITAB, Release 12.1 for Windows). The Tukey multiple comparison test was used to assess where significant differences occurred (Zar, 1996).
3. Results
3.1. Crude lipid content (% wet weight of sample)
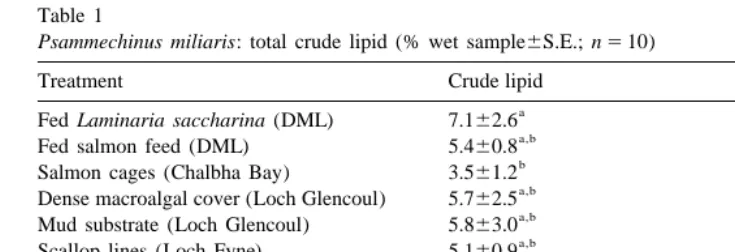
A significant difference in crude lipid within the gonads was found between the dietary treatment / location groups (one-way ANOVA; n510, df55, F53.22, P5 0.013). The gonads of the sea urchins fed L. saccharina were found to contain a significantly higher percentage of crude lipid (7.1%) compared with the gonads of the sea urchins held in the salmon cage in Chalbha Bay (3.5%) (Tukey; P,0.05). There were no significant differences between the other treatment groups (Table 1).
Table 1
Psammechinus miliaris: total crude lipid (% wet sample6S.E.; n510)
Treatment Crude lipid
a
Fed Laminaria saccharina (DML) 7.162.6
a,b
Fed salmon feed (DML) 5.460.8
b
Salmon cages (Chalbha Bay) 3.561.2
a,b
Dense macroalgal cover (Loch Glencoul) 5.762.5
a,b
Mud substrate (Loch Glencoul) 5.863.0
a,b
Scallop lines (Loch Fyne) 5.160.9 Diet
a
Salmon feed 23.965.4
b
Macroalgae — L. saccharina 0.560.4
b
Mussel tissue (Mytilus edulis) 1.560.8
Means followed by the same letter for either treatment or diet are not significantly different (Tukey;
P,0.05).
crude lipid (one-way ANOVA; n59, df52, F5465.67, P,0.001). The salmon pellets contained a higher amount of lipid (23.9% of the weight) compared to the macroalga, L. saccharina (0.5%) and mussel tissue (1.5%) (Tukey; P,0.05). There was no significant difference between the total lipid content in the macroalgae and mussel tissue (Table 1).
3.2. Fatty acid composition
3.2.1. Diets
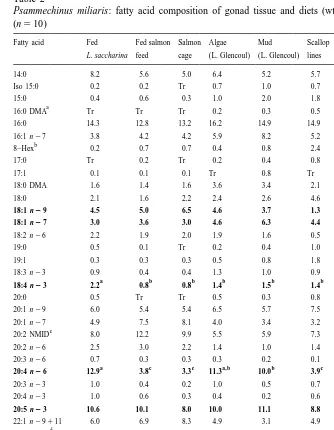
The fatty acid composition of the diets is presented in Table 2. The saturated fatty acid 16:0 was a major component in the fatty acid composition of all the diet types, constituting between 14.6 and 18.5% of the total lipid content. In addition, the main fatty acids in the salmon feed were the monounsaturates, 18:1 n29 and 22:1 n211, although this diet contained higher quantities of the fatty acids, 20:1 n29, 20:1 n27 and 20:4 n23 than the L. saccharina and the mussel tissue. 22:6 n23 was also present in the salmon feed. The macroalga, L. saccharina was characterised by high con-centrations of polyunsaturated fatty acids (PUFAs), particularly 18:4 n23, 20:4 n26 and 20:5 n23, although this diet was found to contain significantly higher quantities of 18:3 n23 than the salmon feed and the mussel tissue (ANOVA; n59; df52; F5190.15; P,0.001). L. saccharina was the only diet to contain 19:0 (Table 2). This diet, however, was devoid of 20:1, 20:2, 20:3 n26 and fatty acids with a chain length greater than C20. The main fatty acids in the mussel tissue were 20:5 n23 and 22:6 n23, although 20:3 n26, 20:3 n23, 22:2, 20:2 n26 and the 20:2 and 22:2 non-methylated interrupted dienoic fatty acids (NMID) were present in substantially higher quantities than in the salmon feed and the macroalgae.
3.2.2. Gonadal tissue
Table 2
Psammechinus miliaris: fatty acid composition of gonad tissue and diets (wt.% of total fatty acids)
(n510)
Fatty acid Fed Fed salmon Salmon Algae Mud Scallop L. Salmon Mussel
L. saccharina feed cage (L. Glencoul) (L. Glencoul) lines saccharina feed tissue
14:0 8.2 5.6 5.0 6.4 5.2 5.7 5.9 7.3 2.6 Iso 15:0 0.2 0.2 Tr 0.7 1.0 0.7 0.1 0.2 – 15:0 0.4 0.6 0.3 1.0 2.0 1.8 Tr 0.4 0.6
a
16:0 DMA Tr Tr Tr 0.2 0.3 0.5 1.4 – 0.5 16:0 14.3 12.8 13.2 16.2 14.9 14.9 14.6 16.7 18.5 16:1 n27 3.8 4.2 4.2 5.9 8.2 5.2 1.9 6.7 6.4
Unidentified 4.0 3.5 3.3 3.6 5.4 6.6 1.6 3.0 3.0 Total Sats. 27.8 22.5 22.7 31.3 30.3 32.8 24.5 26.5 36.1 Total Mono. 28.6 32.9 36.0 31.0 31.9 28.4 7.5 51.7 11.9 PUFA n23 16.0 21.5 21.9 15.9 17.2 21.9 47.3 16.2 38.3 PUFA n26 16.1 6.2 5.8 13.6 12.0 4.72 20.6 4.97 7.64 18:1 n29:18:1 n27 1.5 1.4 2.2 1.0 0.6 0.3 0.0 5.1 0.7
Means followed by the same letter for the same fatty acid in either the gonadal tissue or the diets are
a b
not significantly different (Tukey; P,0.05). Tr5Levels ,0.1%; dimethyl acetal, 8, hexadecenoic-7
c
methyl, methyl ester; 20:2 non-methylene-interrupted-dienoic fatty acids (NMID), includingD5, 11
d
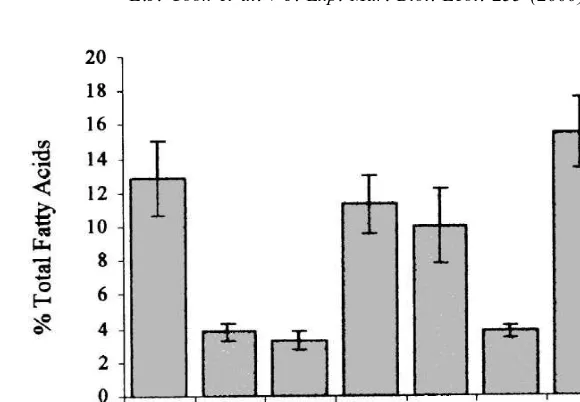
on the west coast of Scotland is presented in Table 2. In general, the gonads contained between 22.5 and 32.8% saturates, 28.4 and 36.0% monounsaturates, 4.7 and 16.1% n26 and 15.9 and 21.9% n23 PUFAs. The gonads of the sea urchins fed L. saccharina and collected from the two inter-tidal sites in Loch Glencoul, however, contained higher amounts of n26 and lower amounts of n23 PUFAs than the gonads of the sea urchins fed salmon feed, held in the salmon cage and attached to the scallop lines (Table 2). All the gonads were characterised by their high content of the saturated fatty acid 16:0 and the polyunsaturated fatty acid 20:5 n23, which comprised 12.8– 16.2 and 8.8–11.1% of the total lipid content, respectively. High concentrations of 22:6 n23 were found in the gonads of the sea urchins fed salmon feed, held in the salmon cage and attached to the scallop lines compared with the gonads of the sea urchins fed L. saccharina and collected from the two intertidal sites (Loch Glencoul) (ANOVA; n510; df55; F527.12; P,0.001) (Fig. 1). A significantly higher proportion of 20:4 n26, however, was found in the gonads of sea urchins fed L. saccharina and collected from the two intertidal sites in Loch Glencoul compared with the gonads of sea urchins fed salmon feed and collected from the salmon cages and scallop lines (ANOVA; n510; df55; F581.39; P,0.001) (Fig. 2). A significantly higher proportion of 18:4 n23 was also found in the gonads of the sea urchins fed L. saccharina than all the other sea urchins analysed (ANOVA; n510; df55; F56.93; P,0.001). The sea urchins collected from the scallop lines and the two intertidal sites in Loch Glencoul did have slightly higher amounts of this fatty acid in their gonads than the sea urchins fed salmon feed or held in the salmon cage (Table 2).
A significantly higher proportion of 16:1 n27 was found in the gonads of the sea urchins collected from the intertidal site, characterised by the mud substratum in Loch
Fig. 2. Relative amounts of 20:4 n26 fatty acid in the gonadal tissue of Psammechinus miliaris and in the macroalga, Laminaria saccharina (L. sacc.), salmon feed (Sal. Feed) and mussel tissue (Mussel). Error bars are standard deviations.
Glencoul than in the gonads from the other treatment groups / locations (ANOVA; n59; df55; F512.66; P,0.001; Tukey; P,0.05). The ratio of 18:1 n29 / 18:1 n27 was also lower in the gonads of the sea urchins collected from this location and from the scallop lines in Loch Fyne compared with the other groups (Table 2). Whereas, the sea urchins held in the salmon cage had a higher 18:1 n29 / 18:1 n27 ratio than the other groups. The proportion of odd-chain and branched fatty acids in the gonads was also greatest in the sea urchins collected from the scallop lines and the intertidal site, characterised by the mud substratum. In contrast, the lowest proportions of these fatty acids were found in the gonads of the sea urchins fed salmon feed and L. saccharina and held in the salmon cages.
The gonads of the sea urchins fed L. saccharina also contained 20:1 n29, 20:1 n27, 20:2 n26, 20:2 and 22:2 non-methylene-interrupted dienoic fatty acids (NMID), whereas the macroalgae was devoid of these fatty acids. The L. saccharina and the salmon feed were also devoid of 20:3 n26, however, low amounts of this fatty acid were found in the gonads of the sea urchins fed these two diet types.
4. Discussion
content. The difference in lipid content of the gonads, particularly between the sea urchins fed L. saccharina and those collected from the salmon cages in Loch Chalbha may, therefore, be the result of differences in uptake rates and / or storage of the different fatty acids within their diets.
In addition, the proportion / ratio of certain fatty acids in the gonads of the sea urchin, Psammechinus miliaris was affected by diet. This suggests that fatty acid composition in P. miliaris could be used as an indicator of diet. For example, a large proportion of the lipid was composed of 22:6 n23 in the gonads of the sea urchins fed salmon feed. This fatty acid was also found in high quantities in the salmon feed. Analysis of the gonads from the sea urchins held in cages stocked with Atlantic salmon also found high levels of 22:6 n23 in the lipid fraction. This fatty acid is a major PUFA in fish oils (Gunstone et al., 1994) and it has been associated with a predominantly carnivorous / necrophagous diet in benthic organisms (Pond et al., 1997). Tagaki et al. (1986) found that the Echinolampas sternopetala (Order Cassiduloidea) contained high amounts of 22:6 n23, which was attributed to its feeding upon the carcasses of marine animals (Lawrence, 1975). The main dietary component in the sea urchins collected from the salmon cage in Chalbha Bay, therefore, is most likely to be the uneaten salmon feed pellets, as they contain a high proportion of animal-based proteins and lipids. This is supported by observations of P. miliaris feeding upon these pellets, when held in cages stocked with Atlantic salmon (Kelly et al., 1998a).
The mussel, Mytilus edulis collected from the scallop lines in Loch Fyne was also found to contain high levels of 22:6 n23, which is in agreement with the findings of Rodegker and Nevenzel (1964) on the fatty acid composition on the mussel, Mytilus californianus. Mussels are filter feeders and Loch Fyne is known to be a site of high plankton productivity (Tett et al., 1986). Certain classes of flagellated microalgae can contain high proportions of 22:6 n23 (Mansour et al., 1999). Analysis of the sea urchins collected from the lines in Loch Fyne found that their gonads also contained high levels of 22:6 n23. P. miliaris are grazers and although, it is known that they can capture plankton with their tube feet (Leighton, 1968), the main source of this fatty acid is probably Mytilus edulis and various other species of encrusting organisms which settle on the outside of the commercial scallop lines. This is supported by observations of P. miliaris feeding upon the mussel, M. edulis covering the scallop lines in Loch Fyne (pers. obs.)
supported by observations of P. miliaris feeding upon fronds of L. saccharina at the site in Loch Glencoul, which is dominated by a dense stand of this macroalga. The second site, however, is characterised by a mud substratum and was devoid of macroalgae when the sea urchins were collected. The high proportions of 18:4 n23 and 20:4 n26 fatty acids in their gonads, therefore, may have been the result of these sea urchins feeding upon drift algae that had become dislodged from the adjacent site, phytodetritus, microbial mats or a combination of all three sources. Further investigation of the fatty acid composition of the mud substratum is now required to determine whether the substratum or the drift algae was the dietary source of the 18:4 n23 and 20:4 n26 fatty acids in the gonads of the sea urchins collected from this site.
The ratio of 18:1 n29 / 18:1 n27 fatty acids in the gonads of P. miliaris also showed a wide range in values, particularly between the sea urchins collected from the different locations. The gonads of the sea urchins collected from the intertidal site in Loch Glencoul, characterised by the mud substratum, and from the scallop lines in Loch Fyne, exhibited a lower ratio for the 18:1 n29 / 18:1 n27 acids and contained a higher proportion of branched and odd-chained fatty acids than the gonads of the sea urchins held in the aquaria or in the salmon cage. Vaccenic acid, 18:1 n27 has been widely proposed as an indicator of bacterial input to the diet, because it is biosynthesised by the anaerobic pathway, reputedly unique to bacteria (Volkman et al., 1980; Gillian et al., 1988). The ratio of 18:1 n29 / 18:1 n27 fatty acids, therefore, has been found to give a useful indication of the contribution of bacteria to the nutrition of marine organisms (Sargent et al., 1987). Pond et al. (1997) found that the hydrothermal vent shrimp, Rimicaris exoculata, which is thought to derive nutrition from bacterial production, contained a significantly lower 18:1 n29 / 18:1 n27 ratio than the vent shrimp, Alvinocaris markensis which has a necrophagous diet. In addition, the proportion of odd-chained and branched fatty acids within the lipid fraction has also been used to indicate the level of dietary bacterial input (Leo and Parker, 1966). It is well established that sediments contain a high level of odd and branched chain fatty acids, which are believed to be of bacterial origin (Leo and Parker, 1966; Sargent et al., 1983; Phillips, 1984). This is supported by the fact that a high proportion of these fatty acids have been found in the gonads of the mud-ingesting sea urchin Strongylocentrotus franciscanus (Hayashi and Takagi, 1977) and the holothurians, Scotoplanes theeli (Lewis, 1967) and Holothuria forkskali (Allen, 1968). This would suggest, therefore, that the sea urchins in Loch Glencoul are deriving some of their fatty acids from the substratum and not feeding exclusively on macroalgae. It also suggests that the sea urchins on the scallop lines in Loch Fyne have a high bacterial and / or diatom input in their diet.
The presence of 20:2 and 22:2 non-methylene-interrupted-dienoic fatty acids (NMID) in the gonads of P. miliaris, fed either salmon feed or L. saccharina, diets which contain miminal amounts of these NMID, however, suggests that this species is able to synthesise 20:2 and 22:2 NMIDs de novo. The 20 carbon NMIDs are formed by a
D5-desaturase acting on 20:1 n-9 and 20:1 n27 to give 20:2 D5,11 and 20:2 D5,13,
respectively. These fatty acids can then be elongated to 22:2D7,13 and 22:2D7,15 while
22:2D5,13 is formed from 22:1 n29 byD5-desaturase. The amounts of NMIDs present
In addition to fatty acids being used to provide an insight into dietary sources, certain fatty acids have been associated with improved growth rates in marine organisms (Enright et al., 1986; Mai et al., 1996). This has led to a number of studies which have attempted to determine the nutritional value of microalgae as food for organisms in mariculture (Langdon and Waldock, 1981; Pillsbury, 1985; Ben-Amotz et al., 1987; Volkman et al., 1989). Enright et al. (1986) found that the daily growth rate was significantly increased by feeding the juvenile oyster, Ostrea edulis, a diet which contained high levels of the essential fatty acids 20:5 n23 and 22:6 n23. Similarly, the addition of 22:6 n23 to algal diets fed to the oyster spat, Crassostrea gigas, increased growth compared with unsupplemented diets (Langdon and Waldock, 1981). Mai et al. (1996) found that the highest specific growth rate in the abalone, Haliotis
tuberculata and H. discus hannai was observed when they were fed the red alga,
Palmaria palmata. This macroalga was characterised by the highest proportion of 20:5 n23 compared with the other algal species examined. Mai et al. (1996) also found that PUFAs of both the n23 and n26 families appeared to be essential for the growth of these two abalone species.
Psammechinus miliaris was found to exhibit significantly greater somatic and gonadal growth rates when fed salmon feed compared to the macroalga, L. saccharina (Cook et al., 1998). High growth rates have also been observed in natural settlements of P. miliaris on the scallop lines in Loch Fyne where they have been observed feeding upon encrusting organisms. L. saccharina contains higher proportions of both n23 and n26 PUFAs than the salmon feed and the mussel, Mytilus edulis. This suggests, therefore, that the proportions of these PUFAs may not be as important as specific fatty acids in promoting growth in P. miliaris. The salmon feed and M. edulis contain a relatively large proportion of the 22:6 n23 acid, and yet this fatty acid is absent in L. saccharina. This fatty acid, therefore, may be important in promoting growth in P. miliaris. Salmon feed, however, contains a higher proportion of total lipid than L. saccharina. Further investigation, therefore, is required to determine whether total dietary lipid availability and / or specific fatty acids, for example 22:6 n23 are important in promoting growth in P. miliaris and to increase our understanding of the nutritional requirements of this species.
In conclusion, this study has shown that the fatty acid composition of the gonads in P. miliaris can be significantly altered by different diets and consequently, it can be used to give an indication of their predominant food. It has also highlighted specific fatty acids, particularly 22:6 n23 which may be important in the growth of this species.
Acknowledgements
from the scallop farm, Mr. John Joyce for his technical advice and Mr. James Dick for his help with the GC–MS analysis. [SS]
References
Allen, W.V., 1968. Fatty acid synthesis in the Echinoderms Asterias rubens, Echinus esculentus and Holothuria
forskali. J. Mar. Biol. Assoc. UK 48, 521–533.
Ben-Amotz, A., Fishler, R., Schneller, A., 1987. Chemical composition of dietary species of marine unicellular algae and rotifers with emphasis on fatty acids. Mar. Biol. 95, 31–36.
Cook, E.J., Kelly, M.S., McKenzie, J.D., 1998. Somatic and gonadal growth of the sea urchin Psammechinus
miliaris (Gmelin) fed artificial salmon feed compared with a macroalgal diet. J. Shell. Res. 17, 1549–1555.
Christie, W.W., 1992. In: Gas Chromatography and Lipids. The Oily Press, Dundee.
Comely, C.A., 1979. Observation on Two Scottish West Coast Populations of Psammechinus miliaris. SMBA Internal Report.
¨
Eichelbaum, E., 1909. Uber nahrung und ernahrungsorgane von echinodermen. Wiss. Meeresunters, Abt. Kiel. 11, 187–275.
Enright, C.T., Newkirk, G.F., Craigie, J.S., Castell, J.D., 1986. Evaluation of phytoplankton as diets for juvenile Ostrea edulis L. J. Exp. Mar. Biol. Ecol. 96, 1–13.
Emson, R.H., Moore, P.G., 1998. Diet and gonad size in three populations of Echinus esculentus. In: Mooi, R., Telford, M. (Eds.), Proceedings of the 9th International Echinoderm Conference, San Francisco. Balkema, Rotterdam, pp. 641–644.
Folch, J., Lees, N., Sloan-Stanley, G.H., 1957. A simple method for the isolation and purification of total lipid. J. Biol. Chem. 226, 287–291.
Gillian, F.T., Stoilov, I.L., Thompson, J.E., Hogg, R.W., Wilkinson, C.R., Djerassi, C., 1988. Fatty acids as biological markers for bacterial symbionts in sponges. Lipids 23, 1139–1145.
Graeve, M., Kattner, G., Hagen, W., 1994. Diet-induced changes in the fatty acid composition of Arctic herbivourous copepods: experimental evidence of trophic markers. J. Exp. Mar. Biol. Ecol. 182, 97–110. Gunstone, F.D., Harwood, J.L., Padley, F.B., 1994. Occurrence and characteristics of oils and fats. In: The
Lipid Handbook. Chapman and Hall, London, pp. 47–223.
Hayashi, K., Takagi, T., 1977. Occurrence of branched- and odd-chain fatty acids in a mud-feeding sea urchin,
Strongylocentrotus franciscanus. Bull. Fac. Fish. Hokkaido Univ. 28, 40–46.
Kelly, M.S., McKenzie, J.D., Brodie, C.C., 1998. Sea urchins in polyculture: the way to enhanced gonad growth. In: Mooi, R., Telford, M. (Eds.), Proceedings of the 9th International Echinoderm Conference, San Francisco. Balkema, Rotterdam, pp. 707–712.
Kharlamenko, V.I., Zhukova, N.V., Khotimchenko, S.V., Svetashev, V.I., Kamenev, G.M., 1995. Fatty acids as markers of food sources in a shallow-water hydrothermal ecosystem (Kraternaya Bight, Yankich Island, Kurile Islands). Mar. Ecol. Prog. Ser. 120, 231–241.
Langdon, C.J., Waldock, M.J., 1981. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. UK 61, 431–448.
Lawrence, J.M., 1975. On the relationships between marine plants and sea urchins. Oceanogr. Mar. Biol. Annu. Rev. 13, 213–286.
Lawrence, J.M., Lane, P., 1982. The utilisation of the resources by post-metamorphic echinoderms. In: Jangoux, M., Lawrence, J.M. (Eds.), Echinoderm Nutrition. Balkema, Rotterdam, pp. 331–371. Leighton, D.L., 1966. Studies of food preference in algivorous invertebrates of southern California kelp beds.
Pac. Sci. 20, 104–113.
Leighton, D.L., 1968. A comparative study of food selection and nutrition in the abalone Haliotis rufescens (Swainson), and the sea urchin, Stongylocentrorus purpuratus (Stimpson). Ph.D. Dissert, Univ California, San Diego, 197 pp.
Leo, R.F., Parker, P.L., 1966. Branched-chain fatty acids in sediments. Science 152, 649–650.
McGee, F., 1996. Lipids as dietary indicators in echinoderms from west coast scottish waters. B.Sc. (Hons) Thesis, University of Stirling.
Mai, K., Mercer, J.P., Donlon, J., 1996. Comparative studies on the nutrition of two species of abalone,Haliotis
tuberculata L. and Haliotis discus hannai Ino.V. The role of polyunsaturated fatty acids of macroalgae in
abalone nutrition. Aquaculture 139, 77–89.
Mansour, M.P., Volkman, J.K., Jackson, A.E., Blackburn, S.I., 1999. The fatty acid and sterol composition of five marine dinoflagellates. J. Phycol. 35, 710–720.
Mortensen, T., 1943. A monograph of the Echinoidea. In: Camarodonta II. C.A. Reitzel, Copenhagen, p. 446. Nichols, P.D., Guckert, J.B., White, D.C., 1986. Determination of monosaturated fatty acid double-bond position and goemetry for microbial monocultures and complex consortia by capillary GC-MS of their dimethyl disulphide adducts. J. Microbiol. Methods 5, 49–55.
Paradis, M., Ackman, R.G., 1977. Potential for employing the distribution of anomalous non-methylene-interrupted dienoic fatty acids in several marine invertebrates as part of food web studies. Lipids 12, 170–176.
Parkes, R.J., 1987. Analysis of microbial communities within sediments using biomarkers. In: Ecology of Microbial Communities. SGM 41. Cambridge University Press, Cambridge.
Phillips, N.W., 1984. Role of different microbes and substrates as potential suppliers of specific, essential nutrients to marine detritivores. Bull. Mar. Sci. 35, 283–298.
Pillsbury, K.S., 1985. The relative food value and biochemical composition of five phytoplankton diets for Queen conch, Strombus gigas (Linne) larvae. J. Exp. Mar. Biol. Ecol. 90, 221–231.
Pond, D.W., Dixon, D.R., Bell, M.V., Fallick, A.E., Sargent, J.R., 1997. Occurrence of 16:2 (n-4) and 18:2 (n-4) fatty acids in the lipids of the hydrothermal vent shrimps Rimicaris exoculata and Alvinocaris markensis: nutritional and trophic implications. Mar. Ecol. Prog. Ser. 156, 167–174.
Rodegker, W., Nevenzel, J.C., 1964. The fatty acid composition of three marine invertebrates. Comp. Biochem. Physiol. 11, 53–60.
Sargent, J.R., Whittle, K.J., 1981. Lipids and hydrocarbons in the marine food web. In: Longhurst, A.R. (Ed.), Analysis of Marine Ecosystems. Academic Press, London, pp. 491–533.
Sargent, J.R., Falk-Petersen, I.-B., Calder, A.G., 1983. Fatty acid compositions of neutral glycerides from the ovaries of the asteroids Ctenodiscus cripatus,Asterias lincki and Pteraster miitaris from Balsfjorden, Northern Norway. Mar. Biol. 72, 257–264.
Sargent, J.R., Parkes, R.J., Mueller-Harvey, I., Henderson, R.J., 1987. Lipid biomarkers in marine ecology. In: Sleigh, M.A. (Ed.), Microbes in The Sea. Ellis Horwood, Chichester, pp. 119–138.
Sargent, J.R., Bell, M.V., Henderson, R.J., 1995. Protists as sources of (n-3) polyunsaturated fatty acids for vertebrate development. protistological actualities. In: Brugerolle, G., Migast, J.-P. (Eds.), Proc. of 2nd European Congress of Protistology, Clermont-Ferrand, pp. 54–64.
Shapiro, S.S., Wilk, M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika 52, 591–611.
Tagaki, T., Kaneniwa, M., Itabashi, Y., Ackman, R.G., 1986. Fatty acids in Echinoidea: unusual cis-5-Olefinic acids as distinctive lipid components in sea urchins. Lipids 21, 558–565.
Tett, P., Gowen, R., Grantham, B., Jones, K., Miller, B.S., 1986. The phytoplankton ecology of the Firth of Clyde sea-lochs Striven and Fyne. Proc. Roy. Soc. Edinburgh 90B, 223–238.
Vadas, R.L., 1977. Preferential feeding: an optimisation strategy in sea urchins. Ecol. Monogr. 47, 337–371. Vashappilly, R., Chen, F., 1998. Heterotrophic production potential of omega-3 polyunsaturated fatty acids by
microalgae and algae-like mico-organisms. Bot. Mar. 41, 553–558.
Volkman, J.K., Johns, R.B., Gillian, F.T., Perry, G.J., Bavor, Jr. H.J., 1980. Microbial lipids of an intertidal sediment — I. Fatty acids and hydrocarbons. Geochim. Cosmochim. Acta 44, 1133–1143.
Volkman, J.K., Jeffrey, S.W., Nichols, P.D., Rogers, G.I., Garland, C.D., 1989. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 128, 219–240.
Walker, C., McGinn, N.A., Harrington, L.M., Lesser, M.P., 1998. New perspectives on sea urchin gametogenesis and their relevance to aquaculture. J. Shell. Res. 17, 1507–1514.