www.elsevier.nlrlocateraqua-online
Performance testing of clonal Oreochromis
niloticus lines
Andreas Muller-Belecke
¨
), Gabriele Horstgen-Schwark
¨
Institut fur Tierzucht und Haustiergenetik, Uni¨ Õersitat Gottingen, Albrecht-Thaer-Weg 3,¨ ¨
D-37075 Gottingen, Germany¨
Accepted 7 September 1999
Abstract
Ž .
Six different homozygous clonal lines 343 adult fish were established by gynogenetic reproduction of mitotic gynogenetic Oreochromis niloticus females. Second generation clones
Ž494 adult fish were obtained by gynogenetic reproduction of clonal females. The clonal status of.
the fish was proven by the alloenzyme adenosine deaminase and DNA fingerprints. Performance testing of the all-female clonal groups was carried out in comparison with adequate heterozygous control groups. Up to first feeding, clones showed a significantly reduced mean survival rate
Ž4.0% if compared with offspring of normal heterozygous fish 43.9% . Reproductive traits of the. Ž .
second generation clones varied between and within clonal lines. Mean body weight differences at
Ž .
136th day of life between all-female clonal groups 45.5 g , kept under three different density
Ž .
classes, and corresponding all-female heterozygous control groups 50.2 g were not significant.
Ž
Variation of body weight was significantly lower in all-female homozygous clones coefficient of
. Ž .
variation: 23.1% than in all-female heterozygous controls coefficient of variation: 34.1% . Possible reasons for phenotypic variation between isogenic O. niloticus individuals are discussed.
q2000 Elsevier Science B.V. All rights reserved.
Keywords: Oreochromis niloticus; Gynogenesis; Clones; Survival; Growth
1. Introduction
Since the beginning of the 1930s, plant breeders succeeded in developing highly
Ž .
productive seeds by crossing homozygous lines Allard, 1960 . Due to the heterozygous isogenic status, the variability of performance traits was reduced in these crosses.
)Corresponding author.
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
Furthermore, in some diallel crosses with clonal lines, high heterosis effects were found. Whether a breeding strategy including clone crosses is relevant for practical applications depends on the performance of the clone crosses as well as the cost-effectiveness of development and maintenance of the clonal lines.
Until now, experiments to produce highly inbred lines of farm animals such as pig and poultry were not very successful. The relatively low increase in the inbreeding level per generation and high losses of lines due to inbreeding depression made the practical
Ž .
application of this strategy too expensive Ameli, 1989; Glodek, 1992 .
In fish, external fertilization and embryonic development allow the development of homozygous clones in only two generations by use of induced gynogenetic or
androge-Ž
netic reproduction Thorgaard, 1986; Horstgen-Schwark, 1991; Horvath and Orban,
¨
´
´
.
1995 . The high fecundity of fish increases the probability of development of homozy-gous genotypes free from expression of harmful genes and, therefore, viable and reproductively competent.
Ž
Although clones have been developed within eight fish species Brachydanio rerio: Streisinger et al., 1981; Oryzias latipes: Naruse et al., 1985; Cyprinus carpio: Komen et al., 1991; Plectoglossus altiÕelis: Han et al., 1991; Paralichtys oliÕaceus: Hara et al.,
1993; Oncorhynchus rhodurus: Kobayashi et al., 1994; Onchorhynchus mykiss: Young
.
et al., 1995; Oreochromis niloticus: Muller-Belecke and Horstgen-Schwark, 1995 , only
¨
¨
few data of their reproductive and growth performance in comparison to normal heterozygous controls have been published. The aim of the present investigation is to supply performance data during the development, maintenance and reproduction of O.
niloticus clones for subsequent evaluation of clone crosses.
2. Materials and methods
2.1. DeÕelopment and reproduction of clones
The production of clone mothers by mitotic gynogenesis and the verification of their homozygous status has been described in detail by Muller-Belecke and Horstgen-Schwark
¨
¨
Ž1995 . These mitotic gynogenetic females, derived from the ‘‘Lake Manzala’’ strain. ŽEgypt have been used to develop homozygous clones by meiotic gynogenetic repro-.
duction. For this purpose, groups of mitotic gynogenetic females have been transferred to glass aquaria when they reached an age of 9 month. When preparation of spawning
Ž .
was observed, eggs of ripe females were stripped into 0.2 l saline solution 0.9% NaCl .
Ž .
Sperm from male fish delivering high quality sperm high density and motility and showing characteristic allele configurations at genetic markers was stripped and mixed in saline solution 1:5. For the inactivation of the paternal genome, this mixture was
Ž . y2
irradiated in a layer of 2 mm with UV-light 254 nm with an intensity of 58 mJ cm .
Ž .
The sperm was stirred 120 rpm throughout the process of irradiation. Five minutes
Ž .
after activation of eggs with genetically inactivated sperm and water 288C , eggs were transferred into a 418C water bath for 4.5 min to induce the retention of the 2nd polar
Ž .
body Puckhaber and Horstgen-Schwark, 1996 . The first generation clonal females thus
¨
2.2. Proof of the clonal status
Successful gynogenetic reproduction was confirmed by the alloenzyme marker system adenosine deaminase analysing blood samples of all potentially clonal adults
ŽG50 g as described by Muller-Belecke and Horstgen-Schwark 1995 . Due to the.
¨
¨
Ž .recombination rate of 100%, which was observed in O. niloticus in this marker system, it allows the proper differentiation between meiotic and mitotic gynogenetic origin
ŽHussain et al., 1994 ..
Fish were assumed to be homozygous clones if the absence of characteristic allele configurations of the sperm donor in their electropherograms proved them to be
Ž
gynogenetic offspring, resulting from mitotic gynogenetic females first generation
. Ž .
clones or clonal females second generation clones . A verification of the clonal status at the DNA level was conducted by multilocus DNA fingerprinting and random
Ž . Ž .
amplified polymorphic DNA RAPD as described by Jenneckens et al. 1999 .
2.3. Control groups
Control groups were produced simultaneously with gynogenetic groups, but control groups differed with regard to their genetic make-up depending on what kind of comparison they were used for.
Ž .1 During the development of first generation clones normal outbred progeny of the
Ž .
Lake Manzala population 72 batches from 42 females and six males was included as control to compare survival rates of gynogenetically reproduced progeny of mitotic gynogenetic females up to the first feeding stage with survival rates of this outbred
Ž .
progeny Table 1 . Incubation of approximately 200 eggs per batch and first feeding of the hatched larvae was conducted, following the same procedure as gynogenetically reproduced eggs of mitotic gynogenetic females.
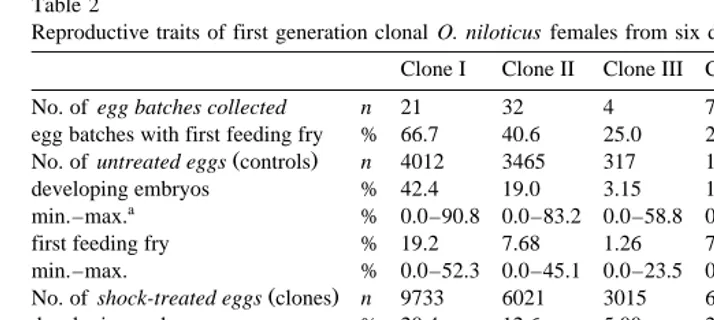
Ž .2 For the evaluation of egg quality of first generation clonal females Table 2Ž .
approximately 200 eggs of each batch from these clonal females undergoing
gyno-Table 1
Survival rates of meiotic gynogenetic O. niloticus offspring of six mitotic gynogenetic clone mothers in comparison to offspring of normal outbred females
Mother Mother Mother Mother Mother Mother Normal
Ž .
Clone I Clone II Clone III Clone IV Clone V Clone VI females ns42
a a a a a a
Number n 2812 1836 1306 3474 958 3691 13334
of eggs
U U U U U U
Developing % 19.8 31.7 15.9 6.30 15.5 6.48 69.4
embryos
U U U U U U
First % 5.26 16.2 8.50 2.13 5.22 1.46 43.9
feeding fry
Adult fish n 73 171 42 14 23 20 data not recorded
Žgeneration 1. % 2.59 9.31 3.22 0.40 2.40 0.54
a
Undergoing meiotic gynogenesis U
Ž .
Table 2
Reproductive traits of first generation clonal O. niloticus females from six different clonal lines
Clone I Clone II Clone III Clone IV Clone V Clone VI
No. of egg batches collected n 21 32 4 7 3 8
egg batches with first feeding fry % 66.7 40.6 25.0 28.6 66.7 25.0
Ž .
No. of untreated eggs controls n 4012 3465 317 1746 343 2121
developing embryos % 42.4 19.0 3.15 15.6 14.3 14.3
a
min.–max. % 0.0–90.8 0.0–83.2 0.0–58.8 0.0–59.8 1.8–27.5 0.0–72.1
first feeding fry % 19.2 7.68 1.26 7.90 2.92 7.40
min.–max. % 0.0–52.3 0.0–45.1 0.0–23.5 0.0–34.1 0.0–6.4 0.0–60.6
Ž .
No. of shock-treated eggs clones n 9733 6021 3015 6365 507 4833
developing embryos % 20.4 12.6 5.00 20.0 8.88 4.95
min.–max. % 0.0–49.2 0.0–77.9 0.0–46.9 0.0–53.4 0.6–15.1 0.0–30.9
first feeding fry % 4.88 2.21 0.99 4.67 0.39 2.09
min.–max. % 0.0–20.5 0.0–13.0 0.0–9.4 0.0–11.4 0.0–0.6 0.0–16.3
adult fish n 159 75 28 158 2 72
Žgeneration 2. % 1.63 1.25 0.93 2.48 0.39 1.49
a
Minimum and maximum values among different egg batches.
genetic reproduction were fertilised with untreated sperm of high quality and raised without any special treatment to serve as controls.
Ž .3 To provide all-female heterozygous control groups for growth comparisons with
Ž .
the all-female homozygous clonal groups Table 3 , normal outbred females of the same age as clonal females were mated with mitotic gynogenetic males that produced
Ž
all-female offspring in earlier experiments Muller-Belecke and Horstgen-Schwark,
¨
¨
.
1995 .
2.4. Performance testing
Reproduction and growth experiments were carried out in the warm water recircula-tion system of the Institut fur Tierzucht und Haustiergenetik under constant environmen-
¨
Ž .
tal conditions water quality parameters, photoperiod the year round. Spawners were consecutively incorporated into reproduction experiments when they reached an age of 9 months. As not all clones have been developed at the same time, growth experiments were performed with first generation and second generation clonal groups ensuring that spawners had a similar age when producing the progenies.
The testing procedure started with the incubation of eggs from clonal and control
3 Ž .
groups for 9 days in 35 cm hatching jars at 288C Habitzky-Biester, 1987 . The number
Ž . Ž .
of eggs 1st day of life , the percentage of developing embryos 2nd day of life and the
Ž .
percentage of first feeding fry 9th day of life were recorded for each group. From the 9th to the 24th day of life, the fry were kept at a density of 50 fish per l in 2 l plastic
Ž .
aquaria, and fed with a high protein diet 47% crude protein . At the 24th day of life, the fish designated for growth performance testing were transferred into 80-l glass aquaria. Depending on the number of available fish per egg batch the 80 l tanks were stocked with a maximum of 80 individuals. Between their 24th and 136th day of life fish were counted every two weeks. All groups were fed three times a day ad libitum with trout
Ž . Ž
Table 3
Mean body weights and coefficients of variation of body weight from all homozygous clonal groups and corresponding all-female heterozygous control groups kept among three different density classes
a
Density class Group No. of tanks No. of fish Mean body CV F-test
weight in g
1–20 fishrtank control 8 86 60.7 45.9
Clone I 6 81 55.4 21.7
all clones 12 139 55.4 n.s. 22.6
21–50 fishrtank control 11 385 49.6 31.1
Clone I 5 140 43.2 18.2
all clones 13 453 48.0 n.s. 22.5
51–80 fishrtank control 3 199 40.3 29.2
Clone I 1 54 29.7 28.3
all clones 4 233 33.0 n.s. 24.2
all density classes control 22 670 50.2 34.1
U
all clones 29 825 45.5 n.s. 23.1
a
Significantly different from control PF0.05 .
.
protein from the 81st to 136th day of life. At the 136th day of life the fish were individually tagged and weighed. After finishing this growth performance testing, fish were communally reared in 700-l tanks. When the fish reached an individual body weight above 50 g, blood samples for genetic marker studies were taken.
2.5. Data analysis
To test differences in the number of surviving fish between clones and controls for their significance, Chi square-tests were applied.
within density classes, between three and 13 replicates were available for the analysis of growth performance data. Differences in mean body weights between clones and controls kept among the three different density classes were evaluated by ANOVA,
Ž .
applying The Generalised Linear Models GLM procedure on the following statistical model:
U U
Yi jk lsmqGiqCjq
Ž
G C.
i jqTkŽ
G C.
i j qei jk lwhere Yi jk lsvalue observed in the ijklth fish; msoverall mean, Giseffect of the ith
Ž
genetic category is1, 2; 1shomozygous isogenic groups; 2sall-female
heterozy-. Ž
gous control groups ; Cjseffect of the jth density class js1, 2, 3; 1sone to 20 fish
. Ž .
per tank; 2s21 to 50 fish per tank; 3s51 to 80 fish per tank ; G)C i jseffect of the
wŽ . x
interaction between the ith genetic category and the jth density class; Tk G)C i j s
random effect of the k th tank within the ith genetic category and the jth density class; and ei jk lserror term.
To prove the effect of the genetic category for its significance the G -term was testedi
wŽ . x
against the Tk G)C i j -term in an F-test.
Differences in variances of body weight observed between clonal- and control groups were analysed using F-tests from pooled within tank variances.
3. Results
Of 77 adult homozygous females derived by induction of a mitotic gynogenesis, only
Ž . Ž
10 females 13% produced eggs that developed Muller-Belecke and Horstgen-Schwark,
¨
¨
.
1995 . Adult first generation clonal fish were derived from meiotic gynogenetic repro-duction of six mitotic gynogenetic females as shown in Table 1. Mothers of the Clones II to VI descended from the same clone grandmother. The clonal genotypes were free from recessive lethal genes as shown by the viability of the clone mothers, which on basis on their mitotic gynogenetic origin were completely homozygous. However, survival rates within clones were extremely low when compared with survival rates of
Ž .
untreated offspring from normal heterozygous females Table 1 . Considerable
differ-Ž
ences were observed between the clones e.g., 16.2% first feeding fry in Clone II vs.
.
1.46% in Clone VI . The clonal status was confirmed by screening genetic markers for all the 343 adult fish that were derived in the first generation of clone production.
The reproductive traits of the first generation clonal fish, important for the
continua-Ž .
tion of the clonal lines, were also on a low level Table 2 . Only 44% of the egg batches that were derived from first generation clonal females showed first feeding fry in untreated control groups. The corresponding parameter in untreated egg batches from
Ž .
normal heterozygous females was 81% data not shown . The reduced quality of eggs received from first generation clonal spawners was manifested by the low survival rates of untreated groups. The survival rates observed in gynogenetically reproduced eggs
Ž .
from first generation clonal females Table 2 ranged at approximately same level as
Ž
was observed during the development of the first generation clones themselves Table
.
1 . Survival rates of different egg batches from different first generation clonal females
Ž .
adult fish that were produced by meiotic gynogenetic reproduction of first generation clonal females were also clonal.
Table 3 presents body weight data recorded from first and second generation clonal groups and corresponding all-female control groups, after finishing the above-described standard performance testing. Calculated over all density classes, clonal groups showed reduced body weights in comparison with control groups. However, this difference was not significant. The variability of body weight was significantly lower in clonal groups than in controls. As would be expected, the body weight of both, clonal and control groups, decreased when the fish were kept under high densities. The variability of body weight was highest in control groups when they were kept in low density and decreased to a comparable level in middle and high density classes. In clonal groups, however, the variability of body weight was comparable among the density classes, with some tendency to increase if they were kept under the highest density. In some cases, considerable differences in body weights and in the variability of body weight were observed within the clones when the clonal groups were kept under different density classes. The mean survival rate during the standard performance testing between 24th and 136th day of life was 72.2% in all-female clonal groups and 70.5% in all-female
Ž .
heterozygous control groups data not shown . Mortality was almost evenly distributed with a slight tendency to increase from the 80th day of life on in clones as well as in controls. Differences in mortality between the three density classes were not significant.
4. Discussion and conclusions
In several investigations with different fish species, the low survival rates among
Ž
mitotic gynogenetic individuals were demonstrated Mair et al., 1987; Komen et al.,
.
1991; Quillet, 1994 . Another hindrance for the development of clonal lines was the drastically reduced fertility of potential mitotic gynogenetic clone mothers, as observed
Ž . Ž
in C. carpio Komen et al., 1992 and O. niloticus Muller-Belecke and Horstgen-
¨
¨
.
Schwark, 1995 . The present experiment showed that in O. niloticus, in addition to these disadvantages, meiotic gynogenetic offspring of mitotic gynogenetic as well as first generation clonal females had extremely low survival rates if compared with offspring of normal outbred females. Moreover, the reproductive traits of clonal females within clones were highly variable. In some cases, this might be explained by missing the optimal time for artificial stripping of ready ovulated eggs andror unnoticed small variations in the method of gynogenetic reproduction. Further seasonal changes in egg quality could influence reproductive traits of clonal females. Seasonal influences on spawning frequency and success, however, could not be found in outbred females of the Lake Manzala population kept for years under the same management in the same warm water recirculation units. A deciding influence on survival rates of isogenic individuals could result from maternal factors like differences in supply of the developing oocytes
Ž .
with yolk material during oogenesis Gartner, 1990; Bongers, 1997 . An ongoing
¨
The body weight differences between clonal groups and the corresponding controls were not as distinct as for reproductive traits. Clonal groups showed slightly lower growth performance up to the 136th day of life compared to controls, but these differences were insignificant. Similar results were obtained in C. carpio clones by
Ž . Ž .
Komen et al. 1993 and Bongers et al. 1995 . The growth performance of clonal P.
Ž .
altiÕelis groups was significantly lower than in controls Taniguchi et al., 1994 . In
Ž .
contrast to investigations in C. carpio Komen et al., 1993; Bongers et al., 1995 , the clonal O. niloticus groups showed lower variabilities of body weights than the control
Ž .
groups, as also was observed in P. altiÕelis Taniguchi et al., 1994 .
Aggression resulting from territorial behaviour could induce deviations in the growth of isogenic O. niloticus within and between clonal groups besides the already above mentioned maternal factors. Territorial behaviour, which mainly was observed in heterozygous control groups that were kept in the lowest density class, provides an explanation for the increased variability of body weight of these groups compared to
Ž .
control groups from higher density classes Table 3 . The influence of aggression on the
Ž
performance under low densities is also discussed for other fish species Refstie and
.
Kittelsen, 1976; Wallace et al., 1988; Hecht and Uys, 1997 . It remains unclear why the dependency of the density class on the variability of body weight could not be found in clonal groups. From observations during the growth performance testing the authors infer that the tendency to show territorial behaviour is reduced in homozygous clones. The present investigations demonstrated the difficulties of development and reproduc-tion of clonal O. niloticus lines by use of induced gynogenesis. However, due to their growth performance, comparable with heterozygous control groups, the maintenance of the developed clonal groups required almost no additional costs and care. It still remains to be determined, whether continuation of clonal lines by natural spawning of females with sex-reversed functional clonal males will be easier than by gynogenetic reproduc-tion. From results of diallel crosses between the developed clonal lines, first conclusions can be drawn if the development and continuation of clonal lines in O. niloticus is worthwhile for practical applications in breeding programmes.
Acknowledgements
This study was supported by the Deutsche Forschungsgesellschaft.
References
Allard, R.W., 1960. Principles of Plant Breeding, Wiley, New York.
Ameli, H., 1989. Inzucht- und Heterosiseffekte sowie genetische Parameter in zwei langjahrig reziproker¨
Ž .
rekurrenter Selektion unterworfenen weißen Leghornlinien LSL und ihren reziproken Kreuzungen. Dissertation, Universitat Gottingen.¨ ¨
Bongers, A.B.J, 1997. Development and Application of Genetically Uniform Strains of Common Carp
Bongers, A.B.J., Boza Abarca, J., Zandieh Doulabi, B., Eding, E.H., Komen, J., Richter, C.J.J., 1995. Maternal influence on development of androgenetic clones of common carp, Cyprinus carpio L. Aquacul-ture 137, 139–147.
Gartner, K., 1990. A third component causing random variability beside environment and genotype. A reason¨
for the limited success of a 30 year long effort to standardize laboratory animals?. Lab. Anim. 24, 71–77. Glodek, P., 1992. Schweinezucht: Grundlagen der Schweineproduktion. Ulmer, Stuttgart.
Habitzky-Biester, H., 1987. Vergleichende Untersuchungen zu Erbrutungs- und Aufzuchtmethoden bei¨
Ž .
Afrikanischen Buntbarschen Oreochromis niloticus . Dissertation, Universitat Gottingen.¨ ¨
Han, H.S., Taniguchi, N., Tsujimura, A., 1991. Production of clonal ayu by chromosome manipulation and
Ž .
confirmation by isoenzyme marker and tissue grafting. Nippon Suisan Gakkaishi 57 5 , 825–832. Hara, M., Dewa, K., Yamamoto, E., 1993. DNA-fingerprinting with non-radioactive probe in clonal flounder
Ž .
Paralichtys oliÕatus. Nippon Suisan Gakkaishi 59 4 , 731.
Hecht, T., Uys, W., 1997. Effect of density on the feeding and aggressive behaviour in juvenile African
Ž .
catfish, Clarias gariepinus. S. Afr. J. Sci. 93 11r12 , 537–541.
Horstgen-Schwark, G., 1991. Biotechnology and genetic improvement of fish. The 2nd ARRC International¨
Symposium, Animal Resources Research Center, Kon-Kuk University, Seoul, pp. 45–48.
Horvath, L., Orban, L., 1995. Genome and gene manipulation in the common carp. Aquaculture 129,´ ´
157–181.
Hussain, M.G., McAndrew, B.J., Penman, D.J., Sodusk, P., 1994. Estimate gene–centromere recombination
Ž .
frequencies in gynogenetic diploids of Oreochromis niloticus L. , using alloenzymes, skin color and a
Ž . Ž .
putative sex-determination locus SDL-2 . In: Beaumont, A.R. Ed. , Genetics and Evolution of Aquatic Organisms. Chapman & Hall, London, pp. 502–509.
Jenneckens, I., Muller-Belecke, A., Horstgen-Schwark, G., Meyer, J.-N., 1999. Proof of the successful¨ ¨
Ž .
development of Nile tilapia Oreochromis niloticus clones by DNA fingerprinting. Aquaculture 173, 377–388.
Kobayashi, T., Ide, A., Hiasha, T., Fushiki, S., Uedo, K., 1994. Production of cloned amago salmon
ŽOnchorhynchus rhodurus . Fish. Sci. 60 3 , 275–281.. Ž .
Komen, J., Bongers, G., Richter, C.J.J., Van Muiswinkel, W.B., Huisman, E.A., 1991. Gynogenesis in
Ž .
common carp Cyprinus carpio L. : II. The production of gynogenetic clones and F hybrids. Aquaculture1
92, 127–142.
Komen, J., Wiegertjes, G.F., Van Ginneken, V.J.T., Eding, E.H., Richter, C.J.J., 1992. Gynogenesis in
Ž .
common carp Cyprinus carpio L. : III. The effects of inbreeding on gonadal development of heterozygous and homozygous gynogenetic offspring. Aquaculture 104, 51–66.
Ž
Komen, J., Eding, E.H., Bongers, A.B.J., Richter, C.J.J., 1993. Gynogenesis in common carp Cyprinus
.
carpio IV: Growth, phenotypic variation and gonad differentiation in normal and methyltestosterone
treated homozygous clones and F hybrids. Aquaculture 111, 271–280.1
Mair, G.C., Scott, A., Beardmore, J.A., Skibinski, D.O.F., 1987. A technique for the induction of diploid
Ž .
gynogenesis in Oreochromis niloticus by the suppresion of the first mitotic division. In: Tiews, K. Ed. , Proc. World Symp. on Selection, Hybridisation and Genetic Engineering in Aquaculture, 27–30 May, 1986, Vol. II, Bordeaux, pp. 289–300.
Ž .
Muller-Belecke, A., Horstgen-Schwark, G., 1995. Sex determination in tilapia Oreochromis niloticus . Sex¨ ¨
ratios in homozygous gynogenetic progeny and their offspring. Aquaculture 137, 57–65.
Ž
Naruse, K., Ijiri, K., Shima, A., Egami, N., 1985. The production of cloned fish in the medaka Oryzias
.
latipes . J. Exp. Zool. 236, 335–341.
Puckhaber, B., Horstgen-Schwark, G., 1996. Growth rate and gonadal development of triploid tilapia¨
ŽOreochromis niloticus . In: The Third International Symposium on Tilapia in Aquaculture , ICLARM. .
Ž .
Makati City Philippines . ICLARM Conference Proceedings, Manila 41, pp. 377–382.
Quillet, E., 1994. Survival, growth and reproductive traits of mitotic gynogenetic rainbow trout females. Aquaculture 123, 223–236.
Refstie, T., Kittelsen, A., 1976. Effect of density on growth and survival of artificially reared atlantic salmon. Aquaculture 8, 319–326.
Streisinger, G., Walker, C., Dower, N., Knauber, D., Singer, F., 1981. Production of clones of homozygous
Ž .
Taniguchi, N., Han, H.S., Tsujimura, A., 1994. Variation in some quantitative traits of clones produced by chromosome manipulation in ayu, Plecoglossus altiÕelis. Aquaculture 120, 53–60.
Thorgaard, G.H., 1986. Ploidy manipulation and performance. Aquaculture 57, 57–64.
Wallace, J.C., Kolbeindhavn, A.G., Reinsnes, T.G., 1988. The effect of stocking density on early growth in
Ž .
arctic charr, SalÕelinus alpinus L. . Aquaculture 73, 101–110.