www.elsevier.comrlocateranireprosci
A glimpse at sperm function in vivo: sperm
transport and epithelial interaction in the female
reproductive tract
Mary A. Scott
)Department of Population Health and Reproduction, School of Veterinary Medicine, UniÕersity of California
at DaÕis, One Shields AÕe., Reproduction, DaÕis, CA 95616, USA
Abstract
The process of sperm transport in the female reproductive tract is more than simply a migration of spermatozoa from the site of insemination to the site of fertilization. Rather, it is a complex and dynamic continuum that encompasses phases of sperm distribution within the tract, the accumula-tion of spermatozoa in reservoirs, the modulaaccumula-tion of sperm physiology and acquisiaccumula-tion of fertilization competence, the ascent of competent spermatozoa to the site of fertilization, and the elimination of the non-fertilizing sperm population. The dynamic interactions that occur between functional spermatozoa and the luminal fluids and epithelial surfaces of the female genital tract during transit and storage enhance sperm survival and regulate sperm function in the female. The universal nature of this interaction highlights it as a key component of the sperm transport process.
q2000 Elsevier Science B.V. All rights reserved.
Keywords: Sperm; Sperm transport; Sperm storage; Uterotubal junction; Oviduct
1. Introduction
The arrival of fully mature and functionally competent spermatozoa at the site of fertilization is not a random event, but is the culmination of a concert of interactions between sperm cells and the female reproductive tract that optimizes the likelihood of conception. These spermatozoa, which comprise only a small fraction of the inseminate,
)Corresponding author. Tel.:q1-530-752-1358; fax:q1-530-752-4278.
Ž .
E-mail address: [email protected] M.A. Scott .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
have completed a long and arduous journey, during which they have surmounted the formidable anatomical barriers within the female tract and have undergone the physio-logical changes that are required to initiate and complete fertilization. The journey begins at insemination and proceeds with a variable timetable that appears to be closely regulated by the female. Sperm distribution and function in the female are influenced by the site of semen deposition, seminal characteristics, the anatomy of the female genital tract, and the microenvironment of the lumen. The duration of sperm transport depends on the interval between insemination and ovulation and the functional lifespan of spermatozoa in the female tract. Accordingly, inter-species variation in sperm transport biology likely reflects the diversity of social behaviors and mating strategies that have evolved in the animal kingdom.
This review provides a general overview of the natural history of mammalian spermatozoa following insemination, with special emphasis on sperm–epithelium inter-action as an integral feature of sperm function in vivo. For a more detailed examination of sperm transport, the reader should consult one or more of the many comprehensive
Ž
reviews that are available in the literature e.g., Hunter, 1975; Overstreet, 1983; Hunter, 1988; Overstreet and Katz, 1990; Drobnis and Overstreet, 1992; Harper, 1994,
Yanagi-. machi, 1994 .
2. Copulatory behavior and insemination
In most domestic animal species, sperm arrival in the female genital tract is temporally associated with the timing of ovulation, either because ovulation is induced
Ž .
by coitus e.g., cat, rabbit, and llama , because ovulation occurs during estrus when the
Ž .
female is receptive to coitus, e.g., dog, horse, sheep, pig, mouse, rat and hamster , or
Ž .
because ovulation occurs shortly after estrus e.g., cattle . Amongst mammals, the most profound example of a prolonged interval between coitus and ovulation is the
hibernat-Ž .
ing bat, in which this interval may exceed 6–7 months Racey et al., 1987 .
The site of semen deposition defines the anatomical barriers that spermatozoa will encounter during transit to the oviducts. These barriers restrict sperm passage, establish a gradient in sperm numbers along the tract, and may function as sperm reservoirs ŽOverstreet and Cooper, 1978b; Hunter, 1988 . The establishment of a sperm gradient.
Ž .
appears to reduce the risk of polyspermy see Hunter, 1988 . For species with uterine
Ž .
deposition of semen e.g., dog, horse, pig, llama, laboratory rodents , the uterotubal Ž .
junction UTJ is the primary physical barrier to the oviducts, and a sharp gradient in
Ž .
sperm numbers occurs cranial to this barrier Hunter, 1988 . For species with vaginal
Ž .
insemination e.g., ruminants, primates, rabbit, hare , the initial barrier is the cervix, and the UTJ serves to further restrict sperm access to the oviducts. In ruminants and primates, the cervical canal is filled with mucus, and the biophysical characteristics of this secretion are affected by the endocrine status of the female. Cervical mucus may
Ž .
block sperm passage luteal phase, progesterone dominance , or, under estrogen
domi-Ž .
nance such as in the periovulatory period , the more hydrated mucus matrix forms
Ž .
Semen composition differs amongst mammalian species with respect to volume and sperm concentration, and these differences are related to the site of insemination ŽHunter, 1988 . Biochemical constituents of seminal plasma, such as prostaglandins can. stimulate smooth muscle activity of the female reproductive tract and thereby assist the
Ž
distribution of semen or spermatozoa within the tract Hunter, 1975; Drobnis and .
Overstreet, 1992; Harper, 1994 . The mechanical stimulus of mating may also enhance
Ž .
visceral contractions and sperm distribution Overstreet and Katz, 1990 . The direct effects of seminal plasma on the female tract may be localized. For ruminants and
Ž .
primates, cervical mucus forms a complete barrier to seminal plasma Katz et al., 1989 . The passage of seminal plasma into the oviducts is blocked by the UTJ in the rat ŽCarballada and Esponda, 1997 but this is apparently not the case in the horse Mann et. Ž
.
al., 1956 . In the pig, biochemical evidence of seminal plasma was not detected in the
Ž .
oviducts following mating Mann et al., 1956 ; however, radiolabelled tracers of different molecular weights will enter the oviducts when combined with sperm-free
Ž .
seminal plasma and artificially inseminated Einarsson et al., 1980 , and this transport
Ž .
across the UTJ is rapid Viring et al., 1980 .
3. Sperm distribution
Sperm distribution within the female tract has classically been described as occurring in phases, which are defined by their relationship in time to the event of insemination and the relative contribution of passive movement caused by visceral contractions of the
Ž .
female genital tract vs. active sperm migration Overstreet and Cooper, 1978a,b . The rapid transport phase is a pericoital event, characterized by the presence of sperm in the
Ž
oviducts within minutes of mating or artificial insemination Overstreet and Cooper, .
1978a . This rate of transport is much faster than sperm swimming speeds; conse-quently, it is attributed to muscular contractility of the female tract and attendant
Ž .
changes in intraluminal pressures see Drobnis and Overstreet, 1992; Harper, 1994 . Rapidly transported spermatozoa do not contribute to the fertilizing population in the
Ž .
oviduct, because they are moribund, dead or disrupted Overstreet and Cooper, 1978a . The rapid transport phase is followed by a prolonged phase of sperm migration ŽOverstreet and Cooper, 1978b , during which the distribution of spermatozoa within the. female tract continues and sperm reservoirs are established. It is during this phase that competent spermatozoa will arrive in the oviducts. This journey takes 1–2 h in the pig ŽHunter, 1988 , 1.5–6 h in rabbits Overstreet and Cooper, 1978b and 6–8 h in sheep. Ž .
Ž .
and cattle Hunter, 1988 . The recovery of highly motile sperm from the mare’s oviduct
Ž .
at 4 h after insemination Scott et al., 1995 and the knowledge that uterine lavage prior
Ž .
to this interval adversely affects conception rates Brinsko et al., 1990, 1991 support the hypothesis that this journey requires 4 h in the horse.
3.1. Sperm motility and sperm transport
contribution of sperm motility to the distribution of spermatozoa along the female tract is most evident during the sustained phase of sperm transport. Within the female tract, sperm motility is modulated by the dynamic forces imposed on the flagellum by spatial constraints, epithelial surface characteristics, and the rheological characteristics of fluid
Ž .
secretions Katz et al., 1989 . Motility is needed for spermatozoa to colonize and cross
Ž . Ž .
the cervix Cooper et al., 1979 and cervical mucus Overstreet and Katz, 1990 , and may also be requisite to traversing the UTJ. Using an elegant preparation for in vitro
Ž .
observation, Gaddum-Rosse 1981 found that motile rat spermatozoa emerged from the cut oviductal end of the UTJ of excised tracts, whereas non-motile spermatozoa andror uterine fluid did not, despite the presence of smooth muscle contractions. Spermatozoa
Ž .
appear to ascend the tract by moving along epithelial surfaces Katz et al., 1989 . This migration has been visualized directly through the transparent wall of excised oviducts
Ž . Ž .
in the hamster Katz and Yanagimachi, 1980 and mouse Suarez, 1987 , and is suggested by the appearance of the flagellar curvature and epithelial orientation of
Ž .
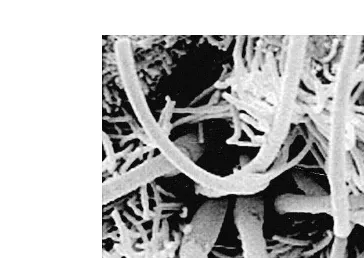
equine spermatozoa fixed in situ at the UTJ Fig. 1; Scott et al., 2000 . Nilsson and Ž .
Reinius 1969 noted that mouse spermatozoa at the ostium of the colliculus tubarius ‘‘almost climb on the surrounding microvilli of the tightly apposed luminal surfaces and follow the longitudinal ridges of the junctura’’ to enter the oviduct, and suggested that a narrow lumen at the UTJ would effectively channel spermatozoa in an adovarian
Ž .
direction Nilsson and Reinius, 1969, p. 79 .
Spermatozoa that move progressively forward are more likely to penetrate the
Ž .
microstructure of cervical mucus see Katz et al., 1989 or successfully cross the UTJ ŽGaddum-Rosse, 1981 . However, transport across the UTJ is significantly impaired for. uterine spermatozoa that display the vigorous but non-progressive motility of
hyperacti-Ž
vation, whether this motility is induced by in vitro capacitation hamster; Shalgi et al.,
. Ž
1992 or preincubation of epididymal spermatozoa with calcium mouse; Olds-Clarke .
and Wivell, 1992 prior to artificial insemination. Furthermore, in horses, even if the insemination doses are balanced for number of progressively motile spermatozoa, fewer spermatozoa from subfertile stallions reach the oviducts of normal mares compared with
Fig. 1. Scanning electron micrograph of equine spermatozoa fixed in situ at the UTJ of a mare, 4 h after preovulatory insemination. The shape of the flagella and orientation of these spermatozoa with the epithelium
Ž .
Ž .
fertile stallion sperm numbers Scott et al., 1995 . Similarly, subfertile mares have fewer spermatozoa in their lower oviducts than fertile mares despite receiving equal numbers
Ž .
of progressively motile fertile stallion spermatozoa at insemination Scott et al., 1995 . Thus, motility per se does not assure successful sperm migration.
The defect may be at the level of sperm–epithelium interaction. Recent studies with transgenic mice have demonstrated that altering proteins on the sperm surface can compromise the migration of spermatozoa that otherwise display normal motility.
Ž .
Fertilin and the testis isozyme of angiotensin converting enzyme ACE are distinct sperm surface proteins. Uterine spermatozoa recovered from female mice mated with
Ž .
mutant males lacking either the gene for fertilin beta Cho et al., 1998 or the gene for
Ž .
testis ACE Hagaman et al., 1998 show normal motility characteristics, capacitation, and acrosome reaction in vitro, but have impaired transport to the oviducts in vivo. The results of these independent studies strongly suggest that sperm surface proteins are important functional elements in the process of sperm transport and stress the signifi-cance of sperm–epithelium interaction as a key component of normal sperm migration. Support for this hypothesis is derived from the knowledge that mouse spermatozoa normally adhere intermittently to the epithelium of the uterus, colliculus, UTJ and
Ž .
isthmus during transit through the female tract Suarez, 1987 .
3.2. Sperm morphology and sperm transport
Morphologically abnormal spermatozoa may also be functionally incompetent. Motile spermatozoa that are morphologically abnormal may be unable to swim through cervical mucus. Exclusion of abnormal spermatozoa at the cervix may be due to inferior motility
Ž .
andror cell surface abnormalities see Katz et al., 1989 . In cattle, abnormal spermato-Ž zoa remaining at oviductal isthmus and UTJ 12 h after intrauterine insemination i.e.,
.
bypassing the cervical barrier had a significantly reduced proportion of knobbed acrosomes than the inseminate, indicating compromised transport in the anterior genital
Ž .
tract for spermatozoa with this defect Mitchell et al., 1985 . Also, few spermatozoa with abnormal heads were recovered from the oviduct or UTJ of heifers 2 h after intrauterine insemination with semen having a post-thaw motility of 50% and a
Ž .
proportion of spermatozoa with abnormal heads exceeding 40% Larsson, 1988 . The UTJ appears to block the passage of most mouse spermatozoa that have severe head
Ž .
abnormalities Krzanowska, 1974 . In mares, more than 90% of spermatozoa visualized in situ at the UTJ using scanning electron microscopy are morphologically normal even when the inseminate contains high numbers of spermatozoa with major morphological
Ž .
defects Scott et al., 2000 . Those results led the authors to suggest that most morpholog-ically abnormal equine spermatozoa either do not reach the UTJ or do not develop
Ž
normal associations with the epithelium during transit through the tract Scott et al., .
4. Sperm reservoirs
A functional interaction between spermatozoa and the female tract is also crucial to the establishment and maintenance of sperm reservoirs, which are needed when mating or insemination precedes ovulation by many hours or days. Within the female genital tract, regions that accumulate and retain a population of viable spermatozoa for an extended period of time are considered to function as sperm reservoirs. In cattle, sheep and goats, the cervix with its cervical mucus maintains a population of viable spermato-zoa that is thought to provide a continued source of spermatospermato-zoa for the upper tract ŽHawk, 1983 . A cervical population adequate to achieve 100% fertilization is estab-.
Ž .
lished within 30–60 min of mating in ewes Hunter and Nichol, 1993 , and motile Ž spermatozoa can be found in cervical mucus as long as 3.5 days after mating Hunter
.
and Nichol, 1983 . In rabbits, the vagina, cervix and uterus each maintain a population
Ž .
of progressively motile spermatozoa for at least 16 h after mating Cooper et al., 1979 , Ž
by which time ovulation has occurred and 98% of eggs are fertilized Overstreet and .
Cooper, 1978b . Motile spermatozoa may persist in the lumen of the dog uterus for as
Ž .
long as 11 days Doak et al., 1967 . In addition, spermatozoa accumulate in the uterine glands of the bitch within 15 min after mating and are found there consistently for 6 days and with variable frequency for up to 9 days, when mating occurs on the first day
Ž .
of estrus Doak et al., 1967 . The disappearance of spermatozoa from the uterine glands prior to the loss of motile luminal spermatozoa, suggests that the glands supplement the
Ž .
luminal population Doak et al., 1967 . In the hare, spermatozoa have been found deep in the uterine glands and at the UTJ at 17 days of pregnancy, and this conservation of spermatozoa is thought to account for the superfetation that occurs in this species ŽMartinet and Raynaud, 1975 ..
In horses, pregnancy can result from a single mating or insemination up to 6 days
Ž .
prior to ovulation Day, 1942; Burkhardt, 1949 . Although this indicates the presence of Ž .
a sperm reservoir in the mare, its anatomical location s has not been confirmed. However, within 4 h after preovulatory insemination, equine spermatozoa accumulate at
Ž .
the UTJ Scott et al., 2000 , and spermatozoa can be found there 18 hours after
Ž .
insemination Scott, unpublished observations . The appearance of the spermatozoa and their intimate contact and orientation with the epithelium of the UTJ are suggestive of
Ž .
sequestration there Fig. 2 .
In the pig, the UTJ and lower oviductal isthmus are considered to be functional sperm reservoirs, because spermatozoa may survive in these regions for as long as 72 h ŽHunter, 1988 . Stable numbers of spermatozoa persist at the UTJ for 24 h following.
Ž .
artificial insemination Rigby, 1966 , and are found within the terminal folds of this
Ž .
region for at least 24 h following coitus Flechon and Hunter, 1981; Mburu et al., 1997 . Based on staining patterns with fluorescent probes, more than 40% of the spermatozoa
Ž
recovered by flushing the UTJ after ovulation have intact plasma membranes Mburu et .
al., 1996 and are presumably viable.
During the sustained phase of sperm transport, competent spermatozoa that cross the UTJ prior to ovulation will accumulate in the lower isthmus of the oviduct and remain
Ž
there until ovulation is imminent. In sheep Hunter et al., 1982; Hunter and Nichol,
. Ž . Ž .
Fig. 2. Scanning electron micrograph of equine spermatozoa fixed in situ at the UTJ of a mare, 18 h after insemination. These spermatozoa are located deep in an epithelial fold of the uterine papilla and show a characteristic side by side arrangement and orientation that is suggestive of a specific interaction with the
Ž .
epithelium of this region bars2mm .
surgical transection of the isthmus at various intervals between mating and ovulation was used to demonstrate that fertilization competent spermatozoa do not progress beyond the first 1.5–2 cm of the lower isthmus until the periovulatory period. In the rabbit, which does not ovulate spontaneously, the isthmic reservoir will accumulate normal numbers of spermatozoa following artificial insemination, but spermatozoa are
Ž
not found in the upper oviduct unless ovulation is induced Overstreet and Cooper, .
1979 . Thus, the lower isthmus not only functions as a sperm reservoir, but also appears to regulate sperm ascent to the ampulla.
4.1. Mechanisms of sperm storage
A close relationship between spermatozoa and the luminal epithelium of the female tract appears to be important for the survival of spermatozoa in reservoirs. This relationship is epitomized in the female bat, which may store viable spermatozoa in the uterus, UTJ, andror oviducts for many weeks to months, depending on the species ŽRacey, 1979 . Regardless of species, or location or duration of storage, during the. period of storage, spermatozoa are found displaying a typical linear, side by side
Ž arrangement, with their heads closely associated with the luminal epithelium Racey,
.
1979; Krutzsch et al., 1982; Racey et al., 1987; Krishna, 1997 . These spermatozoa are structurally intact. This relationship appears to be established soon after insemination
Ž .
and very early in the period of storage Racey et al., 1987 , and it is not found in
Ž .
females inseminated prior to the defined breeding season Krishna, 1997 . The universal nature of these observations in female bats suggests that the observation of similar morphological associations in other mammalian species may also depict sperm storage
Ž .
in a reservoir e.g., Fig. 2 .
In domestic animal species, observations of spermatozoa in situ following insemina-tion have demonstrated similar sperm–epithelium interacinsemina-tions in regions of sperm accumulation. Using scanning electron microscopy, intimate sperm–epithelium contact
Ž .
Ž . Ž pig Flechon and Hunter, 1981; Mburu et al., 1997 , and at the UTJ of the horse Scott
.
et al., 2000 . Spermatozoa have been found to line up in groups within epithelial crypts
Ž .
of the lower isthmus of the hamster Smith et al., 1987; Smith and Yanagimachi, 1990 , where they appear to ‘‘dig in’’ to the mucosa with their hooked heads, indicating a
Ž .
physical attachment Smith and Yanagimachi, 1990 . This close contact appears to maintain sperm viability, since spermatozoa with close epithelial contact maintain
Ž
flagellar activity and survive longer than luminal spermatozoa Smith and Yanagimachi,
. Ž .
1990 . Suarez 1987 observed similar interactions between living mouse spermatozoa and the oviductal epithelium in excised oviducts and proposed two possible mechanisms of sperm retention in the isthmus: adherence to the epithelium or immobilization. In the rabbit, motility is suppressed in sperm recovered from the lower isthmus in native fluid,
Ž .
and this is a proposed mechanism for their retention there Overstreet et al., 1980 . In cattle, a combination of a narrow lumen and the presence of mucus may contribute to
Ž .
sperm retention in the lower isthmus Suarez et al., 1997 .
During the period of sperm storage in female bats, epithelia intimately contacted by spermatozoa are ultrastructurally and histochemically different from epithelia without
Ž .
sperm contact Krutzsch et al., 1982; Krishna, 1997 . Differences have also been observed for epithelia of storage sites between the period of storage and the time of
Ž .
ovulation or arousal from hibernation Krutzsch et al., 1982; Krishna, 1997 . These observations suggest a functional change in the epithelia of storage sites that may regulate sperm attachment and release.
Support for a regulation of sperm physiology or attachment by epithelia comes from in vitro studies of spermatozoa co-cultured with monolayers of oviductal epithelial cells ŽOEC . For example, when equine spermatozoa are incubated with OEC, a lower. intracellular calcium concentration is found in attached spermatozoa compared with spermatozoa in media, suggesting that attachment is associated with a modulation of
Ž .
calcium Dobrinski et al., 1996 . The authors proposed that reduced intracellular calcium levels might prevent premature capacitation and acrosome reaction in spermatozoa
Ž .
during storage Dobrinski et al., 1996 .
4.2. Ascent of competent spermatozoa to the site of fertilization
Fertilization competence in spermatozoa is gained after a period of residence within the female tract. Functional changes in sperm cell physiology, collectively called capacitation, are induced or facilitated by sperm interaction with the luminal fluids and epithelial surfaces during transit, and enable spermatozoa to acrosome react, penetrate
Ž .
the zona pellucida and fuse with the oolemma see Yanagimachi, 1994 . Associated with capacitation are changes in sperm motility, termed hyperactivation, that are
character-Ž .
ized by a highly vigorous asymmetrical flagellar motion see Yanagimachi, 1994 . Hyperactivated motility generates thrusting forces considered great enough to enable a
Ž sperm to gain release from storage and to penetrate the vestments of the oocyte see
.
Katz et al., 1989 . In ruminants and primates, capacitation is initiated in the cervix with the removal of sperm surface proteins as spermatozoa swim through the microstructure
Ž .
of the cervical mucus see Drobnis and Overstreet, 1992 . The final stages of
capacita-Ž .
There is a temporal relationship between the resumption of sperm progress to the oviductal ampulla and the occurrence of ovulation. This coordination may be regulated
Ž .
by the changing hormonal profile that occurs at ovulation see Hunter, 1988 . Release of spermatozoa from the isthmic reservoir is associated with capacitation and the
develop-Ž .
ment of activated motility Overstreet and Cooper, 1979 . Forward progress within the confined spaces of the oviduct is enhanced in spermatozoa that display hyperactivated
Ž .
motility Katz and Yanagimachi, 1980 , which suggests it plays a key role in sperm migration to the ampulla, but also, oviductal contractility is likely to assist sperm transit Žsee Katz and Yanagimachi, 1980 . Spermatozoa ascend from the lower isthmus sooner.
Ž .
if mating occurs closer to ovulation Smith et al., 1987 , and this corresponds to a
Ž .
shortened length of time required for capacitation Smith and Yanagimachi, 1989 .
5. The elimination of excess spermatozoa
Millions to billions of sperm may be inseminated, but, regardless of the site of semen
Ž .
deposition at mating, relatively few gain access to the oviducts Harper, 1994 , and the Ž ratio of spermatozoa to oocytes at the site of fertilization may be only 1:1 see
.
Yanagimachi, 1994 . Most spermatozoa are rapidly eliminated from the lower tract by
Ž .
retrograde efflux through the cervix and vagina Mitchell et al., 1985; see Hunter, 1988 . Excess oviductal sperm may by eliminated via passage into the peritoneal cavity ŽOverstreet and Cooper, 1978a; Larsson, 1986 . In addition, a physiological inflamma-. tory response occurs in response to insemination and phagocytosis of spermatozoa by
Ž .
leukocytes occurs see Mitchell et al., 1985; Drobnis and Overstreet, 1992 . In bats, the leukocyte infiltration and active phagocytosis of spermatozoa which occurs in the lumen of the uterus and UTJ during the spring arousal, may reflect an estrogen surge that is
Ž .
analogous to the post-mating inflammation in other mammals Krutzsch et al., 1982 . Following the period of storage in bats, spermatozoa may be eliminated by engulfment
Ž .
and phagocytosis by epithelial cells Krutzsch et al., 1982; Krishna, 1997 .
6. Concluding comments
The process of sperm transport from the site of insemination to the site of fertilization is complex and involves dynamic interactions between spermatozoa and the female genital tract that assure the arrival of fertilization competent spermatozoa in the ampulla
Ž .
Acknowledgements
The electron micrographs were produced with funding provided by the Center for Equine Health at the University of California, Davis.
References
Brinsko, S.P., Varner, D.D., Blanchard, T.L., 1991. The effect of uterine lavage performed four hours post insemination on pregnancy rate in mares. Theriogenology 35, 1111–1119.
Brinsko, S.P., Varner, D.D., Blanchard, T.L., Meyers, S.A., 1990. The effect of postbreeding uterine lavage on
Ž .
pregnancy rate in mares. Theriogenology 33 2 , 465–475.
Burkhardt, J., 1949. Sperm survival in the genital tract of the mare. J. Agric. Sci. 39, 201–203.
Carballada, R., Esponda, P., 1997. Fate and distribution of seminal plasma proteins in the genital tract of the female rat after natural mating. J. Reprod. Fertil. 109, 325–335.
Cho, C., Bunch, D.O., Faure, J.E., Goulding, E.H., Eddy, E.M., Primakoff, P., Myles, D.G., 1998. Fertilization defects in sperm from mice lacking fertilin b. Science 281, 1857–1859.
Cooper, G.W., Overstreet, J.W., Katz, D.F., 1979. The motility of rabbit spermatozoa recovered from the female reproductive tract. Gamete Res. 2, 35–42.
Day, F.T., 1942. Survival of spermatozoa in the genital tract of the mare. J. Agric. Sci. 32, 108–111. Doak, R.L., Hall, A., Dale, H.E., 1967. Longevity of spermatozoa in the reproductive tract of the bitch. J.
Reprod. Fertil. 13, 51–58.
Dobrinski, I., Suarez, S.S., Ball, B.A., 1996. Intracellular calcium concentration in equine spermatozoa attached to oviductal epithelial cells in vitro. Biol. Reprod. 54, 783–788.
Drobnis, E.Z., Overstreet, J.W., 1992. Natural history of mammalian spermatozoa in the female reproductive
Ž .
tract. In: Mulligan, S.R. Ed. , Oxford Reviews of Reproductive Biology 14 Oxford Univ. Press, Oxford, pp. 1–45.
Einarsson, S., Jones, B., Larsson, K., Viring, S., 1980. Distribution of small- and medium-sized molecules within the genital tract of artificially inseminated gilts. J. Reprod. Fertil. 59, 453–457.
Flechon, J.E., Hunter, R.H.F., 1981. Distribution of spermatozoa in the uterotubal junction and isthmus of pigs and their relationship with the luminal epithelium after mating: a scanning electron microscope study. Tissue Cell 13, 127–139.
Gaddum-Rosse, P., 1981. Some observations on sperm transport through the uterotubal junction of the rat. Am. J. Anat. 160, 333–341.
Hagaman, J.R., Moyer, J.S., Bachman, E.S., Sibony, M., Magyar, P.L., Welch, J.E., Smithies, O., Krege, J.H., O’Brien, D.A., 1998. Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. U. S. A. 95, 2552–2557.
Ž .
Harper, M.J.K., 1994. Gamete and zygote transport. In: Knobil, E., Neill, J.D. Eds. , The Physiology of Reproduction. 2nd edn. Raven Press, New York, pp. 123–187.
Hawk, H.W., 1983. Sperm survival and transport in the female reproductive tract. J. Dairy Sci. 66, 2645–2660.
Hunter, R.H.F., 1975. Transport, migration and survival of spermatozoa in the female genital tract: species
Ž .
with intra-uterine deposition of semen. In: Hafez, E.S.E, Thibault, C.G. Eds. , The Biology of Spermato-zoa: Transport, Survival and Fertilizing Ability, INSERM Int. Symp., Nouzilly 1973. Karger, Basel, pp. 145–155.
Hunter, R.H.F., 1984. Pre-ovulatory arrest and periovulatory redistribution of competent spermatozoa in the isthmus of the pig oviduct. J. Reprod. Fertil. 72, 203–211.
Hunter, R.H.F., 1988. In: The Fallopian Tubes: Their Role in Fertility and Infertility. Springer-Verlag, New York, pp. 53–80.
Hunter, R.H.F., Nichol, R., 1993. Rate of establishment of a fertilizing population of spermatozoa in the sheep cervix after a single mating at the onset of oestrus. J. Exp. Zool. 266, 168–171.
Hunter, R.H.F., Wilmut, I., 1984. Sperm transport in the cow: peri-ovulatory redistribution of viable cells within the oviduct. Reprod., Nutr., Dev. 24, 597–608.
Hunter, R.H.F., Barwise, L., King, R., 1982. Sperm transport, storage, and release in the sheep oviduct in relation to the time of ovulation. Br. Vet. J. 138, 225–232.
Hunter, R.H.F., Flechon, B., Flechon, J.E., 1991. Distribution, morphology and epithelial interactions of bovine spermatozoa in the oviduct before and after ovulation: a scanning electron microscope study. Tissue Cell 23, 641–656.
Katz, D.F., Yanagimachi, R., 1980. Movement characteristics of hamster spermatozoa within the oviduct. Biol. Reprod. 22, 759–764.
Katz, D.F., Drobnis, E.Z., Overstreet, J.W., 1989. Factors regulating mammalian sperm migration through the female reproductive tract and oocyte vestments. Gamete Res. 22, 443–469.
Krishna, A., 1997. The relationship between spermatozoa and epithelium of the female genital tract during
Ž .
sperm storage in the greater yellow bats Scotophilus heathi : the light and electronmicroscopic observa-tions. Proc. Natl. Sci. Counc., Repub. China, Part B: Life Sci. 21, 31–36.
Krutzsch, P.H., Crichton, E.G., Nagle, R.B., 1982. Studies on prolonged spermatozoa survival in chiroptera: a morphological examination of storage and clearance of intrauterine and cauda epididymal spermatozoa in the bats Myotis lucifugus and M.Õelifer. Am. J. Anat. 165, 421–434.
Krzanowska, H., 1974. The passage of abnormal spermatozoa through the uterotubal junction of the mouse. J. Reprod. Fertil. 38, 81–90.
Larsson, B., 1986. Transperitoneal migration of spermatozoa in heifers. J. Vet. Med., Ser. A. 33, 714–718. Larsson, B., 1988. Distribution of spermatozoa in the genital tract of heifers inseminated with large numbers of
abnormal spermatozoa. J. Vet. Med., Ser. A 35, 721–728.
Mann, T., Polge, C., Rowson, L.E.A., 1956. Participation of seminal plasma during the passage of spermatozoa in the female reproductive tract of the pig and horse. J. Endocrinol. 13, 133–140.
Martinet, L., Raynaud, F., 1975. Prolonged spermatozoan survival in the female hare uterus: explanation of
Ž .
superfetation. In: Hafez, E.S.E, Thibault, C.G. Eds. , The Biology of Spermatozoa: Transport, Survival and Fertilizing Ability, INSERM Int. Symp., Nouzilly 1973. Karger, Basel, pp. 143–144.
Mburu, J.N., Einarsson, S., Lundeheim, N., Rodriguez-Martinez, H., 1996. Distribution, number and mem-brane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim. Reprod. Sci. 45, 109–121.
Mburu, J.N., Rodriguez-Martinez, H., Einarsson, S., 1997. Changes in sperm ultrastructure and localisation in the porcine oviduct around ovulation. Anim. Reprod. Sci. 47, 137–148.
Mitchell, J.R., Senger, P.L., Rosenberger, J.L., 1985. Distribution and retention of spermatozoa with acrosomal and nuclear abnormalities in the cow genital tract. J. Anim. Sci. 61, 956–967.
Nilsson, O., Reinius, S., 1969. Light and electron microscopic structure of the oviduct. In: Hafez, E.S.E.,
Ž .
Blandau, R.J. Eds. , The Mammalian Oviduct: Comparative Biology and Methodology. University of Chicago Press, Chicago, pp. 57–83.
Olds-Clarke, P., Wivell, W., 1992. Impaired transport and fertilization in vivo of calcium-treated spermatozoa fromqrqor congenic tw32rqmice. Biol. Reprod. 47, 621–628.
Overstreet, J.W., 1983. Transport of gametes in the reproductive tract of the female mammal. In: Hartmann,
Ž .
J.F. Ed. , Mechanism and Control of Animal Fertilization. Academic Press, New York, pp. 499–543. Overstreet, J.W., Cooper, G.W., 1978a. Sperm transport in the reproductive tract of the female rabbit: I. The
rapid transit phase of transport. Biol. Reprod. 19, 101–114.
Overstreet, J.W., Cooper, G.W., 1978b. Sperm transport in the reproductive tract of the female rabbit: II. The sustained phase of transport. Biol. Reprod. 19, 115–132.
Overstreet, J.W., Cooper, G.W., 1979. Effect of ovulation and sperm motility on the migration of rabbit spermatozoa to the site of fertilization. J. Reprod. Fertil. 55, 53–59.
Overstreet, J.W., Katz, D.F., 1990. Interaction between the female reproductive tract and spermatozoa. In:
Ž .
Gagnon, C. Ed. , Controls of Sperm Motility: Biological and Clinical Aspects. CRC Press, Boca Raton, FL, pp. 63–75.
Racey, P.A., 1979. The prolonged storage and survival of spermatozoa in Chiroptera. J. Reprod. Fertil. 56, 391–402.
Racey, P.A., Uchida, T.A., Mori, T., Avery, M.I., Fenton, M.B., 1987. Sperm-epithelium relationships in
Ž .
relation to the time of insemination in little brown bats Myotis lucifugus . J. Reprod. Fertil. 80, 445–454. Rigby, J.P., 1966. The persistence of spermatozoa at the uterotubal junction of the sow. J. Reprod. Fertil. 11,
153–155.
Scott, M.A., Liu, I.K.M., Overstreet, J.W., 1995. Sperm transport to the oviducts: abnormalities and their clinical implications. Proc. 41st Ann. Conv. Am. Assoc. Eq. Pract. 41, 1–2.
Scott, M.A., Liu, I.K.M., Overstreet, J.W., Enders, A.C., 2000. The structural morphology and epithelial association of spermatozoa at the uterotubal junction: a descriptive study of equine spermatozoa in situ using scanning electron microscopy. J. Reprod. Fertil., Suppl., In press.
Shalgi, R., Smith, T.T., Yanagimachi, R., 1992. A quantitative comparison of the passage of capacitated and uncapacitated hamster spermatozoa through the uterotubal junction. Biol. Reprod. 46, 419–424. Smith, T.T., Koyanagi, F., Yanagimachi, R., 1987. Distribution and number of spermatozoa in the oviduct of
the golden hamster after natural mating and artificial insemination. Biol. Reprod. 37, 225–234.
Smith, T.T., Yanagimachi, R., 1989. Capacitation status of hamster spermatozoa in the oviduct at various times after mating. Biol. Reprod. 86, 255–261.
Smith, T.T., Yanagimachi, R., 1990. The viability of hamster spermatozoa stored in the isthmus of the oviduct: the importance of sperm–epithelium contact for sperm survival. Biol. Reprod. 42, 450–457.
Suarez, S.S., 1987. Sperm transport and motility in the mouse oviduct: observations in situ. Biol. Reprod. 36, 203–210.
Suarez, S.S., Brockman, K., Lefebvre, R., 1997. Distribution of mucus and sperm in bovine oviducts after artificial insemination: the physical environment of the oviductal sperm reservoir. Biol. Reprod. 56, 447–453.
Viring, S., Einarsson, S., Jones, B., Larsson, K., 1980. Transuterine transport of small- and medium-sized molecules deposited in the uterus of gilts. J. Reprod. Fertil. 59, 459–462.
Ž .