www.elsevier.com / locate / bres
Research report
Immunohistochemical distribution of the receptor for advanced
glycation end products in neurons and astrocytes in Alzheimer’s
disease
a ,
*
a a a aNobuyuki Sasaki
, Sadamu Toki , Hiroshi Chowei , Toshikazu Saito , Norihito Nakano ,
a b c
Yorihide Hayashi , Masayoshi Takeuchi , Zenji Makita
a
Department of Neuropsychiatry, Sapporo Medical University, South 1, West 16, Chuo-ku, Sapporo, 060-8543 Japan
b
Department of Biochemistry, Hokuriku University, Kanazawa, Japan
c
Fourth Department of Internal Medicine, Kurume University, Kurume, Japan
Accepted 3 October 2000
Abstract
Advanced glycation end products (AGE) and the receptor for AGE (RAGE) have been implicated in the chronic complications of diabetes mellitus (DM), and have been reported to play an important role in the pathogenesis of Alzheimer’s disease (AD). In this study, we established a polyclonal anti-RAGE antibody, and examined the immunohistochemical localization of amyloidbprotein (Ab), AGE, and RAGE in neurons and astrocytes from patients with AD and DM. Our anti-RAGE antibody recognized full-length RAGE (50 kd) and N-terminal RAGE (35 kd) in human brain tissue. Ab-, AGE-, and RAGE-positive granules were identified in the perikaryon of hippocampal neurons (especially from CA3 and CA4) in all subjects. The distribution and staining pattern of these immunopositive granules showed good concordance with each antibody. In AD, most astrocytes contained both AGE-and RAGE-positive granules and their distribution was almost the same. Ab-positive granules were less common, but Ab-, AGE-, and RAGE-positive granules were colocalized in one part of a single astrocyte. In DM patients and control cases, AGE-and RAGE-positive astrocytes were very rare. These finding support the hypothesis that glycated Abis taken up via RAGE and is degraded through the lysosomal pathway in astrocytes. In addition to the presence of AGE, the process of AGE degradation and receptor-mediated reactions may contribute to neuronal dysfunction and promote the progression of AD. 2001 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Degenerative disease: Alzheimer’s disease – beta amyloid
Keywords: Alzheimer’s disease; Advanced glycation end product; Receptor for advanced glycation end product; Astrocyte; Amyloid beta protein
1. Introduction [4,16], which are considered to play an important role in
the pathogenesis of the chronic complications of diabetes Glucose and other reducing sugars react nonenzymati- mellitus (DM) [1,2]. After several components of receptor cally with protein amino groups to initiate a posttransla- for AGE (RAGE) were characterized [17], it was also tional modification process known as nonenzymatic shown that RAGE and AGE play an important role in glycosylation [4,10,16]. This reaction proceeds from re- diabetic nephropathy and atherosclerotic vasculopathy versible Schiff bases to stable, covalently bonded Amadori [13,18,25].
rearrangement products [4]. Once formed, the Amadori Alzheimer’s disease (AD) is the most common cause of products undergo further chemical rearrangement to form dementia in Western countries and in Japan. Pathological-irreversibly bound advanced glycation end products (AGE) ly, AD is characterized by the presence of senile plaques (SPs), neurofibrillary tangles (NFTs), and sever gliosis. AGE can be identified immunohistochemically in both SPs
*Corresponding author. Tel.:181-11-611-2111 (ext 3518); fax:1
81-and NFTs [20,21]. Furthermore, RAGE is expressed by
11-644-3041.
E-mail address: [email protected] (N. Sasaki). neurons, microglial cells, and astrocytes in the normal
human brain [3,11], while its expression by cortical glucose-derived AGE collagen [14,15]. This antibody does neurons increases and becomes more widespread in AD not recognize unmodified RNase, albumin, hemoglobin, [27]. Since it was reported that RAGE may be the nerve LDL, acetyl LDL, or collagen, as well as previously cell receptor for amyloidbprotein (Ab), the role of RAGE reported AGE structures such as 2-furoyl-4 [5]-[2-furanyl]-in the pathogenesis of AD has attracted considerable 1-H-imidazole (FFI),
1-alkyl-2-formyl-3,4-diglycosyl-attention [6,11,28]. pyrroles (AFGP), pyrraline, pentosidine, or CML [14,15].
We have previous studied the distribution of AGE in AD An anti-Ab antibody (1–42, C-terminal) was purchased and several neurodegenerative diseases, and have sug- from Bachem Feinchemikaline AG (Swiss), and a mouse gested that AGE may be an important factor in the anti-glial fibrillary acidi protein (GFAP) antibody was progression of various neurodegenerative disorders [20]. purchased from Dakopatts (Denmark).
However, little is known about the detailed role of RAGE A polyclonal antibody against RAGE were raised in
in AD [28]. rabbits. The peptides were synthesized according to the
To investigate the role of RAGE in AD and DM, we amino acid sequences of RAGE, residues 167–180 [6]. established an anti-RAGE antibody and performed im- Synthesized peptide was coupled to keyhole limpet munohistochemical studies. Our data demonstrated that hemocyanin, and mixed with an equal volume of Freud’s RAGE was present in astrocytes from AD brains along complete adjuvant. The conjugated peptide were injected with AGE and Ab, suggesting that RAGE-mediated degra- into rabbits 33at 2-week intervals. Serum was obtained 2 dation of Ab occurs in astrocytes. weeks after last injection, and antibody titer was assessed by ELISA. When antibody levels plateaued, the serum was collected and stored at 2808C until required.
2. Materials and methods
2.1. Subjects and specimens 2.3. Tissue preparation and Western blot analysis
Brain tissue specimens were obtained from five Western blot analysis was performed on samples of pathologically verified cases of AD, three cases of DM, bovine lung tissue and human brains obtained at post-and three age-matched controls. Histological sections were mortem from subjects with no history of any neurological prepared from the cerebral cortex (temporal and parietal or psychiatric disorders. Bovine lung powder (Sigma, 3 g) lobes) and the hippocampus. None of AD patients or was incubated with Tris (20 mM), NaCl (0.1 M), PMSF (1 controls had diabetes. The clinical features of the subjects mM), trasylol (0.1%), and octyl-b-glucoside (1%), pH 7.4,
are summarized in Table 1. (total 30 ml) for 16 h at 48C with constant mixing.
Insoluble material was removed by centrifugation
2.2. Antibodies (11,0003g) for 30 min at 48C, and the supernatant was
saved (lung extract). Human brain cortex was homogen-A rabbit anti-homogen-AGE-modified ribonuclease antibody was ized in buffer containing 20 mM HEPES, 0.25 M sucrose, used, which has been described previously [14]. This 0.3 mM phenylmethlsulfonyl fluoride (PMSF), 1 mM antibody detects AGE formed in vivo, such as AGE- dithiothreitol (DTT), 1 mM EGTA, and 1 mM MgCl .2 collagen and AGE-hemoglobin, as well as AGE formed in Samples containing 50 mg (lung extract) or 10 mg vivo, such as glucose-derived AGE RNase, glucose-de- (human brain) of protein were loaded onto 10% acrylamide rived AGE albumin, glucose-derived AGE LDL, and gels. Proteins were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred to nitrocellulose filters, as
Table 1
a described by Towbin et al. [24]. After transfer, the filters
Characteristics of subjects
were blocked by incubation with 5% nonfat dry milk in
Case Diagnosis Sex Age at Durations of disease
PBS-T buffer (phosphate-buffered saline-tween; 140 mM
autopsy (years)
NaCl, 27 mM KCl, 81 mM NaHPO , 15 mM KH PO ,4 2 4
1 AD F 64 7
0.1% Tween 20, pH 7.4) overnight at room temperature.
2 AD M 48 5
Then the filters were incubated for 1 h with PBS-T
3 AD F 68 8
4 AD M 45 5 containing 0.1% nonfat dry milk and anti-RAGE antibody
5 AD F 52 6 at 1:2500 dilution. Next, the filters were washed three
6 DM F 57
times in PBS-T and incubated with PBS-T containing
7 DM F 68
0.1% nonfat dry milk and horseradish peroxidase-linked
8 DM F 75
anti-rabbit Ig [F(ab9) ] (Amersham-Life Science) diluted
9 Control F 73 2
10 Control M 66 1:5000 for 1 h at room temperature. Filters were washed
11 Control F 61 three more times in PBS-T, and immunoreactivity was
a
blot detection system (Amersham), followed by exposure to ECL HYPER film (Amersham).
2.4. Immunohistochemical staining
Serial paraffin sections were immunostained according to the standard streptavidin–biotin peroxidase technique using a Vectastain ABC elite kit (Vector Lab., CA). All sections were treated with 99% formic acid for 3 min, and the sections used for AGE staining were also treated with 0.05% proteinase-K for 30 min. Endogenous peroxidase
was inhibited with 0.3% hydrogen peroxidase (H O ) in2 2 Fig. 1. Characterization of the anti-RAGE antibody by Western blotting of human brain and bovine lung extracts. Lane 1, anti-RAGE antibody
methanol for 30 min. These sections were also incubated
and immobilized human brain (10 mg / lane). Lane 4, anti-RAGE antibody
with 10% horse serum (for GFAP), or 10% goat serum
and immobilized bovine lung extract (50 mg / lane). Bands are observed at
(for Ab, AGE, and RAGE) to eliminate nonspecific approximately 35 kd, 50 kd, and 60 kd in lanes 1 and 4. Lane 2 (human binding. This was followed by incubation overnight at 48C brain) and lane 5 (bovine lung) show that the immunoreaction was
with the primary antibodies diluted to 1:500|1:1000 in 10 diminished after absorption by the synthesized RAGE peptide. Lanes 3 and 6 show non-immune rabbit IgG and immobilized human brain or
mM PBS (pH 7.4). The sections were then sequentially
bovine lung extract, respectively, with no bands being observed.
Migra-incubated with the biotinylated secondary antibody for 1 h,
tion of the protein standards is indicated to the left of lane 1.
with streptavidin–biotin-horseradish peroxidase for 1 h, and with 3,39-diaminobenzidine / H O2 2 until the reaction products were visualized (1–3 min). Then the sections
Western blotting and immunohistochemistry in the same were counterstained with hematoxylin. Specificity was
procedure as described above. confirmed by applying PBS instead of the primary
anti-bodies, or by replacing the primary antibodies with
non-2.6. Semiquantification of RAGE-positive astrocytes immune serum.
The combinations of antibodies used for double staining
The number of astrocytes reacting with anti-Ab, AGE or are summarized in Table 2. We used the anti-GFAP
RAGE antibodies was counted in both serial tissue sections antibody as a marker of reactive astrocytes. It was diluted
and double stained sections. In each subject, immuno-extensively (1:2000–4000), so that observation of
im-reactive astrocytes were counted in the hippocampus using
munopositive granules was not prevented. 2
three photomicrographs (1.3 mm each) obtained at3100 magnification.
2.5. Absorption test
Specificity of the antibody was verified by absorption 3. Results tests using Western blotting and immunohistochemistry.
Western blotting was performed using anti-RAGE antibody 3.1. Western blot analysis (1:2500 dilution) incubated with 10003 molar
concen-tration of synthesized peptides of RAGE (residues 167– Immunoblotting was performed to confirm specificity 180, 1 mg / ml) overnight at 48C. In immunohistochemistry, for RAGE. It demonstrated three major bands at approxi-anti-RAGE antibody (1:1000 dilution) was incubated with mately 35 kd, 50 kd, and 60 kd both in human brain (Fig. 10003 molar concentration of above peptides overnight at 1, lane 1) and in bovine lung extract (Fig. 1, lane 4). Fig. 48C. These pellets were separated by centrifugation at 1, lanes 2 and 5 shows the result of an absorption test. The 30,000 g for 30 min. This supernatant was used for reaction was disappeared by the preincubation of the
Table 2
a
Antibody combinations for double immunostaining
Primary antibody Secondary antibody Third step Substrate (color)
Anti-GFAP mouse IgG APAAP complex Fast Red (red)
Anti-Ab biotinylated streptavidin–biotin- diaminobenzidine
Anti-AGE anti-rabbit IgG horseradish peroxidase (brown)
Anti-RAGE
a
antibody with the synthesized peptides in human brain AGE-positive granules in hippocampal neurons, especially (Fig. 1, lane 2) and bovine lung extract (Fig. 1, lane 5). No in CA3 and CA4, than in cortical neurons. These AGE-immunoreaction was detected with non-immune rabbit IgG positive granules were identified in the presence of NFTs both in human brain and bovine lung extract (Fig. 1, lanes and SPs. In DM and control subjects, the Ab and RAGE
3 and 6). staining patterns were similar to that of AGE, and there
were no remarkable changes compared to the findings in
3.2. Immunohistochemical staining AD.
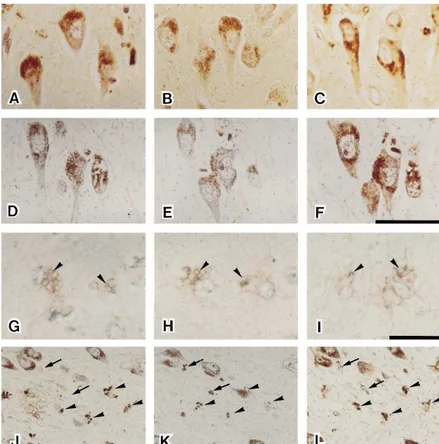
Fig. 3(G–I) shows representative astrocytes immuno-Proteolytic digestion with proteinase-K has been shown stained with anti-Ab (G), AGE (H), and RAGE (I) to expose cross-linked AGE moieties and to enhance AGE antibodies. Astrocytes contained characteristic immuno-immunoreactivity in the human brain [20]. In the present positive granules [12]. Each immunopositive deposit had a study, proteinase-K pretreatment enhanced AGE and very fine dott-like structure, and 2–4 mm granules were RAGE immunoreactivity in astrocytes, but no marked surrounded by these immunopositive structures. Most of improvement was observed for sections stained with other the granules were located in the cytoplasm of astrocytes,
antibodies. but one of them was attached to the surface of an astrocyte.
In AD brains, NFTs and SPs were strongly positive for Fig. 3(J–L) shows adjacent serial sections from an AD the anti-AGE antibody, as has been described elsewhere in brain stained with anti-Ab (J), AGE (K), and RAGE (L)
detail [20]. antibodies. Most astrocytes (approximately 70–80%)
con-There were no or only a few SPs and NFTs in the DM tained both AGE and RAGE-positive granules and their and control subjects. Numerous GFAP-positive astrocytes distribution was almost the same, while fewer astrocytes were observed in AD brains, but very few in DM and contained Ab-positive granules (approximately 20–30%, control brains. The DM subjects had more AGE-positive arrows). Ab-(J), AGE-(K), and RAGE-(L) positive vessels in their brains than the controls. Except for this granules can be seen to colocalize in one part of the same finding, there were no remarkable differences between DM astrocytes (arrowheads). Immunopositive astrocytes
and control subjects. characteristically appeared around SP, but AGE- and
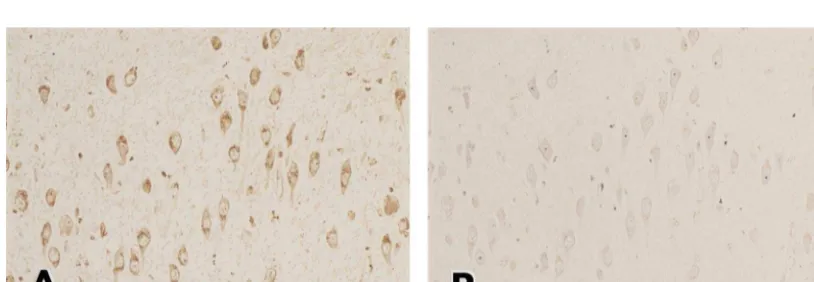
Fig. 2 showed the result of an absorption test. Immuno- RAGE-positive astrocytes were also present in areas reaction in neurons and astrocytes were disappeared by the without SP. In DM and control subjects, AGE- or
RAGE-absorption test (Fig. 2B). positive astrocytes were very rare.
AGE-immunopositive granules were observed in AD (Fig. 3A), DM (Fig. 3B) and control brains (Fig. 3C).
Many AGE-positive granules were identified in the 4. Discussion perikaryon of neurons, and this staining pattern did not
show remarkable differences between the groups. Fig. We characterized a new anti-RAGE antibody by Western 3(D–F) shows representative staining with anti-Ab (D), blot analysis. The bands at approximately 50 kd, observed anti-AGE (E) and anti-RAGE (F) in serial sections of in both brain and lung, is considered to be full length pyramidal neurons from the hippocampus of an AD brain. RAGE [3], while the 35 kd band is considered to be the Many immunopositive granules were identified in the NH -terminal two-thirds of the molecule [3]. Furthermore,2 perikaryon of neurons, and their distribution showed good absorption tests in Western blot analysis and immuno-concordance with each antibody. There were far more histochemical staining showed this antibody has an
Fig. 3. Representative AGE-immuopositive granules in hippocampal neurons from AD (A), DM (B), and control brains (C). Serial sections of hippocampal neurons (D, E, and F) from an AD brain stained with anti-Ab (D), anti-AGE (E), and anti-RAGE (F) antibodies, respectively. Immunopositive granules were identified in the perikaryon of pyramidal neurons, and their distribution did not show remarkable differences between antibodies. Bar in panel F equals 50mm and applies to A–F. Typical staining patterns of immunopositive granules in AD astrocytes obtained with anti-Ab (G), AGE (H) and RAGE (I) antibodies (arrowheads). These sections showing astrocytes (G–I) were also double stained with anti-GFAP antibody (light red). Bar in panel I equals 25mm and applies to G–I. Serial sections showing immunopositive granules detected by anti-Ab(J), AGE (K), and RAGE (L) antibodies. Ab-(J), AGE-(K), and RAGE-(L) positive granules can be seen to colocalize in the same astrocytes (arrowheads). One part of astrocytes that were positive for both anti-AGE (K) and RAGE (L) did not show Ab(J)-immunoreactivity (arrows). Bar in panel L equals 100mm and applies to J–L.
tigenicity against RAGE 167–180 residues. These findings present in human brain, our anti-RAGE antibody may also indicate that this anti-RAGE antibody certainly recognizes recognized the 60-kd AGE binding proteins.
full-length RAGE (50 kd) and N-terminal RAGE (35 kd) It has been reported that AGE are degraded in the
in human brain tissue. lysosomes of macrophages after being taken up via
re-leased into the circulation to be cleared by the kidneys risk factor for AD [9]. These findings would seem to the [2,13]. When glycated proteins bind to RAGE, macro- contradict the hypothesis that AGE contributes to the phages are stimulated to produce proinflammatory cyto- pathogenesis of AD. The present study provided one kines (TNFa, IL-1b, and IL-6) and to induce growth answer for this problem. Since AGE-positive structures are
factors (PDGF and IGF-IA) [25]. little in DM brains [20], it is not necessary to be induced
Astrocytes are resident brain cells of the mononuclear astrocytes, that is a glycated protein scavenger. In conse-phagocytic system, and have some conse-phagocytic capacity quence, DM brains do not contain AGE- and RAGE-under certain conditions [12]. As well as macrophages, positive astrocytes. Therefore, DM neurons should be free RAGE is expressed by neurons and glial cells (microglia from attack by neurotoxic factors (such as cytokines and and astrocytes) in the central nervous system [3,11], and nitric oxide) released by astrocytes. AGE in blood and RAGE expression is upregulated in the regions of AD vessels do not induce astrocytes to brain matter, conse-brains [8]. A cultured astrocyte cell line activated by quently AGE in DM brains do not influence the patho-Ab-peptide can be induced to produce nitric oxide in the genesis of AD. In the onset and progression of AD, the presence of IFN-gor TNF-a[19]. Astrocytes also produce presence of AGE- and RAGE-positive astrocytes is the pro-inflammatory cytokine IL-6, which has been shown thought to be more important than the presence of AGE to be elevated in AD brains and to be a mediator of the itself.
acute phase response [7]. These neurotoxic factors pro- AGE-positive granules were found in the perikaryon of duced by astrocytes may contribute to neuronal dysfunc- neurons in AD, DM, and control brains, and these granules
tion in AD. didn’t show remarkable differences between the groups.
It has been reported that astrocytes contain granules that The neurons with AGE-positive granules were not com-react with anti-C-terminal Ab antibody in AD patients [1] mon in CA1 and CA2 regions with many SPs and NFTs in and aged controls [11,26], and this astroglial Ab im- AD [23]. The AGE-positive granules in neurons have been muoreactivity has been localized to lipofuscin-like reported to accumulate in an age-dependent manner [19]. granules derived from lysosomes [26]. In addition, cultured Therefore, AGE-positive granules in neurons may not have astrocytes rapidly phagocytose Ab [5] or AGE–BSA in a pathological meaning, and are probably related to the vitro [11]. It has been suggested that astrocytes take up Ab normal aging process.
and attempt to degrade it in lysosomes [1,8], but the In conclusion, our immunohistochemical studies demon-pathway involved in this process has been uncleared. It has strated that RAGE-mediated Abdegradation and receptor-already been reported that AGE (carboxymethyl lysine and mediated reactions may contribute to neuronal dysfunction pentosidine)–positive deposits associates with AD as- and death, resulting in the progression of AD.
trocytes are present with Gomori-positive granules, in-dicating chronic oxidative stress generated by AGE [23],
but the relationship with Ab granules has not been Acknowledgements investigated before. The present immunohistochemical
study demonstrated that Ab, AGE, and RAGE were These studies were supported in part of by Grant-in-Aid colocalized in astrocytes from AD brains. This finding for Scientific Research ([09470204) to Zenji Makita from indicates that glycated Ab is taken up via RAGE and is the Japanese Ministry of Education, Science, Sports, and degraded through the lysosomal pathway in astrocytes. Culture, and by a Health Science Research Grants ([ H10-Many astrocytes without Ab granules contained AGE Chozyu-033) to Zenji Makita from the Japanese Ministry and RAGE positive granules, so this finding indicates the of Health and Welfare.
presence of glycated proteins other than Ab. We have already reported that Ab-negative but AGE-positive senile
plaques are present in AD brains, and have suggested that References some novel or unidentified proteins may be glycated [20].
Tabaton et al. failed to detect glycated-Ab and glycated- [1] H. Akiyama, C. Schwab, H. Kondo, H. Mori, F. Kametani, K. Ikeda,
Apo E using immunoprecipitation, and concluded that P.L. McGeer, Granules in glia cells of patients with Alzheimer’s disease are immunopositive for C-terminal sequences ofb-amyloid
other amyloid-associated proteins may be candidates for
protein, Neurosci. Lett. 206 (1996) 169–172.
glycation [22]. Therefore the possible existence of glycated
[2] N. Araki, T. Higashi, T. Mori, R. Shybayama, Y. Kawabe, T.
proteins other than Ab in astrocytes should be kept in Kodama, K. Takahashi, M. Shichiri, S. Horiuchi, Macrophage
mind. scavenger receptor mediates the endocytic uptake and degradation of
AGE and RAGE positive astrocytes were rare in DM advanced glycation end products of the Maillard reaction, Eur. J. Biochem. 227 (1995) 408–415.
and control subjects. In this study, AGE-positive vessels
[3] J. Brett, A.M. Schmidt, S.D. Yan, Y.S. Zou, E. Weidman, D. Pinsky,
were more common in DM than control cases, suggesting
R. Nowygrod, M. Neeper, C. Przysiecki, A. Shaw, A. Migheli, D.
that DM brains contain far more glycated protein than Stern, Survey of the distribution of a newly characterized receptor control brains. Though diabetics have an increase of brain for advanced glycation end products in tissues, Am. J. Pathol. 143
[4] M. Brownlee, H. Vlassara, A. Cerami, Nonenzymatic glycosylation K. Dohi, Immunohistochemical detection of advanced glycosylation and the pathogenesis of diabetic complications, Ann. Intern. Med. end products within the vascular lesions and glomeruli in diabetic 101 (1984) 527–537. nephropathy, Hum. Pathol. 26 (1995) 308–313.
[5] D.A. DeWitt, G. Perry, M. Cohen, C. Doller, J. Silver, Astrocytes [19] F. Rossi, E. Bianchini, Synergistic induction of nitric oxide by regulate microglial phagocytosis of senile plaque cores of Alzheim- b-amyloid and cytokines in astrocytes, Biochem. Biophys. Res. er’s disease, Exp. Neurol. 149 (1998) 329–340. Commun. 225 (1996) 474–478.
[6] D.W. Dickson, The pathogenesis of senile plaques, J. Neuropathol. [20] N. Sasaki, R. Fukatsu, K. Tsuzuki, Y. Hayashi, T. Yoshida, N. Fujii, Exp. Neurol. 56 (1997) 321–339. T. Koike, I. Wakayama, R. Yanagihara, R. Garruto, N. Amano, Z. [7] D.W. Dickson, S.C. Lee, C.F. Brosnan, S. Sinicropi, H. Vlassara, Makita, Advanced glycation end products in Alzheimer’s disease S.H. Yen, Neuroimmuology of Aging and Alzheimer’s Disease with and other neurodegenerative diseases, Am. J. Pathol. 153 (1998) Emphasis on Cytokines. Cytokines and the CNS Defence; Develop- 1149–1155.
ment, Defence, and Disease, CRC Press, 1996, pp. 239–267. [21] M.A. Smith, S. Taneda, P.L. Richey, S. Miyata, S.D. Yan, D. Stern, [8] H. Funato, M. Yoshimura, T. Yamazaki, T.C. Saido, Y. Ito, J. L.M. Sayre, V.M. Monnier, G. Perry, Advanced Maillard reaction Yokofujita, R. Okeda, Y. Ihara, Astrocytes containing amyloid b- end products are associated with Alzheimer disease pathology, Proc. protein (Ab)-positive granules are associated with Ab40-positive Natl. Acad. Sci., USA 91 (1994) 5710–5714.
diffuse plaques in the aged human brain, Am. J. Pathol. 152 (1998) [22] M. Tabaton, G. Perry, M. Smith, M. Vitek, G. Angelini, D. Dapino, 983–992. S. Garibaldi, D. Zaccheo, P. Odetti, Is amyloidb-protein glycated in [9] J. Heitner, D. Dickson, Diabetics do not have increased Alzheimer- Alzheimer’s disease?, Neuroreport 8 (1997) 907–909.
type pathology compared with age-matched control subjects. A [23] A. Takeda, T. Yasuda, T. Miyata, Y. Goto, M. Wakai, M. Watanabe, retrospective postmortem immunocytochemical and histofiluorescent Y. Yasuda, K. Horie, T. Inagaki, M. Doyu, K. Maeda, G. Sobue, study, Neurology 49 (1997) 1306–1311. Advanced glycation end products co-localized with astrocytes and [10] F. Ledl, E. Schleicher, New aspects of the Maillard reaction in foods microglial cells in Alzheimer’s disease brain, Acte. Neuropathol. 95
and in the human body, Angew. Chem. Int. Ed. Engl. 6 (1990) (1998) 555–558.
565–706. [24] H. Towbin, T. Staehlin, J. Gordon, Electrophoretic transfer of [11] J.J. Li, D. Dickson, P.R. Hof, H. Vlassara, Receptors for advanced protein from polyacrylamide gels to nitrocellulose sheets: Procedure glycosylation endproducts in human brain; role in brain homeosta- and some applications, Proc. Natl. Acad. Sci. USA. 83 (1996)
sis, Mol. Med. 4 (1998) 46–60. 5439–5443.
[12] J.J. Li, M. Surini, S. Catsicas, E. Kawashima, C. Bouras, Age- [25] H. Vlassara, R. Bucala, L. Striker, Pathogenic effects of advanced dependent accumulation of advanced glycosylation end products in glycosylation: Biochemical, biologic, and clinical implications for human neurons, Neurobiol. Aging 16 (1995) 69–76. diabetes and aging, Lab. Invest. 70 (1994) 138–151.
[13] Z. Makita, ‘Toxicity of glucose:is AGE the answer?’, Nephrol. Dial. [26] H. Yamaguchi, S. Sugihara, A. Ogawa, T.C. Saido, Y. Ihara, Diffuse Transplant. 10 (Suppl.7) (1995) 33–37. plaques associated with astroglial amyloid b protein, possibly [14] Z. Makita, H. Vlassara, A. Cerami, R. Bucala, Immunochemical showing a disappearing stage of senile plaques, Acta Neuropathol.
detection of advanced glycosylation end products in vivo, J. Biol. 95 (1998) 217–222.
Chem. 267 (1992) 5133–5138. [27] S.D. Yan, X. Chen, J. Fu, M. Chen, H. Zhu, A. Roher, T. Slattery, L. [15] Z. Makita, H. Vlassara, E. Rayfield, K. Cartwright, E. Friedman, R. Zhao, M. Nagashima, J. Morser, A. Migheli, P. Nawroth, D. Stern, Rodby, A. Cerami, R. Bucala, Hemoglobin-AGE: a circulating A.M. Schmidt, RAGE and amyloid-b peptide neurotoxicity in marker of advanced glycosylation, Science 258 (1992) 651–653. Alzheimer’s disease, Nature 382 (1996) 685–691.
[16] V. Monnier, A. Cerami, Nonenzymatic browning in vivo: possible [28] S.D. Yan, D. Stern, A.M. Schmidt, What’s the RAGE? The receptor process for aging of long-lived proteins, Science 211 (1981) 491– for advanced glycation end products (RAGE) and the dark side of
493. glucose, Eur. J. Clin. Invest. 27 (1997) 179–181.