www.elsevier.com / locate / bres
Research report
Effect of food deprivation and leptin repletion on the plasma levels of
estrogen (E2) and NADPH-d reactivity in the ventromedial and
arcuate nuclei of the hypothalamus in the female rats
a ,
*
a a bEffiong Edet Otukonyong
, Fumino Okutani , Seiichi Takahashi , Takuya Murata ,
c a b
Nobuyuki Morioka , Hideto Kaba , Takashi Higuchi
a
Department of Physiology, Kochi Medical School, Nankoku, Kochi 783-8505, Japan
b
Department of Physiology, Fukui Medical University, Matsuoka, Fukui 910-1193, Japan
c
Department of Obstetrics and Gynecology, Kochi Medical School, Nankoku, Kochi 783-8505, Japan Accepted 12 September 2000
Abstract
The exact role of leptin in fasting has not been completely elucidated. To determine whether leptin can act in fasting to influence plasma estrogen levels and nitric oxide synthase reactivity in food regulating centers of the brain, we fasted female rats for 4 days and treated them i.p. with vehicle or 100mg of recombinant mouse leptin as 1 ml on the 3rd and 4th day twice daily (10.00 and 17.00 h). Proestrus blood was collected at 10.00, 14.00, 18.00 and at 22.00 h, plasma obtained and assayed for estrogen (E2) and leptin levels. Verification of ovulation occurrence was by examining the oviduct for extruded ovum. The rat brains were removed and processed for nitric oxide synthase reactivity in the ventromedial hypothalamus (VMH) and arcuate nucleus (ARC) using NADPH-diaphorase histochemistry, a marker for neurons expressing NOS enzyme. Leptin effect on dependable variables such as food intake, water intake and body weight gain was also investigated. Four days fasting significantly decreased body weight, estrogen and postfast leptin levels, nitric oxide reactivity in the VMH and ARC nucleus and stopped ovulation in many (4 out of 5) rats fasted and given vehicle. Leptin treatment significantly increased plasma estrogen and postfast leptin levels, restored ovulation in many (4 out of 5) rats and increased nitric oxide reactivity in the VMH and ARC. Leptin significantly inhibited food intake, water intake and gain in body weight during recommenced feeding. These observations suggest that leptin could act in the pituitary–ovarian axis during fasting to improve reproductive function by partly stimulating estrogen secretion. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Hypothalamic–pituitary–gonadal regulation
Keywords: Arcuate nucleus; Estrogen; Fasting; Leptin; Nitric oxide synthase; Ventromedial hypothalamus
1. Introduction lactational behavior, production of ova and hormones
secretion (see [54] for review).
Reproduction in female mammals is very sensitive to the Leptin and nitric oxide synthase (NOS) have been availability of metabolic fuels and reserves. When there is shown to be involved in the regulation of various physio-a scphysio-arcity of food, reproduction is often suspended in fphysio-avor logical processes and functions. Recent reports revealed of processes essential for survival such as thermoregulation that exogenous leptin administration increased basal LH and cellular maintenance (see [55] for review). In rats, levels, ovarian and uterine weights, testicular, seminal mice, hamsters and other mammals, food deprivation has vesicle weights, sperm counts and corrected the sterility been shown to decrease incidence of ovulation, postovulat- defect in female / male ob / ob mice [5,11,37]. Furthermore, ory vaginal discharge, estrous behavior, onset of puberty, leptin administration to normal female prepubertal rats [12] and mice [2] led to earlier vaginal opening and increased basal LH levels in fasted adult mice [1]. Leptin also acts as
*Corresponding author. Tel. / fax:181-88-880-2307.
E-mail address: [email protected] (E.E. Otukonyong). an afferent satiety hormone by regulating appetite and
weight gain [56,60] through a negative feedback loop leptin to restoring the hormonal levels of several reproduc-involving receptors in the hypothalamus [49]. The recent tive hormones during nutritional distress [1,5]. There is no demonstration of leptin receptors in the ventromedial previous study to date which reported the effect of leptin in hypothalamus (VMH) [47], arcuate nucleus (ARC) starvation on the level of estrogen, an important hormone [31,43], ovary [25,27], paraventricular and periventricular whose release precedes the release of LH. This study was nuclei [15], provides the basis for a neuro-endocrine role designed to examine the possible effects of food depriva-of leptin in the control depriva-of anterior pituitary and ovarian tion and leptin repletion on the plasma levels of estrogen at hormones secretion. Nitric oxide (NO) has been shown to different times on proestrus and NOS reactivity in the be positively correlated to increased food intake and body VMH and ARC.
weight [34,35], and regulates thirst and water intake [9,10]. Recent evidence has implicated NO in many actions of
leptin leading to the enhanced secretion of reproductive 2. Materials and methods
hormones such as LHRH from the hypothalamus [40], LH
and FSH from the adenohypophysis [57,58]. NOS expres- 2.1. Animals and treatments sion and NO generation have been reported in the ovaries
of rats [22,51] and shown to play several roles in ovarian Female Wistar Imamichi rats (Hamamatsu, Japan) steroidogenesis [50] and ovulation [45]. All these findings weighing 230–240 g, were housed under controlled tem-suggest that NO may be involved in the regulation of perature (24618C) and photoperiod (14L:10D), lights on energy metabolism and reproduction. The importance of at (05.00–19.00 h) and free access to food (Oriental Yeast, these hypothalamic sites: the ARC and VMH in the Japan) and water. Estrous cycles were monitored daily regulation of metabolism and feeding behavior has been (09.00–10.00 h) by vaginal lavages. Female Wistar Im-well documented [6,14]. For instance, VMH acting as an amichi rats were used in these experiments because of their important control center for food intake and adiposity, has regular 4-days cycles. Rats were divided into five groups been shown to sensor blood glucose, blood amino acids for use in five different experiments. All rats were fasted and also gives efferent signals to suppress food intake and and deprived of food but not water. Food was withdrawn energy expenditure [7]. Thus, destruction of VMH has from the animals at 09.00 h on diestrus II because this is been reported to be accompanied by the subsequent when food intake and body weight gain in rats are highest increase in food intake and decrease in energy expenditure [53,54]. Leptin (Mouse recombinant, NHPP-NIDDK, which results in massive obesity [8,21]. Central injection USA) or vehicle was given i.p. to all rats (except those fed) of galanin, a feeding-related neuropeptide into the VMH on the 3rd and 4th day at 1000 h and 1700 h. Body weight increased feeding in rats [24]. VMH also contains both was investigated before and after fasting. The fed animals NADPH-diaphorase [52] and estrogen binding sites [39]. were allowed free access to food and water ad libitum. The importance of ARC in the regulation of energy Adequate measures were taken to minimize stress or pain homeostasis has been confirmed and strengthened by a to the animals.
number of recent studies. For example, coexpression
studies indicate that a population of ARC neurons syn- 2.2. Experiment 1 thesizes both agonist for an anabolic neuroendocrine
system neuropeptide Y (NPY) and an antagonist for a In this experiment, the effect of starvation and leptin catabolic system (agouti-related protein) [32]. In addition, repletion on the plasma levels of estrogen (17-b-estradiol) feeding-related neuropeptides such as leptin, pro- was investigated by radio-immunoassay method. All the opiomelanocortin (POMC) and NPY have been shown to reagents used were bought commercially from Nacalai coexpress with neurons of the ARC [13,31]. A recent Tesque Inc., Kyoto, Japan unless otherwise stated. report indicates that damage to ARC produces massive
obesity in birds and mammals [8]. Increased NPY and 2.2.1. Reagents
decreased POMC mRNA in the ARC has been reported in The reagents used in this assay were tracer 2, 4. 6. 16.
fasted animals [26,30,43]. 17-3H E2 (NEN), antiserum 17-b-estradiol-6-CMO-BSA
It is now known that undernutrition affects all levels of (Biogenesis, New Fields, UK), standard 17-b-E2 powder the hypothalamic–pituitary–ovarian axis. The sequential in methanol, 0.01 M sodium phosphate buffer (pH 7.5) in secretion of estradiol and progesterone has been reported 0.9% NaCl, 0.1% sodium azide and 0.1% gelatin (Difco, to be disrupted by food deprivation [33]. A substantial Laboratories, Detroit, Michigan, USA), dextran–charcoal reduction even in the estrogen-binding neurons in the prepared from dextran (Wako Pure Chemical Industries, VMH and in the area lateral to it has also been reported Japan), Norit A in cold buffer, diethylether, aquasol (NET, [28]. Although the exact role of leptin in starvation has not Universal L. S. C) and methanol.
been completely elucidated, evidence for its role in the
restoration of hormonal levels and reproduction in mam- 2.2.2. Experimental protocols
was fed; the second group was fasted and given 0.9% in calculating the level of estradiol. The assay recovery saline (vehicle) and the third group received 100mg leptin rate was 98.7% and the assay sensitivity was 32 pg / ml. as 1 ml. Both the leptin and the vehicle were given i.p. on The inter- and intra-assay coefficients of variation were 4% the 3rd and 4th (last day) of the fasting treatment twice and 3%, respectively.
daily, i.e., at 10.00 h and 17.00 h. Fasting which started at
diestrus was terminated at diestrus covering a one 4-day 2.3. Experiment 2 estrous cycle. Food was returned to all the animals at the
end of the treatments (i.e., 16 h after the last dose of leptin The plasma levels of leptin following fasting and leptin or vehicle). On the following day, proestrus trunk blood treatment were also investigated. The plasma used for E2 was collected into heparinized tubes by neck decapitation RIA were also used to measure leptin levels. This was to with guillotine at 10.00 h (n55), 14.00 h (n55), 18.00 h enable us to know how high the serum leptin rose (n55), and at 22.00 h (n55). The blood was immediately following injections of leptin. Plasma leptin levels were centrifuged at 4000 rpm for 4 min at 48C and the plasma determined by using a specific rat leptin RIA kit purchased stored at 2208C until assayed for estradiol (E2). from Linco Research (St. Louis, MO, USA). The sensitivi-ty of the assay was 0.5 ng / ml. The intra- and inter-assay coefficients of variation were 5 and 6%, respectively. 2.2.3. Estrogen assay procedures
Plasma estrogen was assayed by the method of Albrecht
2.4. Experiment 3 et al. [4] with some modifications. Briefly, the working
tracer (for RIA) was prepared by drying 10 ml of stock
This experiment examined the effect of fasting and tracer in nitrogen gas and adding 8 ml of phosphate buffer
leptin repletion on nitric oxide synthase (NOS) reactivity to the dried up tube. This was stabilized on ice at a final
in the ventromedial (VMH), arcuate (ARC) nuclei using concentration of 10,000 dpm / 100 ml and used for RIA.
NADPH-d histochemistry, a marker for neurons expressing For the assay recovery rate, 1 ml of the working tracer was
NOS enzyme. Rats were divided into three groups of five taken and 9 ml of phosphate buffer added. This was
rats each (n55). The first group was fed, the second group stabilized on ice at a final concentration of 1000 dpm / 100
was fasted and given vehicle, the third was fasted and ml (310 dilution).
received leptin. The treatments procedure and leptin dosing are as described in Experiment 1. Animals in the three 2.2.4. Extraction of E2
groups were killed in random order between 09.00 and The 17-b-estradiol was extracted by diethyl ether. Into a
13.00 h. glass tube, 200ml of plasma, 500ml of buffer and 100ml
of tracer (1000 dpm / 100ml), and 4 ml of ethyl ether were
2.4.1. Tissue preparation added, vortexed and allowed to stand in a freezer for 20
Under pentobarbital anesthesia, animals were transcar-min. The ethyl ether layer was carefully taken to culture
dially perfused with 100 ml of ice-cold buffer (0.1 M glass tubes, dried in nitrogen gas at 378C. One milliter of
phosphate buffer, PB) and 200 ml of ice-cold 4% parafor-methanol was added to the dried up tubes and then
maldehyde (PFA) in 0.2 M PB. The brain tissues were vortexed. One hundred microliter of methanol was added
quickly removed and postfixed in 4% PFA at 48C and then to each vial bottle in 5 ml aquasol and counted for 5 min.
cryoprotected in 30% sucrose solution for 2 days at 48C. The assay recovery rate was calculated using the formula:
Hypothalamic coronal sections (50 mm) were cut on a [(C. sample2C. Bg)310 /(T.C.2C. Bg)]3100% cryostat (Reichert–Jung, Cryocut 1800, Finetec Scientific Instruments, Tokyo, Japan) at 2188C. The VMH and ARC Where: C. sample5sample value; C. Bg.5background levels were identified according to their stereotaxic
coordi-value; T.C.5Total count nates in the rat brain atlas [38]. The sections were placed
in 0.1 M PB after which they were placed to wash on an
2.2.5. E2 RIA procedure electronic shaker (Taitec Instruments, Saitama, Japan) for
Into the dried up tubes, and tubes for the standards, 100 30 min. ml of buffer, 100 ml of tracer and 100 ml of antiserum
(1:4000) were added and incubated in a cold room 2.4.2. NADPH-d histochemistry
and coverslipped using Eukitt reagent (O. Kindler, MA-2091, Japan). The stored images were analyzed for Feiburg, Germany) and examined under a light micro- cell count and intensity by selecting appropriate menus scope. NADPH-d-positive cells were counted per section. such as slice density under the ‘option’ menu at 256 gray The counting and staining intensity quantification was levels / pixel, the ‘tools’ menu to map out the area to be done by using computer software NIH image version 1.58. counted and the ‘lut’ menu to set the intensity threshold Some cells were counted manually at 320 objective using an NIH Image, version 1.58 software with a
mini-magnification. mum particle size setting of 10 pixels and standard
threshold. The cells were counted regardless of the
intensi-2.5. Experiment 4 ty of staining since the weaker intensity observed in some
sections may possibly have resulted from fixation. Some In this experiment, we investigated the effect of fasting cells were counted manually at 320 objective magnifica-treatment and leptin repletion on ovulation. This was to tion. The intensity values which represent the product of know whether leptin treatment was effective in reversing signals that take both the numbers and the intensity of the starvation-induced inhibition of estradiol secretion, and staining into account are presented in arbitrary units. which by implication and extension infers that probably
the secretion of LH (ovulatory hormone) may have 2.8. Statistical analysis occurred in the afternoon of proestrus. The treatment
procedure and the leptin dosing is as described in Experi- At least six sections were counted for each animal and ment 1. Ovariectomy was performed under light ethyl five sections with the highest numbers of NADPH-d-ether in the morning of estrus. The excised ovary and the positive cells were used for statistical analysis. Data are isolated oviduct were covered with drops of saline (0.9%) presented as means6standard error of the mean and coverslipped. The oviduct portion of the ovary was (means6S.E.M.). NADPH-d-positive cells, staining in-examined under a light microscope for an extruded ovum. tensity, estrogen and leptin data were analyzed using the Mann–Whitney U-test. Incidence of ovulation was
ana-2.6. Experiment 5 lyzed by Fisher’s exact probability test. Comparison
between groups for body weight change, food intake and This experiment was designed to investigate the effect water intake was by Student’s t-test.
of leptin treatment on dependable variables such as food intake, water intake and body weight change. This was to
be used as a strong independent measure of ascertaining 3. Results
that the dose of leptin used in this experiment was at least
adequate to alter the neural processes associated with 3.1. Effect of fasting on body weight change and plasma fasting-induced inhibition of the estradiol secretion. Food leptin levels
intake, water intake and body weight change were
25.763 intensity, 261 cells, 11.461 intensity in the fasted vehicle-treated rats and 762 cells, 34.563 intensity in the fasted leptin-treated ones (Fig. 2).
3.3. Effect of fasting and leptin repletion on the plasma estrogen levels
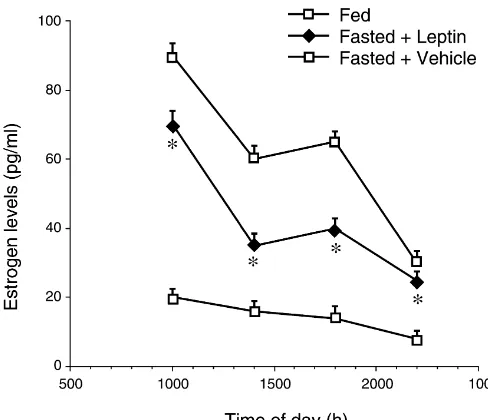
Plasma estrogen levels decreased significantly (P, 0.001, Mann–Whitney U-test) in the fasted vehicle-treated rats at 10.00, 14.00, 18.00 and 22.00 h compared with the fed control. Compared with the fasted vehicle-treated rats, leptin repletion significantly (P,0.001, Mann–Whitney U-test) increased estrogen levels at 10.00, 14.00, 18.00, and 22.00 h (Fig. 3). Leptin may have acted at the pituitary–ovarian axis levels to bring about the increase in estrogen secretion [42].
Fig. 1. Body weight change in fed, fasted vehicle-treated and fasted
leptin-treated female rats. Values are presented as means6S.E.M. (n55 / 3.4. Effect of fasting and leptin repletion on ovulation group). **P,0.01 fasted vehicle-treated; fasted leptin-treated vs. fed
control (Student’s t-test).
Ovulation incidence decreased significantly (P,0.05) in the fasted vehicle-treated rats compared with the fed that the fall of leptin with fasting is sufficient to produce
control as 1 out of 5 rats ovulated (20 vs. 100%). In the these observed changes and signifies the ‘physiological’
fasted lepttreated rats, the incidence of ovulation in-nature of the experiment.
creased significantly (P,0.05) compared with the fasted vehicle-treated rats as 4 out of 5 rats ovulated (80 vs. 20%) 3.2. Effect of fasting and leptin repletion on NADPH-d
(Table 2). reactivity
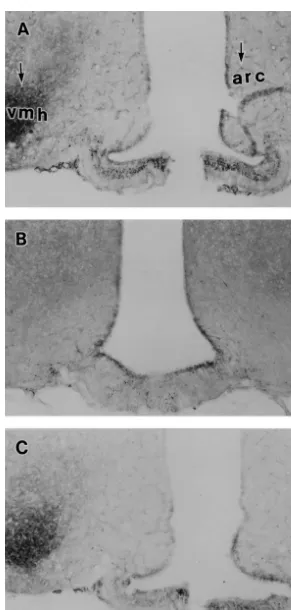
3.5. Effect of leptin repletion after refeeding on In the VMH, NOS-positive cells number and staining
dependable variables intensity increased significantly (P,0.001, Mann–Whitney
U-test) in the fed and fasted leptin-treated rats compared
Food intake, water intake and body weight change were with the fasted vehicle-treated ones. NOS-positive cells
significantly (P,0.05, Student’s t-test) inhibited in the number and staining intensity in the VMH of fed rats was
fasted leptin-treated rats compared with the fasted vehicle-7661 cells, 97.562 intensity, 963 cells, 38.364 intensity
treated rats (Fig. 4). The reduction in food intake and in the fasted-vehicle treated rats, and 7464 cells, 95.263
water intake may have accounted for most of the weight intensity in the fasted leptin-treated ones. Fewer
NOS-loss observed in the leptin-treated animals. It is also positive cells were generally expressed in the ARC area.
possible that an increase in the thermogenesis induced by Compared with fasted vehicle-treated rats, the ARC
NOS-ob protein (leptin) administration may have contributed in positive cells and staining intensity in the fed and fasted
part to the negative energy balance and resultant weight leptin-treated rats increased significantly (P,0.05, Mann–
loss. Another factor could be a reduction in hypothalamic Whitney U-test). In the ARC, NOS-positive cells number
neuropeptide Y levels [23,48] in the leptin-treated rats and the intensity of staining in the fed rats were 661 cells,
which resulted in inhibition of fasting-induced hyperphagia [20].
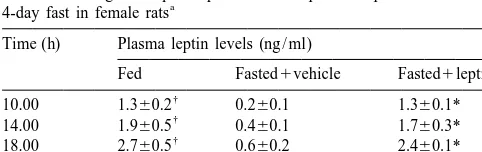
Table 1
Effect of fasting and leptin repletion on the plasma leptin levels during
a
4-day fast in female rats
4. Discussion
Time (h) Plasma leptin levels (ng / ml)
Fed Fasted1vehicle Fasted1leptin Results of the present study indicate that fasting for 4
†
10.00 1.360.2 0.260.1 1.360.1* days started at diestrous was effective in decreasing body
†
14.00 1.960.5 0.460.1 1.760.3* weight, NOS-positive cells number and the staining
in-†
18.00 2.760.5 0.660.2 2.460.1*
tensity in the VMH and ARC. Fasting treatment also †
22.00 3.460.1 0.9860.3 3.160.5*
decreased estrogen and leptin levels and stopped ovulation a
Plasma leptin levels in fed, fasted vehicle-treated, and fasted leptin- in many rats despite that food was returned to the animals treated female rats at 10.00, 14.00, 18.00 and 22.00 h. Data are presented
before being killed, suggesting that fasting may have
as means6S.E.M.; n55 / group.
affected the neuroendocrine processes involved in the
*P,0.01; †P,0.01 vs. fasted vehicle-treated rats (Mann–Whitney
Fig. 3. Plasma estrogen (E2) levels in fed, fasted leptin-treated and fasted vehicle-treated rats at 10.00, 14.00, 18.00 and 22.00 h. Data are presented as means6S.E.M. (n55 / group) and expressed in pg / ml. *P,0.01 vs. fasted vehicle-treated rats (Mann–Whitney U-test).
reversed all these decreases and restored ovulation to all the rats but one. Leptin was potent in inhibiting food intake, water intake and body weight increase after re-commenced feeding, indicating that the dosage of leptin used was at least adequate and pharmacologically potent in altering the neural processes associated with fasting-in-duced inhibition of estradiol secretion. Another plausible explanation that could be given is the possible effect of leptin on the conceivably stimulated NPY in fasting. It has been reported that increased NPY biosynthesis and release is a component of the hypothalamic response to conditions associated with weight loss such as in fasting. NPY activation appears to be a key component of the response to acute reduction in leptin signaling resulting from food deprivation. The ability of leptin to inhibit the activation of the NPY pathway, has been suggested to be a likely integral component of its role in energy regulation. There-fore, leptin inhibition of hypothalamic NPY gene
transcrip-Table 2
Effect of feeding, fasting, fasting plus leptin on ovulation incidence in
Fig. 4. Sustained biological effect of peripherally administered leptin on
a
rats
food intake (A), water intake (B), and body weight change (C), 0.5, 1, 2, Treatment Number of animals ovulated / Number 4, 6, 8, 12, and 24 h of recommenced feeding. *P,0.05 vs. fasted
of animals tested vehicle-treated (control) rats (Student’s t-test).
Fed 5 / 5*
Fasted1 1 / 5 tion is considered an important mechanism by which leptin
Vehicle regulates body weight [44]. Since leptin receptors are
Fasted1 4 / 5*
highly expressed in the ARC [43], as is NPY, a potent
Leptin
stimulator of food intake and inducer of starvation-induced a
Incidence of ovulation in fed, fasted vehicle-treated and fasted leptin- hyperphagia, it is thought that leptin suppressed this treated rats. All the fed (100%, n55) rats, one (20% vs. 100%, n55)
response through a NO mechanism [36] with a resultant
fasted vehicle-treated rat, four (80% vs. 20%, n55) fasted leptin-treated
attenuating effect on food intake, body weight gain and
rats ovulated.
mecha-nism through which leptin administration to fasted rats 10.00 and 14.00 h. Leptin levels started to rise at 18.00 h attenuates the hypothalamic response of increased NPY and peak levels attained at 22.00 h. Leptin secretion production during nutritional stress to exert potent inhib- appeared to be increased in the night, suggesting that its itory effect on all the dependable variables. The decrease secretion pattern might be influenced by circadian rhythms in NOS-positive cells and NADPH-d staining in both the which may be related to the feeding cycle in rodents [3] VMH and ARC nuclei in the fasted vehicle-treated rats, Estrogen plays unique functional roles in mammalian and the subsequent increase in the NOS-positive cells and physiology. Estrogen is considered to be the hormone most NADPH-d staining following leptin repletion, suggests that important for the neural stimulus for the pre-ovulatory LH both the VMH and ARC may be important integrating surge. Estrogen by positive feedback mechanism, is centers in the hypothalamus for NOS and leptin, and their thought to act as a permissive agent in the induction of LH actions may be positively correlated with food intake surge which adequately sensitizes the pituitary gland to the [34,56]. That in the VMH and ARC of fed rats, the stimulating effect of the hypothalamic gonadotropin releas-NOS-positive cells, the intensity of staining and estrogen ing factors [17]. Estrogen exerts its effect on the uterus and levels did not change / decrease as was the case in the vaginal epithelium resulting in hyperemia and hyperplasia fasted rats, suggests that NOS and estrogen levels are of the uterus and increase in the height of the vaginal positively correlated with energy balance (food intake) epithelial cells, and stimulates the adenohypophysis to [28,34]. NOS staining was more marked in the VMH than increase LH release thereby maintaining a normal estrous in the ARC. This observation may be in line with the cycle [17].
functional role of VMH in sexual behavior induction and Like estrogen, leptin also plays important roles in that NOS may be involved in this process. The report by mammalian physiology. Leptin has been demonstrated to Mani et al. [29] that a NO donor sodium nitroprusside modulate appetite and metabolism [56]. Leptin receptors facilitated lordosis tends to lend support to the present are expressed in brain regions associated with the regula-observation. That leptin repletion to fasted rats restored tion of feeding behavior and energy balance, such as the NOS activity, ovulation and estrogen levels, suggests a ARC and VMH [43]. Leptin is thought to act as a signal to positive correlation among leptin, NOS activity, estrogen the brain indicating the level of body fat and induces levels and reproductive function [5,57]. appropriate responses of food intake and energy expendi-The levels of estrogen in the fed, and fasted animals ture. Recent suggestion indicates that leptin may act as a fluctuates at different times of the day indicating the effect starvation signal such that low levels trigger the hypo-of diurnal variation on ovarian function, and suggests the thalamic–pituitary axis to respond to undernutrition [1]. In conformity of its release apparatus to circadian rhythm. the same vein, the effects of leptin on the reproductive Peak estrogen levels was attained in the morning of system suggest that it may act to trigger the hypothalamic– proestrus (10.00 h) and a little rise in the level at 18.00 h. pituitary axis to initiate reproductive cycling and induce a The increased secretion of estrogen in the morning of fertile state [1,5]. As the leptin level is related to the status proestrus often precedes and is needed to trigger LH surge of body fat stores [60], it follows that leptin could which occurs later in the afternoon (2–4 p.m.) [16], whilst influence fertility and the reproductive cycles, either the increased secretion which occurs later, i.e., shortly directly or indirectly [5,7]. Exogenous leptin administra-before dark, is for eliciting lordosis or sexual behavior tion has been reported to rescue the sterility of obese [18]. In this study, a peak estrogen level was recorded in females, elevate serum LH levels and stimulate ovarian the morning (10.00 h) of proestrus. At 14.00 h, the level and uterine histology [5].
[5] L.A. Barash, C.C. Cheung, D.S. Weigle, H. Ren, E.B. Kabigting,
estrogen. The idea that leptin could regulate the functions
J.L. Kuijper, D.K. Clifton, R.A. Steiner, Leptin is a metabolic signal
of reproductive tissues is further strengthened by the
to the reproductive system, Endocrinology 137 (1996) 3144–3147.
expression of leptin receptor mRNAs transcripts in the [6] H.T. Bergen, C.V. Mobbs, Ventromedial hypothalamic lesions pro-endocrine and neuropro-endocrine tissues such as ovary, testis, duced by gold thioglucose do not impair induction of NPY mRNA
anterior pituitary and hypothalamus [59]. Leptin receptor in the arcuate nucleus by fasting, Brain Res. 707 (1996) 266–271. [7] G.A. Bray, D.A. York, Hypothalamic and genetic obesity in
ex-mRNA is also expressed in the immortalized GnRH
perimental animals: an autonomic and endocrine hypothesis,
Phy-(GT1–7 and NLT) neurons and ovarian granulosa cells,
siol. Rev. 59 (1979) 719–809.
suggesting that GnRH neurons and steroid-producing cells [8] G.A. Bray, D.A. York, The MONA LISA hypothesis in the time of of the ovary could be targets for leptin action. Kitawaki et leptin, Recent Prog. Horm. Res. 53 (1998) 95–117.
al. [27] have recently suggested that leptin could stimulate [9] F. Calapai Squadrito, D. Altavilla, B. Zingarelli, G.M. Campo, M. Cilia, A.P. Caputi, Evidence that nitric oxide modulates drinking
estrogen production in the human granulosa cells by
behavior, Neuropharmacology 31 (1992) 761–764.
increasing p450 arom mRNA and p450 arom protein
[10] G. Calapi, G. Mazzaglia, M. Glia, B. zangarelli, F. Squadrito, A.P.
expression and aromatase activity by direct action. We
Caputi, Mediation of nitric oxide formation in the preoptic area of
have demonstrated that peripheral administered recombi- endotoxin and tumour necrosis factor-induced inhibition of water nant mouse leptin to fasted female rats increases plasma intake in the rat, Br. J. Pharmacol. 111 (1994) 1325–1332.
[11] F. Chehab, M. Lim, R. Lu, Correction of the sterility defect in
estrogen levels, the incidence of ovulation and up-regulates
homozygous obese female mice by treatment with human
recombi-NOS activity in the food controlling centers of the brain.
nant leptin, Nat. Genet. 12 (1996) 318–320.
The observations made in this study suggest that leptin
[12] C.C. Cheung, J.E. Thornton, J.L. Kuijper, D.S. Weigle, D.K. Clifton,
could act on the pituitary–ovarian axis to stimulate in- R.A. Steiner, Leptin is a metabolic gate for the onset of puberty in creased secretion of estrogen during nutritional distress. the female rat, Endocrinology 138 (1997) 855–858.
Thus, the concept that leptin serves as a linking signal [13] C.C. Cheung, D.K. Clifton, R.A. Steiner, Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus,
Endo-between nutritional status and neuroendocrine function,
crinology 138 (1997) 4489–4492.
and that the secretion of ovarian estrogen is markedly
[14] K.G. Commons, L.M. Kow, T.A. Milner, D.W. Pfaff, In the
dependent on nutritional status, is further strengthened by ventromedial nucleus of the rat hypothalamus GABA-immuno-the observations arising from this study. Future research on labelled neurons are abundant and are innervated by both enkephalin
the possible effect of leptin on other ovarian hormones and GABA-immunolabelled axon terminals, Brain Res. 816 (1999) 58–67.
under the same experimental paradigm may be necessary
[15] J.K. Elmquist, R.S. Ahima, E. Maratos-Flier, J.S. Flier, C.B. Saper,
to clarify the physiological relevance of some of the
Leptin activates neurons in the ventromedial hypothalamus and
findings. brain stem, Endocrinology 138 (1997) 839–842.
[16] J.W. Everett, D.C. Nichols, The timing of ovulatory release of gonadotropin induced by estrogen in pseudopregnant and diestrus cyclic rats, Anat. Rec. 160 (1968) 346.
Acknowledgements
[17] J.W. Everett, Neuroendocrine aspects of mammalian reproduction, Annu. Rev. Physiol. 31 (1969) 383–417.
The gift of mouse leptin from Dr A.F. Parlow (NHPP, [18] M.E. Freeman, in: E. Knobil, J.D. Neill (Eds.), The Physiology of USA) is gratefully acknowledged. This work was pre- Reproduction, 2nd Edition, Raven Press, New York, 1994, pp.
613–620.
sented in part at the 77th Annual Meeting of the
Physio-[19] L. Hardie, P. Trayhurn, D. Abramovich, P. Fowler, Circulating leptin
logical Society of Japan, Yokohama, Japan 2000, and
in woman: longitudinal study in menstrual cycle and during
preg-supported by Grants-in-Aid for Scientific Research from
nancy, Clin. Endocrinol. 47 (1997) 101–106.
the Ministry of Education, Science, Sports and Culture of [20] R.B.S. Harris, T.R. Kasser, R.J. Martin, Dynamics of recovery of
Japan. body composition after over feeding, food restriction or starvation of
mature female rats, J. Nutr. 116 (1986) 2536–2546.
[21] G.R. Hervey, The effects of lesions in the hypothalamus in parabiotic rats, J. Physiol. 145 (1959) 326–352.
References [22] A. Jablonka-Shariff, L.M. Olson, Hormonal regulation of NOS and
their cell-specific expression during follicular development in rat [1] R.S. Ahima, D. Prabakaran, C. Mantzoros, D. Qu, B. Lowell, E. ovary, Endocrinology 138 (1997) 460–468.
Maratos-Flier, J.S. Flier, Role of leptin in the Neuroendocrine [23] S.P. Kalra, M.G. Dube, A. Sahu, C. Phelps, P.S. Kalra, Neuropeptide response to fasting, Nature 382 (1996) 250–252. Y secretion increases in the paraventricular nucleus in association [2] R.S. Ahima, J. Dushay, S.N. Flier, D. Prabakaran, J.S. Flier, Leptin with increased appetite for food, Proc. Natl. Acad. Sci. USA 88
accelerates the onset of puberty in normal female mice, J. Clin. (1991) 10931–10935.
Invest. 99 (1997) 391–395. [24] S.P. Kalra, P.S. kalra, Nutritional infertility: the role of the inter-[3] R.S. Ahima, D. Prabakaran D, J.S. Flier, Postnatal leptin surge and connected hypothalamic neuropeptide Y-galanin-opioid network,
regulation of circadian rhythm of leptin by feeding: implications for Front. Neuroendocrinol. 17 (1996) 371–401.
energy homeostasis and neuroendocrine function, J. Clin. Invest. [25] C. Karlsson, K. Lindell, E. Svensson, C. Bergh, P. Lind, H. Billig, 101 (1998) 1020–1027. L.M. Carlsson, B. Carlsson, Expression of functional leptin re-[4] E.D. Albrecht, A.L. Haskins, G.J. Pepe, The influence of fetectomy ceptors in the human ovary, J. Clin. Endocrinol. Metab. 82 (1997)
at midgestation upon the serum concentration of progesterone, 4144–4148.
estrone and estradiol in baboons, Endocrinology 107 (1980) 766– [26] E.M. Kim, C.C. Welch, M.K. Grace, C.J. Billington, A.S. Levine,
mRNA levels of opioid peptides in arcuate nucleus, Am. J. Physiol. Identification of targets of leptin action in rat hypothalamus, J. Clin.
270 (1996) R1019–R1024. Invest. 98 (1996) 1101–1106.
[27] J. Kitawaki, I. Kusuki, H. Koshiba, K. Tsukamoto, H. Honjo, Leptin [44] M.W. Schwartz, J.C. Erickson, D.G. Baskin, R.D. Palmiter, Effect of directly stimulates aromatase activity in human luteinized granulosa fasting and leptin deficiency on hypothalamic neuropeptide Y gene cells, Molec. Human Reprod. 5 (1999) 708–713. transcription in vivo revealed by expressing a Lacz reporter gene, [28] H.-Y. Li, G.N. Wade, J.D. Blaustein, Manipulations of metabolic fuel Endocrinology 139 (1998) 2629–2635.
availability alter estrous behaviour and neural estrogen receptor [45] L. Shukovski, A. Tsafriri, The involvement of NO in the ovulatory immunoreactivity in Syrian hamsters, Endocrinology 135 (1994) process in rat, Endocrinology 135 (1994) 2287–2290.
240–247. [46] M.K. Sinha, J.F. Caro, Clinical aspects of leptin, Vit. Horm. 54 [29] S.K. Mani, J.M.C. Allen, V. Rettori, S.M. McCann, B.W. O’Malley, (1998) 1–30.
J.H. Clark, Nitric oxide mediates sexual behavior in female rats, [47] B.M. Spiegelman, J.S. Flier, Adipogenesis and obesity: rounding out Proc. Natl. Acad. Sci. USA 91 (1994) 6468–6472. the big picture, Cell 87 (1996) 377–389.
[30] T.M. McShane, S.L. Petersen, S. McCorne, D.H. Keisler, Influence [48] B.G. Stanley, S.E. Kyrkouli, S. Lampert, S.F. Leibowitz, Neuro-of food restriction on neuropeptide-Y. Proopiomelanocortin and peptide Y chronically injected into the hypothalamus: a powerful luteinizing hormone-releasing hormone gene expression in sheep neurochemical inducer of hyperphagia and obesity, Peptides 7 hypothalami, Biol. Reprod. 49 (1993) 831–839. (1986) 1189–1192.
[31] J.G. Mercer, N. Hoggard, L.M. Williams, C.B. Lawrence, L.T. [49] L.A. Tartaglia, M. Dembski, X. Weng, N. Deng, J. Culpepper, R. Hannah, P.J. Morgan, P. Trayhurn, Coexpression of leptin receptor Devos, G.J. Richards, L.A. Campfield, F.T. Clark, J. Deeds, C. Mui, and preproneuropeptide Y mRNA in arcuate nucleus of mouse S. Sankers, A. Moviarty, K.J. Moore, J.S. Smutko, G.G. Mays, E.A. hypothalamus, J. Neuroendocrinol. 8 (1996) 733–735. Woolf, C.A. Monroe, R.I. Tepper, Identification and expression [32] J.G. Mercer, K.M. Moar, A.W. Ross, N. Hoggard, J.P. Morgan, cloning of a leptin receptor OB-R, Cell 83 (1995) 1263–1271.
Photoperiod regulates arcuate nucleus POMC, AGRP, and leptin [50] B.J. Van Voorhis, M.S. Dunn, G.D. Snyder, C.P. Weiner, Nitric oxide: receptor mRNA in Siberian hamster hypothalamus, Am. J. Physiol. an autocrine regulator of human granulosa-luteal cell steroidogene-278 (2000) R271–R281. sis, Endocrinology 135 (1994) 1799–1806.
[33] L.P. Morin, Environment and hamster reproduction: responses to [51] B.J. Van Voorhis, K. Moore, P.J.L.M. Strijbos, S. Nelson, S.A. phase-specific starvation during estrous cycle, Am. J. Physiol. 251 Baylis, D. Grzybicki, C.P. Weiner, Expression and localization of (1986) R663–R669. inducible and endothelial NOS in the rat ovary, J. Clin. Invest. 96 [34] J.E. Morley, J.F. Flood, Evidence that nitric oxide modulates food (1996) 2719–2726.
intake in mice, Life Sci. 49 (1991) 707–711. [52] S.R. Vincent, H. Kimura, Histochemical mapping of nitric oxide [35] J.E. Morley, J.F. Flood, Competitive antagonism of nitric oxide synthase in the rat brain, Neuroscience 46 (1992) 755–784.
synthase causes weight loss in mice, Life Sci. 51 (1992) 1285– [53] G.N. Wade, Gonadal hormones and behavioural regulation of body
1289. weight, Physiol. Behav. 8 (1972) 523–534.
[36] J.E. Morley, M.M. Alshaher, S.A. Farr, J.F. Flood, V.B. Kumar, [54] G.N. Wade, J.E. Schneider, Metabolic fuels and reproduction in Leptin and neuropeptide Y modulate nitric oxide synthase: further female mammals, Neurosci. Biobehav. Rev. 16 (1992) 235–272. evidence for a role of nitric oxide in feeding, Peptides 20 (1999) [55] G.N. Wade, J.E. Schneider, H.Y. Li, Control of fertility by metabolic
595–600. cues, Am. J. Physiol. 270 (1996) E1–E19.
[37] K. Mounzin, R. Lu, F.F. Chehab, Leptin treatment rescues the [56] D.S. Weigle, T.R. Bukowski, D.C. Foster, S. Holderman, J.M. sterility of genetically obese ob / ob males, Endocrinology 138 Kramer, G. Lasser, C.E. Lofton-Day, D.E. Prunkard, C. Raymond, (1997) 1190–1193. J.L. Kuijper, Recombinant ob protein reduces feeding and body [38] G. Paxinos, C. Watson (Eds.), The Rat Brain Stereotaxic Coordi- weight in the ob / ob mouse, J. Clin. Invest. 96 (1995) 2065–2070. nates, Academic Press, New York, 1982. [57] W.H. Yu, A. Walczewska, S. Karanth, S.M. McCann, Nitric oxide [39] D.W. Pfaff, M. Keiner, Atlas of estradiol concentrating cells in the mediates leptin-induced luteinizing hormone-releasing hormone central nervous system of the female rat, J. Comp. Neurol. 151 (LHRH) and leptin-induced luteinizing hormone (LH) release from (1973) 121–158. the pituitary gland, Endocrinology 138 (1997) 5055–5058. [40] V. Rettori, N. Belova, W.L. Dees, C.L. Nyberg, M. Gimeno, S.M. [58] W.H. Yu, M. Kimura, A. Walczewska, S. Karanth, S.M. McCann, G.
McCann, Role of nitric oxide in the control of luteinizing hormone- Calapai, Role of leptin in hypothalamic–pituitary function, Proc. releasing hormone release in vivo and in vitro, Proc. Natl. Acad. Sci. Natl. Acad. Sci. USA 94 (1997) 1023–1028.
USA 90 (1993) 10130–10134. [59] P.L. Zamorano, V.B. Mahesh, L.M. De Sevilla, L.P. Chorich, G.K. [41] M. Rosenbaum, R.L. Leibel, Leptin: a molecule integrating somatic Bhat, D.W. Brann, Expression and localization of the leptin receptor energy stores, energy expenditure and fertility, Trends Endocrinol. in endocrine and neuroendocrine tissues of the rat,
Neuroendocrinol-Metab. 9 (1998) 117–124. ogy 65 (1997) 223–228.
[42] M.W. Schwartz, M.F. Dallma, S.C. Woods, Hypothalamic response [60] Y. Zhang, R. Proenca, M. Maffei, M. Barone, L. Leopold, J.M. to starvation: implications for the study of wasting disorders, Am. J. Friedman, Positional cloning of the mouse obese gene and its human Physiol. 269 (1995) R949–R957. homologue, Nature 372 (1994) 425–432.