www.elsevier.com/locate/ibmb
Chitin is only a minor component of the peritrophic matrix from
larvae of
Lucilia cuprina
Ross L. Tellam
*, Craig Eisemann
CSIRO Tropical Agriculture, Private Mail Bag 3, Indooroopilly, 4068 Queensland, Australia
Received 10 September 1999; received in revised form 6 April 2000; accepted 27 April 2000
Abstract
The gut of most insects is lined with a peritrophic matrix that facilitates the digestive process and protects insects from invasion by micro-organisms and parasites. It is widely accepted that the matrix is composed of chitin, proteins and proteoglycans. Here we critically re-examine the chitin content of the typical type 2 peritrophic matrix from the larvae of the fly Lucilia cuprinausing a range of techniques. Many of the histochemical and biochemical techniques indicate the presence of chitin, although they are often adversely influenced by the presence of highly glycosylated proteins, a principal component of the matrix. The alkali-stable fraction, which is used as an indicator of the maximum chitin content in a biological sample, is only 7.2% of the weight of the matrix. Larvae fed on the potent chitin synthase inhibitor polyoxin D or the chitin-binding agent Calcofluor White, showed strong concen-tration-dependent inhibition of larval weight and survival but no discernible effects on the matrix structure. A bacterial endochitinase fed to larvae had no effect on larval growth and no observable effect in vitro on the structure of isolated peritrophic matrix. RT– PCR did not detect a chitin synthase mRNA in cardia, the tissue from which PM originates. It is concluded that chitin is a minor structural component of the type 2 peritrophic matrix of this insect. 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Peritrophic matrix; Peritrophic membrane; Peritrophin;Lucilia cuprina; Chitin
1. Introduction
The peritrophic matrix (or peritrophic membrane, PM) lines the guts of most insects at least at some stage dur-ing their lifecycle (Peters, 1992; Tellam, 1996a; Lehane, 1997). This semi-permeable matrix is intimately associa-ted with the insect digestive process by the partitioning of digestive enzymes and partially digested food between the ecto- and endo-PM spaces. In addition, the PM is involved in the protection of insects from invasion by bacteria and parasites. Although the PM can take many diverse forms it typically can be categorised into two classes depending on the site of synthesis. Type 1 PM, which for example is produced in blood-fed adult mosquitoes, is secreted by all of the epithelia lining the midgut usually in response to the ingestion of a meal. This PM envelops the ingested meal in a bag-like struc-ture. In contrast, type 2 PM is produced from a small
* Corresponding author. Tel.:+61-732142476; fax:+61-732142480.
E-mail address:[email protected] (R.L. Tellam).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 9 7 - 7
group of highly specialised cells called the cardia usually situated in the anterior midgut region. This type of PM is constitutively produced as a continuous tube and has a relatively highly ordered structure. The best character-ised type 2 PMs are from the larvae of higher dipteran flies (Peters, 1992).
simi-lar, is difficult to reconcile. A model of the structure of a type 2 PM based on these known components has been proposed (Schorderet et al., 1998). The model shows a protein matrix embedded in a core of chitin fibrils with multivalent noncovalent interactions between the pro-teins and chitin. Proteoglycans are shown bound non-covalently to both proteins and chitin and situated on the periphery of the PM. The basis for the proposed chitin– protein core is the historical evidence that chitin, a β -1-4-linked linear polymer of N-acetyl-d-glucosamine
(GlcNAc), is the major component of the PM and the recent discovery and characterisation of a specific group of intrinsic PM proteins, the peritrophins, which contain multiple non-identical copies of a 6 cysteine domain (the peritrophin-A domain) related to the chitin-binding domains of several insect chitinases (Elvin et al., 1996; Tellam, 1996b; Wang and Granados, 1997; Shen and Jacobs-Lorena, 1998; Tellam et al., 1999). In addition, two peritrophins, peritrophin-44 from the type 2 PM of
Lucilia cuprina larvae and Ag–AperI from the type 1 PM of Anopheles gambiae, have been shown to bind specifically to chitin derivatives in vitro (Elvin et al., 1996; Shen and Jacobs-Lorena, 1998). One of the com-mon physical properties of the peritrophins is that they are glycosylated, sometimes extensively (Adang and Spence, 1982; Peters, 1992; Moskalyk et al., 1996; Wang and Granados, 1997; Tellam et al., 1999). The peritrophin-A domain often has potential N-linked gly-cosylation sites. Some peritrophins, in addition to con-taining multiple peritrophin-A domains, also contain proline and threonine-rich mucin-like domains that are probably substantially glycosylated through O-linked sites.
Previous assessments of the chitin contents of insect PMs have typically relied on individual tests and were made at a time when the presence of the extensively glycosylated peritrophins was not fully appreciated. Consequently, the potentially adverse effects of these proteins on the various tests for chitin were not con-sidered. The current study has critically re-examined the chitin content of a typical type 2 PM from the larvae of the fly L. cuprina using a range of histochemical, bio-chemical, molecular biological and functional analyses.
2. Materials and methods
2.1. Culture of L. cuprina larvae and production of PM
The laboratory culture of L. cuprina larvae and har-vesting of PM from these larvae have been described elsewhere (East et al., 1993). PM was obtained from lar-vae cultured in vitro and was stored at 270°C in PBS (20 mM Na2PO4, 140 mM NaCl, pH 7.2) containing 2.5
mM benzamidine and 5 mM EDTA.
2.2. Preparation of the alkali-stable PM residue
PM (1 g dry weight) was successively homogenised and washed by centrifugation (50,000g, 30 min, 4°C) with 40 ml of each of the following buffers: water; 100 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 5 mM benzamidine, 0.1 mM phenylmethylsulfonyl fluor-ide (PMSF) (buffer); 20 mM Tris–HCl, pH 7.4, 140 mM NaCl (TBS) containing 0.1 mM PMSF and 2% Zwitterg-ent 3–14 (detergZwitterg-ent buffer); TBS containing 6 M urea and 0.1 mM PMSF (urea buffer); TBS containing 6 M guanidine HCl (guanidine buffer) and; a solution con-taining 5% SDS and 10 mM DTT (SDS/DTT solution). These extractions were designed to remove non-coval-ently bound proteins and also any proteins bound by disulphide bonds to other components in the PM. The residual solid material was then extensively washed with water and incubated with 10 ml of 7 N KOH at 60°C for 16 h. The alkali extraction was repeated twice more using identical conditions. After these extractions the remaining solid material was washed extensively with water and its dry weight measured after oven drying in vacuo at 60°C for 24 h.
2.3. Compositional analyses of PM
Amino acid analysis (Ou et al., 1996) was used to determine the mass of protein or amino acids remaining in the alkali-stable PM residue. The protein and GlcNAc contents of whole unextracted PM could not be accu-rately determined because of sample charring under the hydrolysis conditions (i.e. 4 N HCl, 100°C for 4 h). This charring effect is typical of samples containing highly glycosylated mucin-like proteins, which are known to be present in PM (Wang and Granados, 1997; Tellam et al., 1999). The GlcNAc content of the alkali-stable PM residue was determined using the following procedure. The residue (0.5 mg) was hydrolysed by 4 N HCl at 100°C for 4 h in a sealed screw cap tube, lyophilised and then dissolved in water. Monosaccharides were sep-arated by high performance anion exchange chromato-graphy (Dionex DX 500 Carbohydrate System) with pulsed amperometric detection. Quantitation was perfor-med using an internal standard (2-deoxyglucose).
2.4. In vitro feeding assays
The number of surviving larvae and their weights were measured after 20 h of feeding.
2.5. Assessment of the effect of polyoxin D on PM structure
Electron microscopy was used to determine the effects of ingested polyoxin D on PM structure. PM was obtained by dissection from larvae allowed to feed on a diet containing 50 µM polyoxin D for 20 h at 26°C. These larvae were substantially smaller in size than con-trol larvae (|70% smaller). Consequently, larvae that were not exposed to polyoxin D, but which were the same size (and at approximately the same developmental state) as the polyoxin D-treated larvae were chosen as appropriate controls. The control larvae were grown for only 15 h to achieve this comparable size. In each case the larvae were prepared for electron microscopy as pre-viously described (Eisemann et al., 1994) and examined in a Jeol 1010 transmission electron microscope.
2.6. Assessment of the effect of endochitinase on PM structure
Cardiae with attached lengths of PM were dissected from third instar larvae of L. cuprina. After washing in PBS, five of these cardiae were incubated in 1 ml of 50 mM citrate–phosphate buffer, pH 6.1 (Brurberg et al., 1996) containing 1 U of S. marcescens endochitinase (Sigma Chem. Co., St Louis, MO) for 5.5 h at room temperature. Control preparations were incubated in the same buffer without the endochitinase. After subsequent washing in PBS, all preparations were incubated in 0.1% Calcofluor White in PBS, washed extensively and exam-ined in a fluorescence microscope as described below to visualise the PM.
2.7. Localisation of chitin using wheatgerm lectin
L. cuprina larvae were grown to second instar on a synthetic diet (Eisemann et al., 1994) before being dis-sected open and the PM removed, and fixed in 4% par-aformaldehyde in 100 mM phosphate-buffered saline (PBS) for 1 h at room temperature. The sample was then embedded in LR white medium grade acrylic resin (London Resin Co., Reading, UK). Thin sections through the sample were cut using an ultramicrotome and taken up on gold grids, which were incubated for 1–1.5 h on drops of 100 mM PBS with 0.1% Tween 20 containing successively wheatgerm lectin (36 µg/ml) then either rabbit serum raised to wheatgerm lectin or normal rabbit serum (control), both diluted 1:100, and finally goat anti-rabbit antibody conjugated to 10 nm diameter colloidal gold (British Biocell, Cardiff, UK), diluted 1:100. Wheatgerm lectin was purified and serum raised to it essentially as described for an unrelated
pro-tein (Allen et al., 1973; Elvin et al., 1996). In two prep-arations, the PM samples were incubated with wheat-germ lectin and either 0.5 M GlcNAc or 10 mM triacetyl chitotriose for 1 h at room temperature in an attempt to inhibit binding of wheatgerm lectin to glycoproteins and to glycoproteins plus chitin, respectively. After incu-bation sections were washed extensively, stained with uranyl acetate and lead citrate and examined in a Jeol 1010 transmission electron microscope.
2.8. Chitosan test
The chitosan test for chitin was performed essentially as described by Peters (1992). Two samples of cultured PM were used for the test. The first was unextracted water-washed PM while the second sample was sub-jected to the complete extraction procedure described above for the preparation of the alkali-stable PM residue before the chitosan test was performed. Briefly the samples were then extracted with alkali (7 N KOH, 60°C, 16 h, 3 changes), acidified to pH 2.5 with sul-phuric acid and flooded with undiluted Lugol’s reagent (0.33% w/v iodine and 0.67% w/v potassium iodide in water) after removal of the acid solution. After the col-our change (,5 min), the Lugol’s reagent was replaced with 3% w/v acetic acid and further colour changes noted.
2.9. Localisation of chitin using Calcofluor White
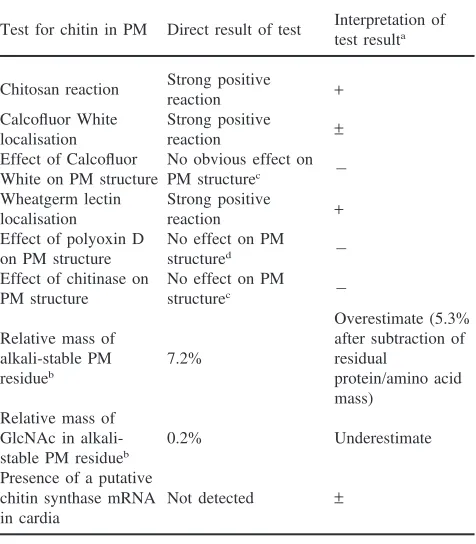
Larvae ofL. cuprinawere fed for 20 h on a diet that included 2.5 mM Calcofluor White (Sigma Chem. Co.). Larval growth at this time was approximately 40% of normal growth. Surviving larvae were dissected and the PM and digestive epithelia isolated and washed with 3 changes of PBS (15 min each). These tissues were then examined using a fluorescence microscope with a violet excitation filter and a 470 nm barrier filter. There was no intrinsic fluorescence (under these conditions) associated with the same tissues when larvae were grown in the absence of Calcofluor White.
2.10. Fluorescence spectrum of Calcofluor White
excitation peak for peritrophin-95. All spectra are uncor-rected.
2.11. Reverse transcriptase PCR
RT–PCR was used to determine whether cardia expresses mRNA coding for a putative chitin synthase (Vuocolo and Tellam, manuscript submitted). Oligo dT-primed first strand cDNA was prepared from RNA iso-lated from cardia, midgut, and carcass (i.e. whole larva minus gut tissues) of L. cuprina2nd instar larvae (Casu et al., 1996). The RNA was initially treated with DNase I to aid in the removal of any contaminating genomic DNA. Two oligonucleotide primers for use in PCR, were designed to amplify a DNA fragment of 1022 bp encompassing a region coding for the putative catalytic domain of this protein. The sense primer was 59 -GAT-CAATCTATGGCCATGAATAGG-39 and the
anti-sense primer was 59
-GACGAGCTTCATCGGATTGCG-39. PCR was perfor-med using 3.5 ng of first strand cDNA in a total reaction volume of 100 µl containing 10 mM Tris–HCl, pH 8.3, 50 mM KCl, 1.1 mM MgCl2, 0.1% gelatin, 250 µM of
each dNTP, 2.5 U RedTaq (Sigma Chem. Co.) and 100 pmol of each oligonucleotide primer. The DNA coding for the putative chitin synthase was amplified in an Omnigene Thermal Cycler (Hybaid, Middlesex, United Kingdom) using the following conditions: one cycle of 1 min at 94°C; 33 cycles of 1 min at 94°C, 1 min at 60°C, 2 min at 72°C; and one cycle of 1 min at 94°C, 1 min at 60°C, 10 min at 72°C. Two β-actin-specific primers were designed to amplify a 330 bp DNA frag-ment at the 59 end of theβ-actin coding region (Casu et al., 1996). The DNA coding for β-actin was amplified as previously described and used to validate the constant quantity of first strand cDNA used in the analysis (Casu et al., 1996). The amplified DNA fragments were separ-ated on a 1% agarose gel in 0.5×TAE (Sambrook et al., 1989). The putative chitin synthase genomic sequence encompassing the region amplified by PCR, contains 2 small introns (21 bp and 58 bp), which allows differen-tiation between DNA amplified from cDNA (1022 bp) and genomic DNA (1101 bp). The DNA fragments amplified from whole second instar larval cDNA and from hindgut cDNA were also cloned and sequenced to confirm that they encoded the putative chitin synthase.
3. Results and discussion
A range of histochemical, biochemical, molecular bio-logical and functional assays have been used to deter-mine whether chitin is present in the type 2 PM from the larvae of L. cuprina. The direct results of these assays and their interpretations are summarised in Table 1.
Table 1
Summary of the results of various tests for the presence of chitin in the type 2 peritrophic membrane fromL. cuprinalarvae
Interpretation of Test for chitin in PM Direct result of test
test resulta
Strong positive
Chitosan reaction +
reaction
Calcofluor White Strong positive ± localisation reaction
Effect of Calcofluor No obvious effect on 2 White on PM structure PM structurec
Wheatgerm lectin Strong positive + localisation reaction
Effect of polyoxin D No effect on PM 2 on PM structure structured
Effect of chitinase on No effect on PM 2 PM structure structurec
Overestimate (5.3% Relative mass of after subtraction of alkali-stable PM 7.2% residual
residueb protein/amino acid
mass) Relative mass of
GlcNAc in alkali- 0.2% Underestimate stable PM residueb
Presence of a putative
chitin synthase mRNA Not detected ± in cardia
a The interpretations of the direct results are discussed in the text. +, the test is unequivocally positive for the presence of chitin; 2, unequivocally negative;±, the interpretation of the test is not conclus-ive.
b Mass measured relative to the dry weight of water-washed PM obtained by larval culture.
c Visualised by low magnification fluorescence microscopy. d Visualised by electron microscopy.
3.1. Chitosan test
The chitosan reaction is probably the most widely used method to detect chitin in biological samples (Peters, 1992). The method involves treatment of bio-logical samples with alkali to extract masking proteins and conversion of the exposed chitin to chitosan by deacetylation. The chitosan is then reacted with potass-ium iodide under acidic conditions, producing a charac-teristic reddish-violet colour which fades with sub-sequent exposure to acidic solutions, as the reacted chitosan dissolves. Whole PM, obtained by larval cul-ture, resulted in a positive reaction with this reagent (result not shown). The colour produced was however, somewhat atypical, being a rust-like reddish brown rather than the more usual reddish violet. A similar col-our was reported for the type 1 PMs of the mosquitoes
chi-tin is present. The cultured PM was also progressively extracted with buffer, detergent buffer, urea buffer, guanidine buffer, SDS/DTT solution and extensive alkali treatment to aid in the removal of glycoproteins before the chitosan test was performed. The alkali-stable material remaining after these extensive extraction pro-cedures also showed a strong positive chitosan reaction indicating the presence of chitin.
It should be noted however that there are several reports in the literature commenting on the lack of speci-ficity of this test particularly in the presence of glyco-proteins, which are a significant component of the PM (Peters, 1992; Tellam et al., 1999). It is assumed that all glycoproteins are removed by the alkali conditions of the chitosan reaction. Surprisingly, amino acid analysis of the alkali-stable residue remaining after the extensive extraction procedure indicated that the residue still con-tained 28% protein probably in the form of amino acids derived from hydrolysed proteins. The presence of amino acids or proteins in the alkali-stable PM residue infers that proteins may be covalently cross-linked to other components of the PM, possibly chitin. This struc-ture could represent a unique proteo-GlcNAc glycan.
3.2. Calcofluor White localisation of chitin
The fluorescent brightening agent, Calcofluor White has been used to detect chitin in many biological samples (Maeda and Ishida, 1967; Huber et al., 1991; Peters, 1992) and has been shown to inhibit chitin synthesis in yeast (Cos et al., 1998). The fluorescence of Calcofluor White is enhanced when it is intercalated between sugar ring structures, particularly the linear polymers of GlcNAc that constitute chitin. Calcofluor White was fed to larvae using an in vitro feeding bioassay. Fig. 1 shows the effect of increasing concentrations of Calcofluor White on mean larval weight and survival. Calcofluor White caused strong concentration-dependent inhibition of larval survival and weight. The concentrations of Cal-cofluor White causing half-maximal effects on larval growth and survival were 2.8 mM and 3.5 mM, respect-ively. The major effects on larval survival occurred over a relatively narrow concentration range compared to the effects of Calcofluor White on larval weights. Dissection of larvae reared on a diet that included 2.5 mM Calco-fluor White and examination by low power microscopy revealed the presence of a PM apparently identical to that found in similar sized control larvae. Calcofluor White fluorescence was strongly associated with the PM [Fig. 2(a)] and also the cell membranes of the midgut digestive epithelial cells [Fig. 2(b)]. There was no Calco-fluor White Calco-fluorescence associated with internal tissues of the larvae or fluorescence remaining on the external surface of washed larvae (results not shown). This infor-mation suggests that Calcofluor White does not penetrate cells and is exerting its anti-larval activity via effects on
Fig. 1. Effect of Calcofluor White on the growth and survival ofL. cuprinalarvae. Increasing concentrations of Calcofluor White were fed to larvae using an in vitro feeding system. After 20 h the mean number of surviving larvae (out of 10) and their mean weights were measured. (a) Mean larval survival; (b) mean larval weight. Error bars denote 1 SD.
the functions of the midgut epithelia or PM. One possi-bility is that chitin synthesis or chitin fibril crystallisation required for normal PM production, was inhibited by Calcofluor White and this had reduced larval growth and survival. However, apparently normal PM was present in treated larvae suggesting that Calcofluor White did not cause gross abnormalities in the PM. More detailed studies of the ultrastructure and function of the PM are required to confirm this conclusion. The midgut epithelia posterior to the cardia does not produce PM or any cuticular layer in larvae of flies (Peters, 1992). This indi-cates that the binding of Calcofluor White to the midgut epithelia is not an indication that chitin is synthesised by this tissue.
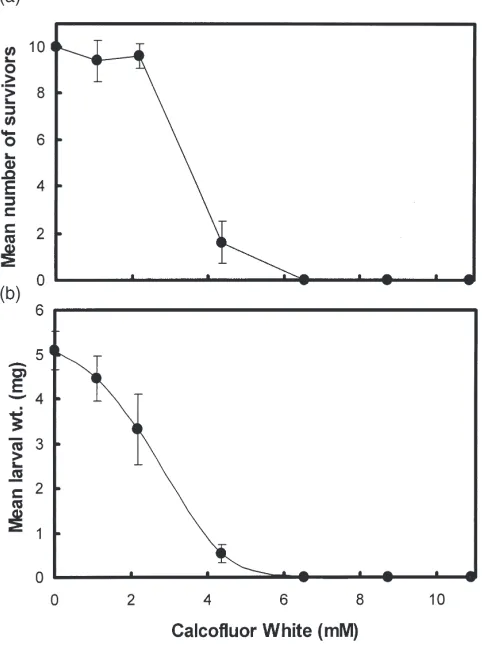
It is possible that Calcofluor White may be binding to oligosaccharides attached to glycoproteins in the PM and on the cell membranes of the digestive epithelial cells. Fig. 3 shows the fluorescence emission spectra of Calco-fluor White in the presence and absence of the purified
Fig. 2. Ingested Calcofluor White associated withL. cuprinalarval tissues. Larvae were allowed to feed on a diet containing 2.5 mM Calcofluor White after which they were dissected and tissues examined for Calcofluor White fluorescence. (a) PM; (b) midgut epithelia stripped of PM; (c) PM from a control larva not fed Calcofluor White. The bars represent 50µm.
Fig. 3. Fluorescence emission spectra of Calcofluor White in the presence and absence of peritrophin-95. The concentrations of Calco-fluor White (CW) and peritrophin-95 (PM95) were 1.8 µM and 10 µg/ml, respectively. The excitation wavelength was 295 nm.
indi-cating that this agent has broad polysaccharide-binding specificity (Maeda and Ishida, 1967). The specific identi-fication of chitin in PM using this fluorescence method is therefore inconclusive.
3.3. Wheatgerm lectin localisation
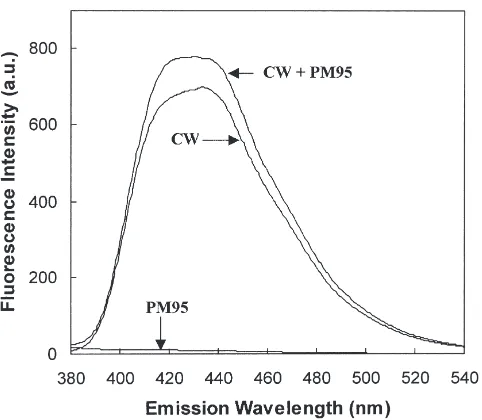
Wheatgerm lectin binds to GlcNAc-containing oligo-saccharides attached to glycoproteins but has much greater affinity for linear oligosaccharides of GlcNAc such as that present in chitin (Allen et al., 1973; Peters and Latka, 1986). The binding of fluorescently-labelled wheatgerm lectin or gold-labelled wheatgerm lectin has been used as an indicator of the presence of chitin in a tissue (Peters and Latka, 1986; Rudin and Hecker, 1989; Ryerse et al. 1992, 1994; Walters et al., 1993). However, simply examining wheatgerm lectin localisation in a tissue cannot differentiate between the lectin binding to chitin or glycoproteins. It has been suggested that wheat-germ lectin can be selectively displaced from oligo-saccharides attached to glycoproteins, but not chitin, using high concentrations of GlcNAc (0.2–0.5 M) and completely displaced from glycoproteins and chitin using triacetyl chitotriose (5–10 mM) (Peters and Latka, 1986; Rudin and Hecker, 1989). The basis of this selec-tivity is the much greater affinity of triacetyl chitotriose compared to GlcNAc for binding to wheatgerm lectin (Allen et al., 1973). The selective use of these sugars allows differential localisations of chitin and glyco-proteins in a tissue (Peters, 1992). Fig. 4(b) shows the indirect immuno-localisation of wheatgerm lectin, in the presence of 0.3 M GlcNAc, on PM fromL. cuprina lar-vae. There was substantial labelling of the PM even in the presence of this sugar. A pre-vaccination control serum showed very little labelling thereby demonstrating the specificity of the immuno-localisation [Fig. 4(a)]. Incubation of the lectin with 10 mM triacetyl chitotriose [Fig. 4(c)] reduced the density of labelling but did not completely inhibit binding of wheatgerm lectin to the PM. The mean densities of gold particles on the PM were 35.2±7.8 (n=4) particles/µm2 and 17.7±1.4 (n=3)
particles/µm2for the localisation of wheatgerm lectin in
the presence of GlcNAc and triacetyl chitotriose, respectively i.e. a 50% reduction of labelling in the pres-ence of triacetyl chitotriose. In comparison, the labelling density of the PM using pre-vaccination serum was 0.7±0.4 (n=5) particles/µm2. The presence of wheatgerm
lectin bound to PM even in the presence of GlcNAc and the reduced labelling of bound wheatgerm lectin in the presence of triacetyl chitotriose compared to GlcNAc indicate that chitin is present in the PM. The reason why triacetyl chitotriose was unable to completely displace wheatgerm lectin from the PM is not clear. All three panels of Fig. 4 show a meshwork of electron-dense fibrils that are thought to be typical of chitin fibrils (Peters, 1992). Another interpretation is that the
mesh-Fig. 4. Binding of wheatgerm lectin to L. cuprina PM. PM was obtained by dissection of second instar larvae and incubated with wheatgerm lectin in the presence of GlcNAc or triacetyl chitotriose. The bound wheatgerm lectin was then visualised using indirect immuno-gold labelling. (a) Control localisation of wheatgerm lectin alone using pre-vaccination serum; (b–c) localisation using serum raised to wheatgerm lectin; (b) incubation with wheatgerm lectin and 0.3 M GlcNAc; (c) incubation with wheatgerm lectin and 10 mM tria-cetyl chitotriose. The bar represents 500 nm.
work consists of fibrils composed of proteins alone or proteins attached to chitin fibrils.
A number of L. cuprina larval peritrophins bind wheatgerm lectin (East et al., 1993; Casu et al., 1997). It is possible that wheatgerm lectin is binding to oligo-saccharides on some of these peritrophins with suf-ficiently strong affinity that the lectin resists displace-ment by high concentrations of GlcNAc or triacetyl chitotriose.
Overall, the binding of wheatgerm lectin to PM is con-sistent with the presence of chitin. However the binding of this lectin to PM glycoproteins under the same con-ditions that define the binding of the lectin to chitin, can-not be ruled out.
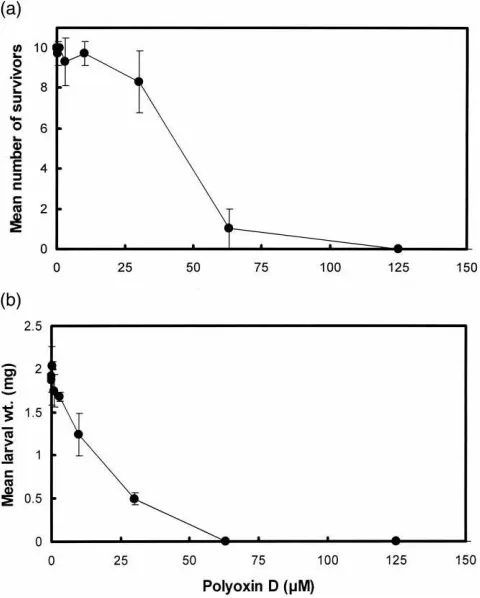
3.4. Effect of polyoxin D on larvae and PM
structural morphology of any PM that does form. Polyoxin D is a potent competitive inhibitor of fungal chitin synthases (Ohta et al., 1970; Hori et al., 1971) and also inhibits insect chitin synthase activity (Sowa and Marks, 1975). The inhibitor was fed to larvae using an in vitro feeding system. Fig. 5 shows the effects of increasing concentrations of polyoxin D on mean larval survival and mean larval weight. Polyoxin D had strong inhibitory effects on the larvae with half-maximal effects at concentrations of 16µM for larval weight and 44µM for larval survival. The shapes of these two curves are different, with strong effects on larval weight and little effect on larval survival at polyoxin D concentrations less than 20µM. The inhibitory effect of polyoxin D on larval growth is consistent with the proposal that chitin is required for the growth and development ofL. cuprina
larvae and that these larvae contain a chitin synthase sensitive to polyoxin D.
Surviving larvae fed 50 µM polyoxin D, were dis-sected and examined by microscopy for the presence of PM. All larvae contained PMs. Electron microscopy was then used to examine the structural integrity of these PMs (Fig. 6). PMs were also examined from control lar-vae grown to the same weight. Even though this
concen-Fig. 5. Effect of polyoxin D on the growth and survival ofL. cuprina
larvae. Increasing concentrations of polyoxin D were fed to larvae using anin vitrofeeding system. After 20 h the mean number of sur-viving larvae (out of 10) and their mean weights were measured. (a) Mean larval survival; (b) mean larval weight. The error bars denote 1 SD.
Fig. 6. Effect of polyoxin D on PM. Larvae were fed 50µM polyoxin D for 20 h after which the PM was examined using electron microscopy. (a) PM from larvae fed polyoxin D; (b) PM from larvae grown to the same size and developmental state but in the absence of polyoxin D. The bar represents 500 nm.
tration of polyoxin D had a strong effect on larval weight (|75% reduction in weight), the PM was normally developed for this sized larva and there were no obvious indications of any structural abnormalities in the PM when examined by electron microscopy. It can be con-cluded from these results that either chitin is not required for PM formation or that chitin is not an important con-tributor to the structural integrity of the PM. Another possibility is that there is a polyoxin D-sensitive chitin synthase responsible for chitin production in the cuticle and a polyoxin D-insensitive chitin synthase in the cardia that is responsible for chitin production in the PM. In this instance the inhibitory effects of polyoxin D on lar-val growth would be a consequence of inhibition of the former enzyme.
Polyoxin D markedly reduced the dry weight of type 2 PM fromCalliphora erythrocephalalarvae by as much as 60% and at concentrations in excess of 40 µM increased the permeability of the PM (Becker, 1980; Zimmermann and Peters, 1987). Furthermore, Dimilin and Captan, which are inhibitors of the synthesis of chi-tin, but not necessarily the enzyme chitin synthase, have also been shown to inhibit PM production, but only par-tially (Becker, 1978; Clarke et al., 1977). These pre-viously reported results suggest that chitin is present in larval type 2 PM from a closely related higher dipteran species. Another interpretation of many of these results is that these chemicals inhibit the growth of larvae, which then indirectly decreases the rate of PM pro-duction from the cardia.
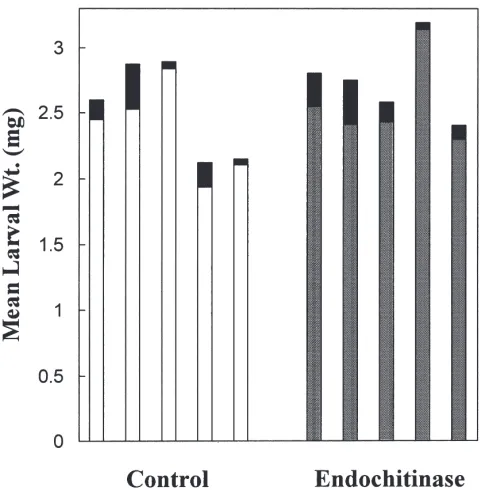
3.5. Effect of chitinase on larvae and PM
Fig. 7. Effect ofS. marcescens endochitinase on the survival and growth ofL. cuprina larvae. The endochitinase (1 U/ml) was fed to larvae using anin vitrofeeding system. After 20 h larval weights were measured. Each assay contained 10 larvae and was repeated 5 times. The growth assay was performed with 5 independent formulations of the culture medium and the mean larval weight from each of these formulations is reported. Control group, open rectangles; group fed endochitinase, shaded rectangles. The fully shaded boxes represent one standard deviation (n=5).
unaffected by the chitinase (Fig. 7). It may be argued that the ingested chitinase was inactivated by endogen-ous gut digestive proteases. However, direct incubation of the chitinase (1 U/ml) with isolated PM showed no apparent changes to its structure when visualised with the aid of Calcofluor White and low magnification flu-orescence microscopy (Fig. 8). These results suggest that chitin is not present in PM. The use of a relatively high
Fig. 8. Effect ofS. marcescansendochitinase on PM obtained by dissection fromL. cuprinathird instar larvae. (a) Isolated PM incubated with 1 U/ml of endochitinase for 4 h; (b) PM incubated with buffer (control). In both instances the PM was visualised with the aid of Calcofluor White fluorescence. The bar represents 50µm.
concentration of chitinase and extended incubation per-iod should have facilitated any enzymatic degradation of chitin present in the PM. More detailed structural and functional analyses of the PM incubated with chitinase are required to eliminate the possibility of subtle changes to the PM. An alternative explanation is that the chitin fibrils in the PM, particularly theirβ-1-4 glycosidic link-ages, are protected from the action of the endochitinase by the presence of chitin-binding peritrophins or by chi-tin fibril crystallisation. In contrast to the results shown in Figs. 7 and 8, the same endochitinase used at a con-centration of 0.1µg/ml, caused easily discernible perfor-ations in the type 1 PM from Spodoptera littoralis
(Regev et al., 1996) and fully digested the type 1 PM from the mosquito Aedes aegypti (Huber et al., 1991).
3.6. Analysis of the alkali-stable insoluble component of PM
residual alkali-insoluble material was 7.2% of the dry weight of the PM. It is assumed that all of this material is chitosan (i.e. deacetylated chitin). However, non-chi-tinous material may be also present in this residue (Hackman, 1974) suggesting that this value may be an overestimate of the chitin content. Indeed, amino acid analysis of this residue revealed that 28% of its weight was still protein or amino acids suggesting that the maximum chitin content of the PM is 5.3%. Thus, this empirical measure strongly indicates that chitin is at most, only a minor component of the PM. This figure is in good agreement with a value of 7.5% (w/w) calculated for the chitin content of type 2 PM from adults of the closely related fly C. erythrocephala(Becker, 1980).
The monosaccharide composition of the alkali-stable PM residue was determined by chemical analysis. Strong acid conditions (4 N HCl, 100°C, 4 h) were used to hydrolyse glycosidic bonds. Under these conditions chi-tin is hydrolysed releasing glucosamine, a GlcNAc breakdown product. The glucosamine content of the alk-ali-stable PM residue was only 0.2% of the original PM mass and 2.8% of the mass of the alkali-stable PM resi-due. What is difficult to quantitate in this analysis how-ever, is the efficiency of the acid hydrolysis. It has been reported that the quantities of glucosamine released from chitin after acid hydrolysis are variable and strongly depend on the nature of residual components in the sam-ple (Hackman, 1974; Muzzarelli, 1977; Hackman and Goldberg, 1981). As already mentioned, the alkali-stable residue still contains 28% protein or amino acids. Thus, the figure of 2.8% for the GlcNAc content of the alkali-stable residue is probably a substantial underestimate. Attempts to directly quantitate the GlcNAc content of unextracted PM were thwarted by charring of the sample under the prolonged high temperature and acidic con-ditions used for the hydrolysis. Incubation of the alkali-stable residue with S. marcescenschitinase (1 U/ml) for 20 h at 30°C had no significant effect on the dry weight of the residue nor was there release of glucosamine, GlcNAc or chitobiose as judged by HPLC analysis (results not shown). Similarly trypsin treatment had no effect on the dry weight of the alkali-stable residue. This latter result is expected since the hot alkali treatment used to produce the residue probably hydrolysed any proteins into amino acids.
3.7. Presence of chitin synthase in cardia
Perhaps one of the best indirect indicators of chitin in PMs is the presence of the enzyme chitin synthase in cardia, the insect organ producing type 2 PM. Until now no insect chitin synthase has been characterised at the molecular level. Recently, we have cloned and sequenced a cDNA coding for a putative chitin synthase fromL. cuprinalarvae (Vuocolo and Tellam; manuscript submitted). This sequence, which codes for a protein
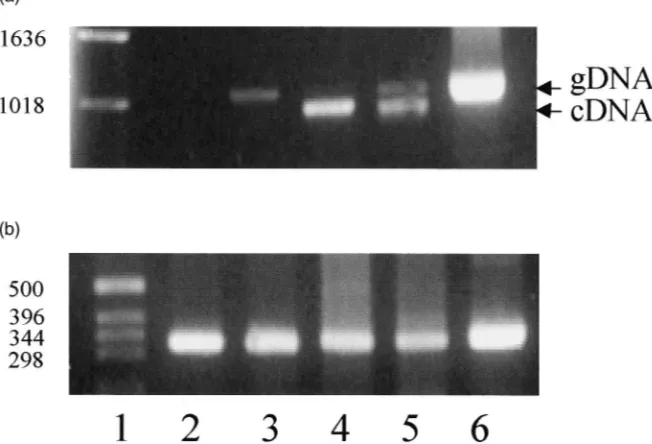
containing 1592 amino acids, has highly significant homology to a number of yeast and fungal chitin synth-ases. The mRNA coding for this protein is expressed in all life stages. RT–PCR has been used to determine whether mRNA coding for this putative chitin synthase is expressed in cardia and various larval gut tissues from second instar larvae. Fig. 9(a) shows that the expected DNA fragment (1022 bp) was amplified from samples from hindgut and carcass (i.e. whole larvae minus all gut tissues) but not cardia or midgut. Correspondingβ-actin controls [Fig. 9(b)] demonstrate the presence of approxi-mately equal quantities of first strand cDNA in each of these reactions. The midgut sample did show a weak 1100 bp band but this was consistent with the size of the corresponding fragment amplified from genomic DNA (1101 bp) and probably represents contaminating gen-omic DNA in this particular sample. The sample from larval carcass [Fig. 9(a), lane 5] shows a major band consistent with the expected size of the DNA amplified from cDNA as well as a minor band consistent with amplification of genomic DNA. Presumably, the latter band also arose from contamination by genomic DNA despite treatment of the total RNA with DNase I. mRNA coding for the putative larval chitin synthase was not detected in cardia, the primary site of synthesis of PM. This result is consistent with the view that the PM does not contain chitin. An alternative explanation is that a different chitin synthase is expressed in cardia, which is not detected with the specific primers used in PCR.
The chitin contents of PMs have also been examined using X-ray crystallography (Rudall and Kenchington, 1973) and more recently solids NMR (Kramer et al., 1995). These techniques indicated the presence of GlcNAc in the PMs from several larvae of the Coleop-tera, silkworm fly, sawfly larvae and M. sexta. The impact of potentially relatively large quantities of glyco-proteins containing GlcNAc oligosaccharides on these techniques is not clear.
3.8. Conclusions
Fig. 9. Detection of chitin synthase mRNA in larval gut tissues. (a) The expression of a putative chitin synthase mRNA in tissues from second instar larvae was measured by RT–PCR. Lane 1, standards; lane 2, first strand cDNA from cardia; lane 3, midgut; lane 4, hindgut; lane 5, carcass (whole second instar larvae minus gut tissues); lane 6, genomic DNA. Each reaction contained the same quantity of first strand cDNA (3.5 ng). (b) Correspondingβ-actin controls for each of the cDNA samples shown in (a).
there is the possibility that its functional role is dispro-portionably important. The lack of any obvious effects on larvae fed chitinase discounts this latter possibility.
The strong anti-larval effects of polyoxin D and Cal-cofluor White could be due to two alternative mech-anisms. First, if the PM contains no chitin, then these agents may be interfering with chitin production else-where within the larvae. This could lead to inhibition of larval growth with corresponding but indirect reduction in the rate of normal PM formation. The current study found no evidence that Calcofluor White fluorescence was present in internal larval tissues or within the cyto-plasm of gut digestive cells. This indicates that Calco-fluor White is exerting its anti-larval effects either through its direct binding to chitin or glycoproteins in the PM or via binding to the extracellular surface glyco-proteins of the midgut epithelia. Second, if the PM does contain small quantities of chitin, then polyoxin D and Calcofluor White could be limiting the amount of chitin available for PM formation such that the primary response of the larvae was to produce only small quan-tities of normal PM. In this model, larval growth would be tightly coupled to normal PM formation, which explains the larval growth inhibition but apparently nor-mal PM in the snor-maller larvae. Very recently it was dem-onstrated that Calcofluor White inhibited the growth of
Trichoplusia nilarvae. That study concluded that Calco-fluor White exerted its effect via dissociation of peritro-phins from the chitin matrix of the PM (Wang and Gran-ados, 2000). While re-emphasising the importance of the peritrophins in the function of the PM, the study also
demonstrated that Calcofluor White grossly altered the PM structure. In contrast, we have seen no evidence for gross abnormalities of the PM from L. cuprina larvae fed Calcofluor White. It is interesting to note that three unrelated agents, which bind to PM (Calcofluor White, wheatgerm lectin (Eisemann et al., 1994) and specific antibodies to PM proteins (Casu et al., 1997)) all have detrimental effects on larval growth. This information strongly emphasises the important contribution of the PM to normal growth and development of the larvae and hints at novel insect control strategies.
Unlike the type 2 PM there are strong functional indi-cators that chitin is a major component of type 1 PM. First, polyoxin D fed to mosquitoes completely inhibited PM production (Shahabuddin et al., 1995). Second, exogenous chitinase fed to Phlebotomus papatasi or mosquitoes inhibited PM production although it was not toxic to these insects (Huber et al., 1991; Shahabuddin et al., 1995; Pimenta et al., 1997). An endochitinase also caused perforations in the PM fromS. littoratis (Regev et al., 1996). Third, the insect invasion strategy of a number of parasites involves a parasite-derived chitinase that facilitates movement of the parasite across the physical barrier of the PM (Huber et al., 1991; Schlein, 1993; Shahabuddin and Kaslow, 1993). Fourth, many plants produce chitinases in response to stress parti-cularly fungal invasion and insect feeding (Flach et al., 1992). This is thought to be a defensive adaptation of plants. Indeed, a chitinase fragment fromManduca sexta
measured in an in vitro feeding bioassay (Wang et al., 1996). The most likely target for the ingested chitinase was the gut PM. However, this postulated effect on PM structure and/or function has not yet been demonstrated. Despite this strong evidence for the presence of chitin there are also some reports that cast doubt on the pres-ence of chitin in type 1 PMs. Chemical analysis of the type 1 PM from An. stephensi (Liston) did not reveal any GlcNAc. Further, it was shown that this PM was soluble in distilled water (Berner et al., 1983). Both of these properties are inconsistent with the presence of chi-tin, a highly stable linear polymer of GlcNAc. Further analysis of insect type 1 PMs using a variety of tech-niques may clarify this contradictory evidence.
The results presented herein suggest that the chitin contents of insect PMs may need re-evaluation. More-over, one strategy used for the development of trans-genic plants that are resistant to insect pests involves the expression of a chitinase. The implied rationale for this approach is that the chitinase ingested by the feeding insect destroys the integrity of the PM. This strategy may need reassessment if chitin is found to be absent or present in very small amounts in the PMs of these insect pests.
Acknowledgements
We thank Lee Cadogan, Janine Jarmey and Allan Donaldson for expert technical assistance. This research was made possible by financial support from the L.W. Bett Trust and Australian wool growers through the International Wool Secretariat. The research was also facilitated by access to the Australian Proteome Analysis Facility established under the Australian Government’s Major National Research Facilities Program. We also thank one of the reviewers for the suggestion that the PM may contain a unique proteo-GlcNAc glycan.
References
Adang, M.J., Spence, K.D., 1982. Biochemical comparisons of the per-itrophic membrane of the lepidopteransOrgyia pseudotsugataand
Manduca sexta. Comp. Biochem. Physiol. 73B, 645–649. Allen, K., Neuberger, A., Sharon, N., 1973. The purification,
compo-sition and specificity of wheatgerm agglutinin. Biochem. J. 131, 155–162.
Becker, B., 1978. Determination of the formation rate of peritrophic membranes in some Diptera. J. Insect Physiol. 24, 529–533. Becker, B., 1980. Effects of polyoxin D on in vitro synthesis of
per-itrophic membranes inCalliphora erythrocephala. Insect Biochem. 10, 101–106.
Berner, R., Rudin, W., Hecker, H., 1983. Peritrophic membranes and protease activity in the midgut of the malaria mosquito,Anopheles stephensi (Liston) (Insecta: Diptera) under normal and experi-mental conditions. J. Ultrastruct. Res. 83, 195–204.
Brurberg, M.B., Nes, I.F., Eijsink, G.H., 1996. Comparative studies of
chitinase a and b from Serratia marcescens. Microbiology 142, 1581–1589.
Casu, R., Eisemann, C., Vuocolo, T., Tellam, R.L., 1996. The major excretory/secretory protease fromLucilia cuprinalarvae is also a gut digestive protease. Int. J. Parasitol. 26, 623–628.
Casu, R., Eisemann, C., Pearson, R., Riding, G., East, I., Donaldson, A., Cadogan, L., Tellam, R., 1997. Antibody-mediated inhibition of the growth of larvae from an insect causing cutaneous myiasis in a mammalian host. Proc. Natl. Acad. Sci. 94, 8939–8944. Clarke, L., Temple, G.H.R., Vincent, J.F.V., 1977. The effects of a
chitin inhibitor — dimilin — on the production of peritrophic mem-brane in the locust, Locusta migratoria. J. Insect Physiol. 23, 241–246.
Cos, T., Ford, R.A., Trilla, J.A., Duran, A., Cabib, E., Roncero, C., 1998. Molecular analysis of CH3P participation in chitin synthase III activity. Eur. J. Biochem. 256, 419–426.
East, I., Fitzgerald, C.J., Pearson, R.D., Donaldson, R.A., Vuocolo, T., Cadogan, L.C., Eisemann, C.H., Tellam, R.L., 1993.Lucilia cup-rina: inhibition of larval growth induced by immunisation of host sheep with extracts of larval peritrophic membrane. Int. J. Parasitol. 23, 221–229.
Eisemann, C., Johnston, L., Broadmeadow, M., O’Sullivan, B.M., Donaldson, R.A., Pearson, R.D., Vuocolo, T., Kerr, J.D., 1990. Acquired resistance of sheep to larvae ofLucilia cuprina, assessed in vivo and in vitro. Int. J. Parasitol. 20, 299–305.
Eisemann, C.H., Donaldson, R.A., Pearson, R.D., Cadogan, L.C., Vuo-colo, T., Tellam, R.L., 1994. Larvicidal activity of lectins on Luci-lia cuprina: mechanism of action. Entomol. Exp. Appl. 72, 1–10. Elvin, C.M., Vuocolo, T., Pearson, R.D., Riding, G.A., East, I., Eisem-ann, C.H., Tellam, R.L., 1996. Characterisation of a major per-itrophic membrane protein, peritrophin-44, from the larvae of Luci-lia cuprina: cDNA and deduced amino acid sequences. J. Biol. Chem. 271, 8925–8935.
Flach, J., Pilet, P.-E., Jolles, P., 1992. What’s new in chitinase research? Experienta 48, 701–716.
Freyvogel, T.A., Staubli, W., 1965. The formation of the peritrophic membrane inCulicidae. Acta Tropica 22, 118–147.
Hackman, R.H., 1974. Chemistry of the insect cuticle. In: Rockstein, M. (Ed.), The Physiology of Insecta. Academic Press, New York/London, pp. 215–270.
Hackman, R.H., Goldberg, M., 1981. A method for determinations of microgram amounts of chitin in arthropod cuticles. Anal. Biochem. 110, 277–280.
Hori, M., Kakiki, K., Misato, T., 1971. Studies on the mode of action of polyoxins: III. Relation of polyoxin structure to chitin synthase inhibition. Agric. Biol. Chem. 35, 1280–1291.
Huber, M., Cabib, E., Miller, L.H., 1991. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc. Natl Acad. Sci. USA 88, 2807–2810.
Kramer, K.J., Hopkins, T.L., Schaefer, J., 1995. Applications of solids NMR to the analysis of insect sclerotised structures. Insect Biochem. Molec. Biol. 25, 1067–1080.
Lehane, M.J., 1997. Peritrophic membrane structure and function. Annu. Rev. Entomol. 42, 525–550.
Maeda, H., Ishida, N., 1967. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J. Biochem. 62, 276–277.
Moskalyk, L.A., Oo, M.M., Jacobs-Lorena, M., 1996. Peritrophic matrix proteins of Anopheles gambiaeand Aedes aegypti. Insect Mol. Biol. 5, 261–268.
Muzzarelli, R.A., 1977. In: Muzzarelli, R.A. (Ed.), Chitin. Pergamon Press, Oxford, pp. 1–154.
Ohta, N., Kakiki, K., Misato, T., 1970. Mode of action of polyoxin D: II. Effect of polyoxin D on the synthesis of fungal cell wall. Agric. Biol. Chem. 34, 1224–1234.
chro-matography of amino acids derivitised with 9-fluorenylmethyl chloroformate. J. Chrom. 723, 219–225.
Peters, W., 1992. Peritrophic membranes. In: Bradshaw, D., Burggren, W., Heller, H.C., Ishii, S., Langer, H., Neuweiler, G., Randall, D.J. (Eds.), Zoophysiology, vol. 130. Springer-Verlag, Berlin, pp. 1– 238.
Peters, W., Latka, I., 1986. Electron microscopic localisation of chitin using colloidal gold labelled with wheat germ agglutinin. Histoch-emistry 84, 155–160.
Pimenta, P.F.P., Modi, G.B., Pereira, S.T., Shahabuddin, M., Sacks, D.L., 1997. Novel role for the peritrophic matrix in protecting
Leishmania from the hydrolytic activities of the sand fly midgut. Parasitology 115, 359–369.
Regev, A., Keller, M., Strizhov, N., Sneh, B., Prudovsky, E., Chet, I., Ginzberg, I., Koncz-Kalman, Z., Koncz, C., Schell, J., Zilberstein, A., 1996. Synergistic activity of a Bacillus thuringiensis delta-endotoxin and a bacterial endochitinase against Spodoptera lit-toralislarvae. Appl. Environ. Micro. 62, 3581–3586.
Rudall, K.M., Kenchington, W., 1973. The chitin system. Biol. Rev. 49, 597–636.
Rudin, W., Hecker, H., 1989. Lectin-binding sites in the midgut of the mosquitoesAnopheles stephensi(Liston) andAedes aegyptiL. (Diptera: Cullicidae). Parasitol. Res. 75, 268–279.
Ryerse, J.S., Purcell, J.P., Sammons, R.D., Lavrik, P.B., 1992. Per-itrophic membrane structure and formation in the larvae of a moth,
Heliothis. Tissue Cell 24, 751–771.
Ryerse, J.S., Purcell, J.P., Sammons, R.D., 1994. Structure and forma-tion of the peritrophic membrane in the larva of the southern corn rootworm,Diabrotica undecimpunctata. Tissue Cell 26, 431–437. Sambrook, T., Fritsch, E.F., Maniatis, T., 1989. In: Sambrook, T., Fritsch, E.F., Maniatis, T. (Eds.), Molecular Cloning: a Laboratory Manual, second ed. Cold Spring Harbour Laboratory Press, New York.
Schlein, Y., 1993.Leishmaniaand sandflies: interactions in the lifecy-cle and transmission. Parasitol. Today 9, 255–258.
Schorderet, S., Pearson, R.D., Vuocolo, T., Eisemann, C., Riding, G.A., Tellam, R.L., 1998. cDNA and deduced amino acid sequences of a peritrophic membrane glycoprotein, “peritrophin-48”, from the larvae ofLucilia cuprina. Insect Biochem. Molec. Biol. 28, 99–111.
Shahabuddin, M., Kaslow, D.C., 1993. Chitinase: a novel target for blocking parasite transmission. Parasitol. Today 9, 252–255. Shahabuddin, M., Kaidoh, T., Aikawa, M., Kaslow, D.C., 1995.
Plas-modium gallinaceum— mosquito peritrophic matrix and the para-site–vector compatibility. Exp. Parasitol. 81, 386–393.
Shen, Z., Jacobs-Lorena, M., 1998. A type 1 peritrophic matrix protein from the malaria vectorAnopheles gambiaebinds chitin — cloning, expression and characterisation. J. Biol. Chem. 273, 17665–17670. Sowa, B.A., Marks, E.P., 1975. An in vitro system for the quantitative measurement of chitin synthesis in the cockroach: inhibition by TH 6040 and polyoxin D. Insect Biochem. 5, 855–859.
Tellam, R.L., 1996a. The peritrophic membrane. In: Lehane, M.J., Billingsley, P.F. (Eds.), The Biology of the Insect Midgut. Chap-man and Hall, London, pp. 86–114.
Tellam, R.L., 1996b. Protein motifs in filarial chitinases: an alternative view. Parasitol. Today 12, 291–292.
Tellam, R.L., Wijffels, G., Willadsen, P., 1999. Peritrophic membrane proteins. Insect Biochem. Mol. Biol. 29, 87–101.
Walters, L.L., Irons, K.P., Guzman, H., Tesh, R.B., 1993. Formation and composition of the peritrophic membrane in the sand fly, Phle-botomus perniciosus(Diptera: Psychodidae). J. Med. Entomol. 30, 179–198.
Wang, P., Granados, R.R., 1997. Molecular cloning and sequencing of a novel invertebrate intestinal mucin. J. Biol. Chem. 272, 16663–16669.
Wang, P., Granados, R.R., 2000. Calcofluor disrupts the midgut defense system in insects. Insect Biochem. Molec. Biol. 30, 135– 143.
Wang, X., Ding, X., Gopalakrishnan, B., Morgan, T.D., Johnson, L., White, F.F., Muthukrishnan, S., Kramer, K.J., 1996. Characteris-ation of a 46 kDa insect chitinase from transgenic tobacco. Insect Biochem. Molec. Biol. 26, 1055–1064.
Zimmermann, U., Peters, W., 1987. Fine structure and permeability of peritrophic membranes of Callipora erythrocephala
(Meigen)(Insecta: Diptera) after inhibition of chitin and protein synthesis. Comp. Biochem. Physiol. 86B, 353–360.
Zimmermann, U., Mehlan, D., Peters, W., 1975. Investigations on the transport function and structure of peritrophic membranes. V. Amino acid analysis and electron microscopic investigations of the peritrophic membranes of the blowflyCalliphora erythrocephala