Brain Research 879 (2000) 13–16
www.elsevier.com / locate / bres
Research report
Bilirubin does not modulate ionotropic glutamate receptors or
glutamate transporters
*
Orpheus Warr, Dominic Mort, David Attwell
Department of Physiology, University College London, Gower Street, London, WC1E 6BT, UK Accepted 5 July 2000
Abstract
Bilirubin, a product of haemoglobin metabolism, has been suggested to damage neurons by increasing activation of N-methyl-D
-aspartate (NMDA) receptors when it reaches high levels in the blood [15,19], as occurs in neonatal jaundice [7]. Bilirubin is also generated in the brain following synthesis of the messenger carbon monoxide (CO) by haem oxygenase, and haem oxygenase is upregulated in Alzheimer’s disease [23]. We examined the effect of bilirubin on currents generated by NMDA anda -amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors in hippocampal pyramidal cells, and on glutamate transporter currents in retinal glial cells. Bilirubin did not modulate either receptor-gated currents or transporter currents. These data show the negative, but important result that bilirubin does not induce neuronal death by acting directly on NMDA or AMPA receptors, nor indirectly by blocking glutamate uptake and raising the extracellular concentration of glutamate. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Neurotoxicity
Keywords: Bilirubin; Glutamate; NMDA; Haem oxygenase; Jaundice; Carbon monoxide
1. Introduction conditions, the basal ganglia, hippocampus, brainstem
nuclei and Purkinje cells of the cerebellum are most vulnerable to damage, and such pathology is associated Bilirubin is formed during the degradation of
haemo-with the development of cerebral palsy, paralysis of globin, by the sequence of reactions haem biliverdin
upward gaze and deafness [6]. Bilirubin normally binds to bilirubin
albumin in the blood, and is probably only neurotoxic in its free (unbound) form [3,8], which occurs when the con-centration ratio of bilirubin to albumin exceeds approxi-mately unity [1,9]. The concentration of free bilirubin achieved in this situation is uncertain: its solubility is less than 0.1mM, but it can exist as a supersaturated solution at In many neonates, particularly those born prematurely, higher concentrations [13].
high levels of unconjugated bilirubin occur in the blood, Bilirubin is also produced during normal brain function causing jaundice. This can lead to neuronal damage and as a by-product of synthesis of the messenger carbon even death, especially when it occurs concurrently (as is monoxide (CO) by haem oxygenase [25]. Biliverdin, often the case with pre-term infants) with complications produced from haem with CO, is converted to bilirubin by such as acidosis and hypoxaemia [7,14]. Under these biliverdin reductase (see reaction scheme above) which is found in the same cells as haem oxygenase [12]. Endogen-ous CO acts on guanylate cyclase at low micromolar levels [17], suggesting the production of bilirubin at low mi-*Corresponding author. Tel.:144-20-7679-7342; fax: 1
44-20-7413-cromolar levels in normal brain (where there is no albumin 8395.
E-mail address: [email protected] (D. Attwell). to bind it). Interestingly haem oxygenase is upregulated in
14 O. Warr et al. / Brain Research 879 (2000) 13 –16
Alzheimers’ disease [23], raising the possibility of a ment. Bilirubin is photosensitive and was kept in the dark, contribution to this disorder from bilirubin toxicity. both as a stock solution and in the experimental solution Although several mechanisms have been proposed for (until 10 s before being applied to cells), by covering its the neurotoxic actions of bilirubin [1,3,5,10], bilirubin may container with silver foil.
¨
mediate much of its neurotoxicity by acting upon N- Glutamate transport was studied in Muller cells [4] methyl-D-aspartate (NMDA) receptors. Damage caused by obtained by enzymatic dissociation of retinae from tiger high levels of free bilirubin can be reduced by treatment salamanders (Ambystoma tigrinum, killed by concussion with MK-801, an open channel blocker of NMDA re- followed by immediate destruction of the brain, in accord-ceptors, and bilirubin-treated neurons show a greater ance with UK animal care regulations). The pipette
solu-¨
degree of MK-801 binding than untreated cells implying tion for whole-cell clamping Muller cells contained (mM) more opening of NMDA receptor channels [15,19]. Fur- KCl 95, NaCl 5, CaCl 1, MgCl 1, K EGTA 5, HEPES 52 2 2
thermore, NMDA injection into the brain causes greater (pH adjusted to 7 with KOH, osmolarity 211 mOsmol). damage in jaundiced rats than in non-jaundiced controls The superfusion solution contained (mM) NaCl 105, KCl
1
[19]. A possible substrate for a potentiating effect of 2.5, BaCl 6 (to block K2 channels), CaCl 3, MgCl 0.5,2 2
bilirubin on NMDA receptor activation is provided by the HEPES 5, glucose 15 (pH adjusted to 7.3 with NaOH, fact that bilirubin often binds to sites on proteins that bind osmolarity 248 mOsmol.).
arachidonic acid [16,21], and arachidonic acid potentiates All drugs were applied in the superfusate at the con-NMDA receptor channel opening [20]. Similarly, since centrations stated (and for the durations shown in the arachidonic acid inhibits glutamate uptake [2], bilirubin figures). Peak glutamate-, AMPA- and NMDA-evoked might also increase NMDA receptor activation indirectly, currents were measured 30–60 s after drug application to by inhibiting glutamate uptake and raising the extracellular slices, and 5 s after application to isolated cells. Data in
glutamate concentration. bilirubin were measured relative to the average of pre- and
We therefore investigated the effect of bilirubin on post-control responses and are expressed as mean6SEM. glutamate receptors (in hippocampal pyramidal cells) and Statistical comparisons were performed using Student’s transporters (in retinal glial cells). a-amino-3-hydroxy-5- t-test.
methyl-4-isoxazole propionate (AMPA) receptors were studied as well as NMDA receptors, since any potentiation
of their opening would lead to a larger potentially neuro- 3. Results
21
toxic Ca influx through NMDA receptor channels by
21
removal of the NMDA channels’ Mg -block. Our data Agonist doses used to evoke NMDA, AMPA or trans-show a negative, but important result: bilirubin neuro- porter currents were chosen to be subsaturating on their toxicity is not caused by short-term modulation of NMDA steady-state dose–response curves [4,22] so that bilirubin-or AMPA receptbilirubin-ors, nbilirubin-or of glutamate transpbilirubin-orters. evoked current changes due to alteration of either the agonist affinity or Imax could potentially be detected (NMDA receptor EC50 for NMDA535 mM, dose used5
2. Materials and methods 10 mM; AMPA receptor EC50 for AMPA511 mM, dose
used55 mM; glutamate transporter EC50 for glutamate5
Glutamate receptor and transporter activity was moni- 20mM, dose used520 mM).
tored electrically using whole cell clamping. All experi- NMDA-evoked currents were recorded at a membrane ments were carried out at room temperature (23–258C). potential of 230 mV to prevent the block of the NMDA
21
Glutamate receptor responses were studied in area CA1 receptor channel by Mg in the bath solution (the pyramidal cells of rat hippocampal slices (200–250 mm increase in MK-801 binding reported previously [15] was
21
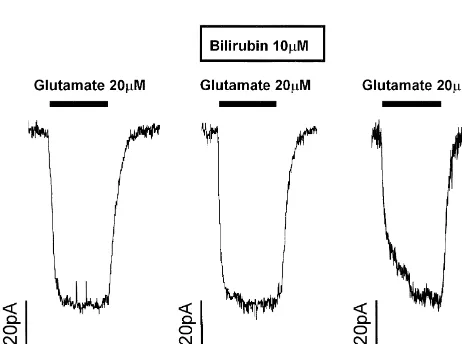
thick) cut with a vibrating tissue slicer from 12 day-old seen in Mg -free solution). Despite previous reports that Sprague–Dawley rats (killed by cervical dislocation in NMDA receptors open more after exposure to bilirubin, accordance with UK animal care regulations). The pipette the application of free bilirubin (10mM) to hippocampal solution for whole-cell clamping contained (mM) CsCl slices did not significantly increase the response of CA1 140, NaCl 4, CaCl 0.5, (N-methyl-2 D-glucamine) -EGTA2 pyramidal cells to 10mM NMDA (Fig. 1). In six cells the 5, MgATP 2, HEPES 10 (pH adjusted to 7 with CsOH, current was insignificantly increased by 466%, P.0.3). osmolarity measured as 300 mOsmol.). The superfusion The application of free bilirubin (10mM) also failed to solution for slices was oxygenated with 100% O2 and modulate significantly the current evoked by 5mM AMPA contained (mM) NaCl 140, KCl 2.5, NaH PO 1, CaCl2 4 2 at 260 mV (current increased in three cells by 169%, 2.5, MgCl2 2, HEPES 10, bicuculline 0.02 (to block P.0.9). Fig. 2 shows a specimen trace from one of these GABAA receptors), glucose 10 (pH adjusted to 7.4 with cells.
¨
arach-O. Warr et al. / Brain Research 879 (2000) 13 –16 15
effect of 10 mM bilirubin was seen on glutamate-evoked currents in CHO cells [18] expressing GLT-1 transporters (n52, data not shown).
4. Discussion
Previous work [15] suggested that bilirubin increases the amount of NMDA receptor activation produced by a fixed concentration of glutamate. Despite the presence of a Fig. 1. Bilirubin does not modulate NMDA receptor currents in rat
potentiating arachidonic acid-binding site on NMDA re-hippocampal pyramidal cells. Current responses to 10mM NMDA are
shown in ordinary external solution, in the presence of 10mM bilirubin, ceptors [20], to which bilirubin might be expected to bind and then in control solution again. Holding potential was 230 mV. [16,21], we found no effect of bilirubin on NMDA-evoked
currents in hippocampal pyramidal cells. Bilirubin also had no effect on AMPA-evoked currents, nor on the rate of glutamate uptake by transporters, ruling out potentiation of AMPA receptor-mediated depolarization, or a rise of extracellular glutamate concentration produced by uptake inhibition, as explanations for the observation [19] that blocking NMDA receptor channels with MK-801 reduced bilirubin-evoked neuronal death.
Although our data show no short-term direct effect of bilirubin on glutamate receptors or transporters, it is possible that bilirubin could have an action that was too slow to be seen in our experiments, for example increasing Fig. 2. Bilirubin does not modulate AMPA receptor currents in rat
expression of NMDA receptors (although no change in hippocampal pyramidal cells. Current responses to 5 mM AMPA are
MK-801 binding site density was seen in Ref. [15]) or shown in ordinary external solution, in the presence of 10mM bilirubin,
and then in control solution again. Holding potential260 mV. decreasing transporter expression. Alternatively, bilirubin may have an action that was negated by the conditions of our experiment, for example an alteration of channel idonic acid [2] and so might be inhibited by bilirubin. phosphorylation that might not occur when the cell is However, in six cells, the addition of 10mM free bilirubin dialysed with the pipette solution (although previous work to the bath solution did not significantly reduce the uptake on NMDA receptor phosphorylation has used whole-cell current generated by 10 mM L-glutamate at 240 mV clamping). Finally, although it would not explain the (current increased by 565%, P.0.3). A specimen trace increased MK-801 binding seen in bilirubin [15] (since that from one of these cells is shown in Fig. 3. Similarly, no was seen in membranes removed from brain), it is possible that bilirubin produces NMDA receptor-dependent neuro-nal death by potentiating glutamate release from
presynap-21 tic terminals or sensitising the postsynaptic cell to the Ca influx which occurs through NMDA receptors.
In conclusion, our results suggest that, to explain previous data showing that bilirubin kills neurons in an NMDA receptor-dependent manner, it is necessary to look for long-term effects of bilirubin on components of gluta-matergic signalling.
Acknowledgements
Supported by the Wellcome Trust and MRC.
Fig. 3. Bilirubin does not modulate GLAST glutamate transporter
References currents in salamander retinal glial cells. Current responses to 20mM
glutamate are shown in ordinary external solution, in the presence of 10
mM bilirubin, and then in control solution again. Holding potential 240 [1] Y. Amit, G. Chan, S. Fedunec, M.J. Poznansky, D. Schiff, Bilirubin
16 O. Warr et al. / Brain Research 879 (2000) 13 –16
3 35
ATPase, [ H]-thymidine uptake, L-[ S]-methionine incorporation, Diseases of the Fetus and Infant, Vol. 2, Mosby, St. Louis, MO, and mitochondrial function, Pediatr. Res. 25 (1989) 364–368. 1977, pp. 1345–1372.
[2] B. Barbour, M. Szatkowski, N. Ingledew, D. Attwell, Arachidonic [15] D.J. Hoffmann, S.A. Zanelli, J. Kubin, O.P. Mishra, M. Delivoria-acid induces a prolonged block of glial cell glutamate uptake, Nature Papadopoulos, The in vivo effect of bilirubin on the N-methyl-D -342 (1989) 918–920. aspartate receptor / ion channel complex in the brains of newborn [3] D. Bratlid, How bilirubin gets into the brain, Clin. Perinatol. 17 piglets, Pediatr. Res. 40 (1996) 804–808.
(1990) 449–465. [16] J. Hsia, S.S. Er, C.T. Tan, T. Estes, E. Ruoslahti, a-fetoprotein [4] H. Brew, D. Attwell, Electrogenic glutamate uptake is a major binding specificity for arachidonate, bilirubin, docosahexaenoate, current carrier in the membrane of axolotl retinal glial cells, Nature and palmitate. A spin label study, J. Biol. Chem. 255 (1980)
327 (1987) 707–709. 4224–4227.
[5] R. Brodersen, Bilirubin. Solubility and interaction with albumin and [17] T. Ingi, J. Cheng, G.V. Ronnett, Carbon monoxide: an endogenous phospholipid, J. Biol. Chem. 254 (1979) 2364–2369. modulator of the nitric oxide-cyclic GMP signaling system, Neuron [6] R.K. Byers, R.S. Paine, B. Crothers, Extrapyramidal cerebral palsy 16 (1996) 835–842.
with hearing loss following erythroblastosis, Pediatrics 15 (1955) [18] L. Levy, O. Warr, D. Attwell, Stoichiometry of the glial glutamate 248–254. transporter GLT-1 expressed inducibly in a Chinese hamster ovary
1
[7] W.J. Cashore, The neurotoxicity of bilirubin, Clin. Perinatol. 17 cell line selected for low endogenous Na -dependent glutamate
(1990) 437–447. uptake, J. Neurosci. 18 (1998) 9620–9628.
[8] W.J. Cashore, W. Oh, Unbound bilirubin and kernicterus in low- [19] J.W. McDonald, S.M. Shapiro, F.S. Silverstein, M.V. Johnston, Role birth-weight infants, Pediatrics 69 (1982) 481–485. of glutamate receptor-mediated excitotoxicity in bilirubin-induced [9] L. Chuniaud, M. Dessante, F. Chantoux, J.P. Blondeau, J. Francon, brain injury in the Gunn rat model, Exp. Neurol. 150 (1998) 21–29. F. Trivin, Cytotoxicity of bilirubin for human fibroblasts and rat [20] B. Miller, B. Sarantis, S. Traynelis, D. Attwell, Potentiation of astrocytes in culture. Effect of the ratio of bilirubin to serum NMDA receptor currents by arachidonic acid, Nature 355 (1992) albumin, Clin. Chim. Acta 256 (1996) 103–114. 722–725.
[10] S.B. Churn, R.J. DeLorenzo, S.M. Shapiro, Bilirubin induces a [21] G.B. Odell, J.O. Cukier, J.M. Ostrea Jr., A.C. Maglalang, R.L. calcium-dependent inhibition of multifunctional Ca21/ calmodulin- Poland, The influence of fatty acids on the binding of bilirubin to dependent kinase II activity in vitro, Pediatr. Res. 38 (1995) 949– albumin, J. Lab. Clin. Med. 89 (1977) 295–307.
954. [22] D. Patneau, M. Mayer, Structure-activity relationships for amino [11] S. Eliasof, J.L. Arriza, B.H. Leighton, M.P. Kavanaugh, S.G. Amara, acid transmitter candidates acting at N-methyl-D-aspartate and
Excitatory amino acid transporters of the salamander retina: identifi- quisqualate receptors, J. Neurosci. 10 (1990) 2385–2399. cation, localization, and function, J. Neurosci. 18 (1998) 698–712. [23] M.A. Smith, R.K. Kutty, P.L. Richey, S.D. Yan, D. Stern, G.J. [12] J.F. Ewing, C.M. Weber, M.D. Maines, Biliverdin reductase is heat Chader, B. Wiggert, R.B. Petersen, G. Perry, Heme oxygenase-1 is resistant and coexpressed with constitutive and heat shock forms of associated with the neurofibrillary pathology of Alzheimer’s disease, heme oxygenase in brain, J. Neurochem. 61 (1993) 1015–1023. Am. J. Pathol. 145 (1994) 42–47.
[13] J.-S. Hahm, J.D. Ostrow, P. Mukerjee, L. Celic, Ionization and [24] M. Spiridon, D. Kamm, B. Billups, P. Mobbs, D. Attwell, Modula-self-association of unconjugated bilirubin, determined by rapid tion by zinc of the glutamate transporter in glial cells isolated from solvent partition from chloroform, with further studies of bilirubin the tiger Salamander retina, J. Physiol. 506 (1998) 363–376. solubility, J. Lipid Res. 33 (1992) 1123–1137. [25] A. Verma, D.J. Hirsch, C.E. Glatt, G.V. Ronnett, S.H. Snyder, Carbon [14] L.P. Halamek, D.K. Stevenson, Neonatal jaundice and liver disease, monoxide: a putative neural messenger, Science 259 (1993) 381–