19 Kempton, J.H. (1920) Heritable characters of maize V. Adherence, J. Hered.
11, 317–322
20 Becraft, P.W., Stinard, P.S. and McCarty, D.R. (1996) CRINKLY4: a
TNFR-like receptor kinase involved in maize epidermal differentiation, Science 273, 1406–1409
21 Jenks, M.A. et al. (1996) Mutants in Arabidopsis thaliana altered in
epicuticular wax and leaf morphology, Plant Physiol. 110, 377–385
22 Lolle, S.J., Hsu, W. and Pruitt, R.E. (1998) Genetic analysis of organ fusion in
Arabidopsis thaliana, Genetics 149, 607–619
23 Sinha, N. (1998) Organ and cell fusions in the adherent1 mutant in maize, Int.
J. Plant Sci. 159, 702–715
24 Sinha, N. and Lynch, M. (1998) Fused organs in the adherent1 mutation in
maize show altered epidermal walls with no perturbations in tissue identities, Planta 206, 184–195
25 Lolle, S.J., Cheung, A.Y. and Sussex, I.M. (1992) Fiddlehead: An Arabidopsis
mutant constitutively expressing an organ fusion program that involves interactions between epidermal cells, Dev. Biol. 152, 383–392
26 Lolle, S.J. and Cheung, A.Y. (1993) Promiscuous germination and growth of
wild-type pollen from Arabidopsis and related species on the shoot of the Arabidopsis mutant, fiddlehead, Dev. Biol. 155, 250–258
27 Lolle, S.J. et al. (1997) Developmental regulation of cell interactions in the
Arabidopsis fiddlehead-1 mutant: a role for the epidermal cell wall and cuticle, Dev. Biol. 189, 311–321
28 Aida, M. et al. (1997) Genes involved in organ separation in Arabidopsis: an
analysis of the cup-shaped cotyledon mutant, Plant Cell 9, 841–857
29 Koornneef, M., Hanhart, C.J. and Thiel, F. (1989) A genetic and phenotypic
description of eceriferum (cer) mutants in Arabidopsis thaliana, J. Hered. 80, 118–122
30 Levin, J.Z. et al. (1998) A genetic screen for modifiers of UFO meristem
activity identifies three novel FUSED FLORAL ORGANS genes required for early flower development in Arabidopsis, Genetics 149, 579–595
31 Neuffer, M.G., Coe, E.H. and Wessler, S.R. (1997) Mutants of Maize, Cold
Spring Harbor Laboratory Press
32 Becraft, P.W. (1998) Receptor kinases in plant development, Trends Plant Sci.
3, 384–388
33 Hülskamp, M. et al. (1995) Identification of genes required for pollen–stigma
recognition in Arabidopsis thaliana, Plant J. 8, 703–714
34 Heslop-Harrison, J., Knox, R.B. and Heslop-Harrison, Y. (1974) Pollen-wall
proteins: exine–held fractions associated with the incompatibility response in Cruciferae, Theor. Appl. Genet. 44, 133–137
35 Verbeke, J.A. (1989) Stereological analysis of ultrastructural changes during
induced epidermal cell redifferentiation in developing flowers of Catharanthus roseus (Apocynaceae), Am. J. Bot. 76, 952–957
36 Preuss, D. et al. (1993) A conditional sterile mutation eliminates surface
components from Arabidopsis pollen and disrupts cell signaling during fertilization, Genes Dev. 7, 974–985
37 Taylor, L.P. and Hepler, P.K. (1997) Pollen germination and tube growth,
Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 461–491
38 Wolters-Arts, M., Lush, W.M. and Mariani, C. (1998) Lipids are required for
directional pollen-tube growth, Nature 392, 818–821
39 Bell, P.R. (1995) Incompatibility in flowering plants: adaptation of an ancient
response, Plant Cell 7, 5–6
40 Conner, J.A. et al. (1998) Comparative mapping of the Brassica S locus
region and its homeolog in Arabidopsis: implications for the evolution of mating systems in the Brassicaceae, Plant Cell 10, 801–812
T
he genomes of all living things are constantly subject to dam-age and decay. Some of the damdam-age occurs as an inevitable consequence of the chemical nature of DNA and its aqueous environment, or as a result of errors of metabolism (i.e. the donation of a methyl group to DNA rather than its intended target, or the presence of stray radicals mistakenly formed during respiration or photosynthesis). This endogenously generated damage is often termed ‘spontaneous’ DNA damage to distinguish it from damage due to exogenous sources, such as UV radiation and chemical DNA damaging agents.The wide variety of DNA damaging agents results in the formation of an equally wide variety of damage products. Although DNA damage is often associated with mutagenesis, the actual biological consequences of these damaged products depends on the chemical nature of the lesion. For example, the widely used artificial mutagen,
ethyl methane sulfonate (EMS) can donate its alkyl group with vary-ing efficiencies to almost all of the nitrogen and oxygen atoms present on all of the bases1. Its most frequently induced product, N7
-alkylguanine, is also its most innocuous; this lesion apparently has no biological consequences, and behaves as a proper guanine both in terms of its ability to form base pairs and its accuracy in being read as a ‘G’. In contrast, the second most frequently in-duced damage product, N3
-alkyladenine, is highly toxic; because it is incapable of being recognized at all by DNA polymerase it acts as a block to DNA replication. This is in contrast to yet another alkyl-ated base, O6-alkylguanine, which base pairs very efficiently during replication, but with poor accuracy; DNA polymerase is as likely to insert a T as a C opposite this lesion. The consequence of this ten-dency of O6
-alkylguanine to mispair is readily visible in the dozen or so EMS-induced alleles that have been sequenced from plants:
Susan J. Lolle*and Robert E. Pruitt are at the Dept of Molecular and Cellular Biology, Harvard University, 16 Divinity Avenue, Cambridge, MA 02138, USA.
*Author for correspondence (tel 11 617 495 0568; fax 11 617 496 6702; e-mail [email protected]).
Molecular genetics of DNA repair in
higher plants
Anne B. Britt
all of these mutations are point mutations involving the transition of G to A. Thus, a single DNA damaging agent can produce lesions that are inconsequential, mutagenic (due to mispairing), or cyto-toxic (due to the blockage of transcription or DNA replication).
Of course, the toxicity or mutagenicity of any particular lesion also depends on the efficiency with which it is eliminated by the cell. All organisms employ a wide variety of strategies to either reverse, excise, or tolerate the persistence of DNA damage products. Even the extremely compact genomes of bacteriophage, where space is at a premium, often encode a repair enzyme. Some of these damage resistance mechanisms are ancient and almost ubiquitous, whereas others seem to be of more recent origin. Although repair and damage tolerance mechanisms have been thoroughly described in E. coli,
Saccharomyces cerevisiae, humans and rodents, remarkably little is
known about these processes in plants. However, in recent years, in-creasing interest in the effects of enhanced UV-B radiation has pro-vided a lot of information on the repair of UV-induced pyrimidine dimers. Similarly, interest in the development of genetic engineering techniques with which to manipulate the plant genome, has resulted in promising advances in research into the repair of double strand breaks and homologous recombination. The potential ‘applications’ of these DNA repair processes have resulted in additional investi-gations into interesting and more basic areas including the mecha-nisms of mutagenesis, the control of the cell cycle by DNA damage, and the developmental and environmental regulation of repair.
Recent developments in the field of DNA repair are described be-low, with particular emphasis on the molecular genetics of repair. In order to be concise, some aspects of repair, and the entire field of damage tolerance (the surface of which has hardly been scratched in plants) have been omitted and the reader is referred to comprehen-sive reviews of this field2–4
, and reviews of the physiological effects of UV radiation on plants5,6.
Repair of UV-induced DNA damage
UV radiation induces a degree of oxidative damage (pyrimidine hydrates) and crosslinks (both DNA–protein and DNA–DNA). How-ever, the predominant, and probably most significant, lesions are various types of pyrimidine dimers. Cyclobutane pyrimidine dimers (CPDs) make up the bulk of the damage (perhaps 75%, depending on the sequence context), and pyrimidine [6-4]pyrimidinone dimers (known as 6-4 products) make up the rest (Fig. 1). Both classes of dimers act as blocks to transcription in mammalian cells, and in-hibit DNA replication in bacteria and eukaryotes. Pyrimidine dimers are also premutagenic lesions: C-containing dimers are subject to conversion to TT mutations via a process termed ‘dimer bypass’. The existence of this process has yet to be established in plants, and little is known about the spectrum of mutations induced by UV radiation in plants.
Because of their effect on transcription, the persistence of pyri-midine dimers is highly toxic, and a mechanism for their efficient re-moval, even from nonreplicating, terminally differentiated somatic cells, might be regarded as an essential function for any living or-ganism that is exposed to sunlight. This is especially true for plants, because as obligate phototrophs there is no natural environment in which visible light is not accompanied by UV radiation, and no UV-absorbing pigment can absorb 100% of the incident radiation. There are two major categories of mechanisms for the repair of DNA damage: the damage can be directly reversed, or the damage can be excised from the genome, and the resulting gap repaired using the undamaged strand as a template. In the case of pyrimi-dine dimers, both classes of mechanisms exist in most organisms, and plants, not unexpectedly, are able to repair dimers via both mechanisms.
Photolyases and photoreactivation (live by the sword, die by the sword)
Just as there is no natural environment in which plants can enjoy visible radiation without having to endure UV radiation, so UV radi-ation never naturally occurs without accompanying visible light. It has long been observed (in microbial systems) that the toxic and mutagenic effects of UV radiation can be reversed by subsequent exposure to radiation in the 360–420 nm range (UV-A to blue). This phenomenon is termed ‘photoreactivation’ and is due to the ac-tions of one or more proteins termed ‘photolyases’. These enzymes specifically recognize and bind to pyrimidine dimers. The enzymes carry two chromophores. The first is a flavin cofactor (FADH2), which acts as a transient electron donor to reverse the crosslink be-tween the bases. The second chromophore, which acts as an an-tenna pigment to excite the electron donor, is of varying chemical composition in various species, and largely determines the action spectrum of the enzyme7
. Through the action of photolyases, pyri-midine dimers can be directly reversed, in an error-free fashion, to pyrimidine monomers without excision of the damaged bases. Photolyases are one of the handful of examples of repair enzymes that are at once very simple, efficient and error free, because of their ability to reverse rather than excise damage. In addition, photolyases (as with all damage-reversal enzymes) are extremely specialized in terms of their substrate-specificity.
Until the early 1990s only a handful of microbial CPD-specific photolyases had been identified and characterized, either geneti-cally or biochemigeneti-cally. The last decade has seen the discovery of several new classes of photolyases and related enzymes as a con-sequence of studies of this protein in a wider range of species. The first higher eukaryotic photolyase, a CPD-specific enzyme, was cloned from goldfish (Carassius auratus) and found to have a sequence that is related to, but highly diverged from, the microbial genes (Fig. 2). The two classes of CPD photolyases share only 10–15% sequence identity8. Homologs of this so-called ‘metazoan’, or ‘class II’ CPD photolyase were subsequently identified in insects, several vertebrates (including marsupials, although not placental mammals), some archaebacteria, some eubacteria and Arabidopsis. The Arabidopsis homolog of this class II photolyase sequence (PHR1) corresponds to a gene (UVR2) that was identified via classical genetic analysis as being required for photoreactivation of CPDs in vivo9,10
.
At the same time, there was the unexpected discovery of a class of photolyases specific for 6-4 photoproducts. First observed bio-chemically in extracts from Drosophila11, the repair activity was also detected in Arabidopsis in vivo12
. Arabidopsis mutants defec-tive in this activity (uvr3 mutants) were isolated, and the UVR3 gene was shown to correspond to the Arabidopsis homolog of sequences encoding a 6-4 photolyase in other species, including Fig. 1. Two common UV-induced photoproducts: a cyclobutane
pyrimidine (in this example T-T) dimer and a pyrimidine [6-4] pyrimidinone (in this example T-C) dimer. The dimers are formed between adjacent (59and 39) bases on the same DNA strand.
HN
N O
O
CH3
HN
N O
O
CH3 HN
N O
CH3
NH2
O N
N O H3C
Drosophila and Xenopus13
. The 6-4 photolyase is clearly related in sequence to the CPD photolyases, and in fact is more closely related to the class I ‘microbial’ CPD photolyase sequence than the class II CPD photolyase (Fig. 2).
The genomes of Chlamydomonas and Arabidopsis encode yet another type of class I photolyase homolog. These homologs (CRY1/HY4 and CRY2/PHH1) are not involved in DNA repair but instead play a role in blue-light perception14. Interestingly, the hu-man and mouse genomes also encode two 6-4 photolyase homo-logs15. However, these proteins lack any repair activity in vitro, and expression studies suggest that the genes, hCRY1 and hCRY2, are involved in circadian rhythm entrainment16.
In summary, the family of photolyase-related proteins is a very ancient one. The duplication of the CPD photolyase to produce both class I and class II CPD photolyases may have occurred be-fore the archaebacterial/eubacterial split (examples of both classes of photolyases can be observed in both kingdoms of bacteria, although this might also be an example of horizontal gene trans-fer). Similarly, the 6-4 photolyase is not a recent development, but is observed in both the plant and animal kingdoms. The early evo-lution of repair activity is not particularly surprising, if one bears in mind that the Earth’s early atmosphere had a very low oxygen content17
, resulting in a much higher level of UV radiation at the Earth’s surface. In this remarkably harsh environment, nucleic-acid based life was probably only possible in well-protected en-vironments (i.e. under a murky water column). The evolution of photolyases may have been an important step on the path to phototrophy.
‘Dark’ repair of pyrimidine dimers
Most organisms possess both substrate-specific repair mechanisms and a more general pathway, termed nucleotide excision repair (NER). This mechanism involves the recognition, with varying efficiencies, of a very broad range of DNA damage prod-ucts, including pyrimidine dimers (Fig. 3). The damaged DNA strand is then nicked 59
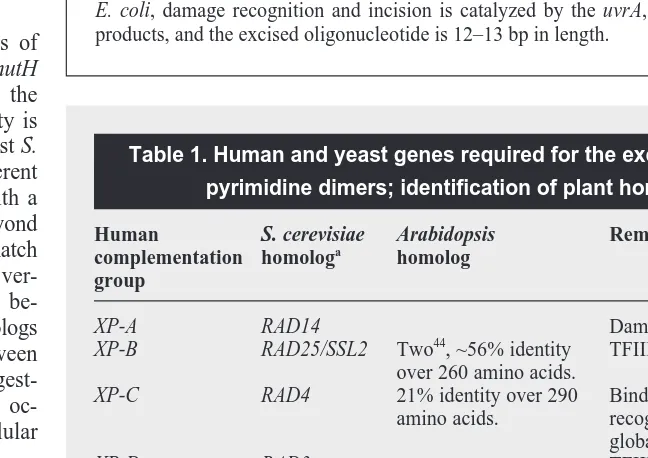
and 39of the damage product, the oligonu-cleotide removed, and the undamaged strand used as a template to restore the original sequence. This pathway is fairly error-free, although it is of course subject to the same sort of errors inherent in any DNA repli-cation process. In human cells, NER is an essential repair process (the existence of a CPD photolyase in human cells is still a subject of debate), as shown by the ex-treme UV-sensitivity of individuals afflicted with Xeroderma pigmentosum (XP), a heri-table disease affecting nucleotide excision repair. In contrast, NER probably only acts as a minor pathway for dimer repair in or-ganisms that possess functional photolyases (although it may be essential for the repair of rare DNA damage products that lack a dedicated repair pathway). Most of the genes (approximately a dozen) required for NER in mammals and yeast have been identi-fied via classical genetics, cloned and se-quenced18. There is clear homology between the fungal and animal genes. Although bac-teria possess a functionally similar, but less complex, repair pathway, the homology does not extend to the DNA sequence level. Plants are capable of repairing UV-induced dimers in the dark, indicating that some sort of repair pathway exists in addition to photolyases3,19,20
. Classical genetic analysis has resulted in the identification of at least four complementation groups required for this repair in Arabidopsis (UVR1, UVR5, UVR7, and UVH1)21,22
, and many more UV-sensitive mutants await further genetic and pheno-typic characterization2
. The classical genetics of the dark repair of dimers remains unsaturated (i.e. few representatives of each comple-mentation group have been identified), suggesting that additional complementation groups exist. Some of these mutations result in a gamma-radiation sensitive phenotype, as well as UV-sensitivity, indicating that this repair pathway recognizes a variety of substrates. All of this is consistent with the existence of some sort of NER path-way in plants. A search of GenBank, using the human XP genes as probes, reveals several very convincing homologs of these excision repair genes (Table 1). Furthermore, a homolog of the NER endo-nuclease, ERCC1, was recently cloned from Lilium longiflorum. When expressed in ERCC1 deficient CHO cells, this gene was able to enhance the cells’ resistance to the crosslinking agent mito-mycin C (Ref. 23). Thus the gene almost certainly plays a similar role in DNA repair in plants.
Homologs of a second gene, RAD23, which influences the rate of NER in yeast but is not absolutely required for repair24
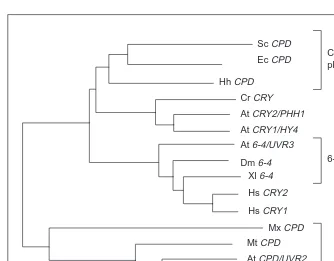
, have been identified in several plant species, and in one case shown to partially complement the UV-sensitive phenotype of yeast rad23 mutants25. It remains to be seen whether any of these excision re-pair gene homologs are able to complement the rere-pair deficiency of the uvr/uvh mutants isolated in Arabidopsis, but it appears likely that Fig. 2. Phylogenetic analysis of photolyase homologs. The data presented is from Ref. 13:
their tree was generated by the ODEN software package (National Institute of Genetics, Japan). Labeling of genes as ‘photolyases’ is based either on their ability to encode a func-tional photolyase on expression in E. coli, or genetic analysis of their role in vivo. Genes labeled ‘CRY’ (crytochrome) are known (via genetic analysis) or suspected to encode photoreceptors. Abbreviations: Sc, Saccharomyces cerevisiae; Ec, Escherichia coli; Hh, Halobacterium halobium; Cr, Chlamydomonas reinhartii; At, Arabidopsis thaliana; Dm, Drosophila melanogaster; Xl, Xenopus laevis; Hs, Homo sapiens; Mx, Myxococcus xanthus; Mt, Methanobacterium thermoautotrophicum; Ca, Carassius auratus; Pt, Potorous tridactylus.
6-4 photolyase
Class II CPD photolyase Class I CPD photolyase Sc CPD
Ec CPD
Hh CPD
Cr CRY
At CRY2/PHH1
At CRY1/HY4
At 6-4/UVR3
Dm 6-4 Xl 6-4
Hs CRY2
Hs CRY1
Mx CPD
Mt CPD
At CPD/UVR2
Dm CPD
Ca CPD
the ‘dark’ repair of UV-induced dimers ob-served in plants is driven by a mechanism directly related to NER in other eukaryotes.
Mismatch repair
Mismatched bases, regularly produced through errors of replication or through homologous recombination, pose a unique problem to the repair systems of the cell4
. Both bases are legitimate and neither is a rec-ognizable DNA damage product. Although it is easy to conceive of enzymes that can recognize mispaired bases (and the enzymes involved in NER can do this to some extent) the ‘trick’ to mismatch repair is the dis-crimination between the correct and incor-rect base. In E. coli this problem is solved by methylating specific sequences (G5mATC) in its genome at some time after DNA replication. The original template strands (presumably correct in sequence) can then, for a brief interval, be distinguished from newly synthesized (error-prone) unmethyl-ated strands. Once a mismatch is recognized by the MutS protein, the MutL protein binds to the complex and helps to promote an interaction between the complex and the MutH protein, an endonuclease which lo-cates the nearest unmethylated GATC and guides the degradation of the unmethylated strand from the GATC to the mismatched base. The original template strand can then be replicated to fill the gap.
Eukaryotes possess multiple copies of obvious mutS and mutL homologs. No mutH homologs have been identified, and the mechanism by which strand-specificity is generated is unclear. The budding yeast S.
cerevisiae possesses at least six different mutS homolog (MSH) genes, each with a
specialized function extending well beyond the original function of MutS as a mismatch repair protein. Several mammalian ver-sions of MSH also exist. Similarities be-tween the human and yeast MSH2 homologs are greater than the similarities between the various yeast MSH homologs, suggest-ing that the duplication of this locus oc-curred before the evolution of multicellular organisms26.
Both mutS homologs and mutL homologs have been found to play an important role in the prevention of somatic mutagenesis; hu-mans defective in some of these genes have a heritable predisposition to certain types of cancers. Other MSH and MLH genes are required for meiosis. In addition, mutS,
mutL and some of their eukaryotic
homo-logs have been found to have a powerful anti-recombinogenic effect on homeologous (similar, but not identical) sequences in bac-teria, yeast, and mammals, without affecting recombination between identical stretches of DNA27. It is possible that mismatch repair
Fig. 3. Nucleotide excision repair. DNA damage is excised as an oligonucleotide. In yeast
and mammals damage recognition and incision requires the coordinate actions of approxi-mately 11 gene products and the excision tracts range in size from 23 to 32 nucleotides. In E. coli, damage recognition and incision is catalyzed by the uvrA, uvrB, and uvrC gene products, and the excised oligonucleotide is 12–13 bp in length.
Recognition and incision enzymes
DNA polymerase, ligase Helicases
Table 1. Human and yeast genes required for the excision repair of pyrimidine dimers; identification of plant homologs
Human S. cerevisiae Arabidopsis Remarks/function complementation homologa
homolog group
XP-A RAD14 Damage recognition.
XP-B RAD25/SSL2 Two44, ~56% identity TFIIH subunit. over 260 amino acids.
XP-C RAD4 21% identity over 290 Binds ssDNA, damage
amino acids. recognition, required for global repair only.
XP-D RAD3 TFIIH subunit.
XP-F RAD1 39% identity over 1100 5′endonuclease. amino acids.
XP-G RAD2 3′endonuclease.
CS-A RAD28 Required for transcription-coupled repair only. CS-B RAD26 Required for
transcription-coupled repair only. ERCC-1 RAD10 Lily homolog23, 5′endonuclease.
53% identity over 216 amino acids.
a
homologs might determine the degree of sequence identity required for meiotic recombination: there is evidence to this effect in MSH2 and PMS1 (a mutL homolog) mutants of yeast28
. If mismatch repair proteins play a similar role in inhibiting recombination between diverged sequences in plants and animals, the study of mismatch repair proteins in plants has obvious applications to plant breed-ing, as well as research into speciation.
Although several research groups have informally reported the cloning of plant mismatch repair gene homologs, to date only one sequence has been published, an MSH homolog from Arabidopsis26. Sequence analysis indicates that this is an MSH2 homolog. In both humans and yeast, the MSH2 gene product acts as a heterodimer with MSH3 or MSH6 gene products to recognize and correct mis-matched bases. It also serves to inhibit both meiotic and mitotic recombination between diverged sequences in yeast. The function of this gene in plants has yet to be determined.
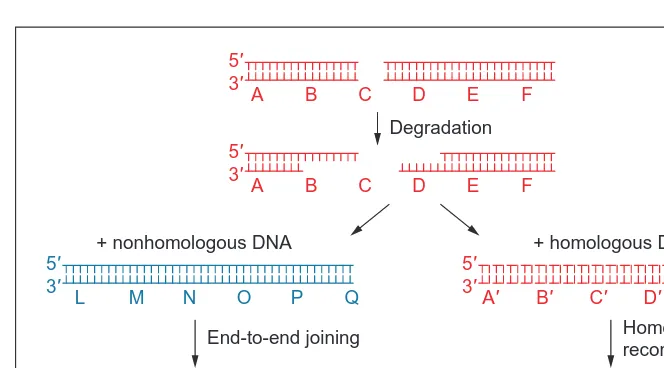
Repair of double strand breaks
Double strand breaks (DBSs) can occur spontaneously in the cell as a result of a nick in a single stranded region, due to mechanical stress of a chromosome, or as part of the initiation of crossing over during meiosis4. Artificial sources of DSBs include ionizing radi-ation, certain radiomimetic chemicals, and transgenic DNAs. There are two basic classes of pathways for the repair of double strand breaks (DSBs; Fig. 4). The first, repair via homologous recombi-nation, uses a gene conversion mechanism to replace the break with intact sequence from a sister chromatid, homolog or other identical (or nearly identical) sequence. The second mechanism, nonhomolo-gous end-joining (NHEJ), involves the resealing of two broken ends. Although there are often ‘microhomologies’ of two to five bases at the resealed joint, these sequences probably only act to stabilize the ligation reaction, and do not reflect the kind of true homology search involved in homologous recombination. Because there is no mechanism to ensure the pairing of the two original chromosome ends, NHEJ can produce chromosomal inversions, deletions, trans-locations, and partial duplications. Although all organisms in which DSB repair has been studied have been found to possess both repair mechanisms, the rate at which one or the other mechanism is actually employed varies widely. As a general rule, organisms with relatively compact genomes (bacteria and yeast) will virtually always repair
breaks via homologous recombination, pro-vided sufficient homology is available. Or-ganisms with larger genomes will virtually always repair breaks via NHEJ, regardless of how much homology is provided. There are notable exceptions to this rule. For ex-ample, mouse embryonic stem cells will integrate homologous transgenes at their homologous chromosomal locus with good efficiency; this capacity for gene replace-ment has spawned a minor industry for the generation of ‘knockout mice’.
Analysis of the products of double strand break repair can provide clues as to the mechanism of repair. In plants, these ‘prod-ucts’ have largely been characterized as ex-cision products of transposable elements, or insertion products of T-DNAs29–31. The classification of these molecules as the products of DSB repair involves certain as-sumptions and inherent logical circularities (i.e. transposable element excision products look like mammalian DSB repair products, therefore they are the products of DSB re-pair, therefore plant DSB repair is similar to that in mammals). More recently, experiments involving the creation and healing, via NHEJ, of true DSBs at predetermined chromosomal loci (using unique restriction sites, with transient introduction of the restric-tion enzyme32,33
, or by sequencing of repaired lineared plasmids34 ) have confirmed that the kinds of products observed as a result of T-DNA integration or TE excision do indeed resemble genuine plant DSB repair products in many ways:
• Some degradation of the broken ends usually occurs before ligation, resulting in a loss of information.
• Microhomologies are generally utilized during NHEJ. • ‘Filler’ DNAs are often integrated into the break. These may be
recognizable sequences from elsewhere in the genome, dupli-cations or inversions of local sequences, or simple sequences that seem to be generated at random.
An interesting and important question is whether the majority of genuine chromosomal DSBs are repaired via NHEJ, rather than through homologous recombination. There is some difficulty in comparing the actual rate of homologous versus nonhomologous re-pair of DSBs, because, unfortunately, experiments are set up to res-cue only those events that regenerate a selectable marker (through homologous recombination) or only those events that destroy a counterselectable marker (through NHEJ). Experiments have not been performed that directly compare the relative rates of the two kinds of events. Surveys of T-DNA integration events and TE exci-sion products certainly suggest that in higher plants, as in mammals, breaks are repaired by nonhomologous recombination far more frequently than via homologous recombination. In Arabidopsis, T-DNAs integrate at random positions: the ratio of gene replace-ment events to random insertion events is approximately 1:1000 (Ref. 35); in tobacco this ratio is closer to 1:10 000 (Ref. 36). Sur-prisingly, even in experimental designs in which the repair of one end of a linear transgene is ‘forced’ to occur via homologous recom-bination (through selection for the reconstitution of a fragmented drug resistance marker), the remaining end is often (in about one out of four cases) repaired nonhomologously, in spite of the proxim-ity of homologous sequences32
. This suggests that even in a case where the search for homology is greatly simplified, the enzymes involved in NHEJ often ‘out compete’ those required for homolo-gous recombination in the race to process the double strand break. Fig. 4. Double strand break repair. Repair can occur via two independent pathways.
Homologous recombination restores the original sequence, whereas nonhomologous end joining (NHEJ) produces chromosomal deletions, translocations, and inversions. Evidence from transformed plants suggests that the majority of transformed DNAs are integrated via NHEJ. However, plants are capable of repairing double strand breaks via either pathway.
Degradation
+ homologous DNA + nonhomologous DNA
5′ 3′
A B C D E F
5′ 3′
A B C D E F
End-to-end joining
5′ 3′
L M N O P Q
5′ 3′
A′ B′ C′ D′ E′ F′
5′ 3′
A B C:N O P Q
5′ 3′
A′ B′ C′ D′/D E F
Homologous recombination
Very few of the plant genes involved in DSB repair have been identified. Homologs of the recA genes (DMC1/LIM15, RAD51)37–39 have been cloned from plants, and the L. longiflorum homologs lo-calized to meiotic prophase chromosomes40, but their activity has not been determined through biochemical or mutational analysis. Mutants sensitive to ionizing radiation41,42have been isolated, but the genes involved have not yet been cloned. Some of these mu-tants exhibit altered meiotic and extrachromosomal recombination rates, suggesting that the genes are involved in either homologous recombination or NHEJ (Ref. 43).
The rate of meiotic recombination, and the relative rates of gene replacement versus gene addition events, are governed by the enzymology of the pathways that are involved in these processes. An understanding of these mechanisms, and the identification of the genes involved, would enable us to truly ‘engineer’ the plant genome, rather than mutating or transforming plants in the essen-tially random manner employed today.
Acknowledgements
Work cited from the author’s lab was supported by USDA NRICGP grant 94-37301-0564 and NSF grant 90-19159.
References
1 Lawley, P. (1974) Some chemical aspects of dose-response relationships in
alkylation mutagenesis, Mutat. Res. 23, 283–295
2 Vonarx, E.J. et al. (1998) DNA repair in higher plants, Mutat. Res. 400, 187–200 3 Britt, A. (1998) DNA repair in higher plants, in DNA Damage and Repair
(Vol. 1) (Nickoloff, J. and Hoekstra, M., eds), pp. 577–595, Humana Press
4 Friedberg, E.C., Walker, G.C. and Siede, W. (1995) DNA Repair and
Mutagenesis, ASM Press
5 Rozema, J. et al. (1997) UV-B as an environmental factor in plant life: stress
and regulation, Trends Ecol. Evol. 12, 22–28
6 Jansen, M., Gaba, V. and Greenberg, B. (1998) Higher plants and UV-B
radiation: balancing damage, repair and acclimation, Trends Plant Sci. 3, 131–135
7 Sancar, A. (1994) Structure and function of DNA photolyase,Biochemistry 33, 2–9 8 Yasui, A. et al. (1994) A new class of DNA photolyases present in various
organisms including aplacental mammals, EMBO J. 13, 6143–6151
9 Ahmad, M. et al. (1997) An enzyme similar to animal type II photolyases
mediates photoreactivation in Arabidopsis, Plant Cell 9, 199–207
10 Jiang, C-Z. et al. (1997) Photorepair mutants of Arabidopsis, Proc. Natl. Acad.
Sci. U. S. A. 94, 7441–7445
11 Todo, T. et al. (1993) A new photoreactivating enzyme that specifically
repairs ultraviolet light-induced (6-4) photoproducts, Nature 361, 371–374
12 Chen, J-J., Mitchell, D. and Britt, A.B. (1994) A light-dependent pathway for
the elimination of UV-induced pyrimidine (6-4) pyrimidinone photoproducts in Arabidopsis thaliana, Plant Cell 6, 1311–1317
13 Nakajima, S. et al. (1998) Cloning and characterization of a gene (UVR3)
required for photorepair of 6-4 products in Arabidopsis thaliana, Nucleic Acids Res. 26, 638–644
14 Cashmore, A. (1997) The cryptochrome family of photoreceptors, Plant Cell
Environ. 20, 764–767
15 Hsu, D.S. et al. (1996) Putative human blue-light photoreceptors hCRY1 and
hCRY2 are flavoproteins, Biochemistry 35, 13871–13877
16 Miyamoto, Y. and Sancar, A. (1998) Vitamin B-2-based blue-light
photo-receptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals, Proc. Natl. Acad. Sci. U. S. A. 95, 6097–6102
17 Caldwell, M. (1979) Plant life and ultraviolet radiation: some perspective in
the history of the earth’s UV climate, BioScience 29, 520–525
18 Sancar, A. (1996) DNA excision repair, Annu. Rev. Biochem. 65, 43–81 19 Britt, A.B. et al. (1993) A UV-sensitive mutant of Arabidopsis defective in the
repair of pyrimidine-pyrimidinone (6-4) dimers, Science 261, 1571–1574
20 Quaite, F.E. et al. (1994) DNA damage levels determine cyclobutyl pyrimidine
dimer repair mechanisms in alfalfa seedlings, Plant Cell 6, 1635–1641
21 Jenkins, M.E. et al. (1995) Radiation-sensitive mutants of Arabidopsis
thaliana, Genetics 140, 725–732
22 Jiang, C-Z. et al. (1997) UV- and gamma-radiation sensitive mutants of
Arabidopsis, Genetics 147, 1401–1409
23 Xu, H. et al. (1998) Plant homologue of human excision repair gene ERCC1
points to conservation of DNA repair mechanisms, Plant J. 13, 823–829
24 Mueller, J.P. and Smerdon, M.J. (1996) Rad23 is required for
transcription-coupled repair and efficient overall repair in Saccharomyces cerevisiae, Mol. Cell. Biol. 16, 2361–2368
25 Sturm, A. and Lienhard, S. (1998) Two isoforms of plant RAD23 complement
a UV-sensitive rad23 mutant in yeast, Plant J. 13, 815–821
26 Culligan, K.M. and Hays, J.B. (1997) DNA mismatch repair in plants. An
Arabidopsis thaliana gene that predicts a protein belonging to the MSH2 subfamily of eukaryotic mutS homologs, Plant Physiol. 115, 833–839
27 Modrich, P. and Lahue, R. (1996) Mismatch repair in replication fidelity,
genetic recombination, and cancer biology, Annu. Rev. Biochem. 65, 101–133
28 Hunter, N. et al. (1996) The mismatch repair system contributes to meiotic
sterility in an interspecific yeast hybrid, EMBO J. 15, 1726–1733
29 Scott, L., LaFoe, D. and Weil, C.F. (1996) Adjacent sequences influence DNA
repair accompanying transposon excision in maize, Genetics 142, 237–246
30 Ohba, T. et al. (1995) DNA rearrangement associated with the integration of
T-DNA in tobacco: an example for multiple duplications of DNA around the integration target, Plant J. 7, 157–164
31 Takano, M. et al. (1997) The structures of integration sites in transgenic rice,
Plant J. 11, 353–361
32 Puchta, H., Dujon, B. and Hohn, B. (1996) Two different but related
mechanisms are used in plants for the repair of genomic double-strand breaks by homologous recombination, Proc. Natl. Acad. Sci. U. S. A. 93, 5055–5060
33 Salomon, S. and Puchta, H. (1998) Capture of genomic and T-DNA sequences
during double-strand break repair in somatic plant cells, EMBO J. 17, 6086–6095
34 Gorbunova, V. and Levy, A.A. (1997) Non-homologous DNA end joining in
plant cells is associated with deletions and filler DNA insertions, Nucleic Acids Res. 25, 4650–4657
35 Kempin, S. et al. (1997) Targeted disruption in Arabidopsis, Nature 389, 802–803 36 Paszkowski, J. et al. (1988) Gene targeting in plants, EMBO J. 7, 4021–4026 37 Kobayashi, T., Hotta, Y. and Tabata, S. (1993) Isolation and characterization
of a yeast gene that is homologous with a meiosis-specific cDNA from a plant, Mol. Gen. Genet. 237, 225–232
38 Klimyuk, V. and Jones, J. (1996) AtDMC1, the Arabidopsis homologue of the
yeast DMC1 gene: characterisation, transposon-induced allelic variation and meiosis-specific expression of a pAtDMC1:GUS fusion, Plant J. 11, 1–14
39 Doutriaux, M-P. et al. (1998) Isolation and characterisation of the RAD51 and
DMC1 homologs from Arabidopsis thaliana, Mol. Gen. Genet. 257, 283–291
40 Terasawa, M. et al. (1995) Localization of RecA-like recombination proteins
on chromosomes of the lily at various meiotic stages, Genes Dev. 9, 925–934
41 Davies, C. et al. (1994) Isolation of Arabidopsis thaliana mutants
hypersensitive to gamma radiation, Mol. Gen. Genet. 243, 660–665
42 Masson, J., King, P. and Paszkowski, J. (1997) Mutants of Arabidopsis
thaliana hypersensitive to DNA damaging treatments, Genetics 146, 401–407
43 Masson, J. and Paszkowski, J. (1997) Arabidopsis thaliana mutants altered in
homologous recombination, Proc. Natl. Acad. Sci. U. S. A. 94, 11731–11735
44 Ribeiro, D.T. et al. (1998) Cloning of a cDNA from Arabidopsis thaliana
homologous to the human XPB gene, Gene 208, 207–213
Anne B. Britt is at the Section of Plant Biology, University of California, Davis, CA 95616, USA (tel 11 530 752 0699; fax 11 530 752 5410; e-mail [email protected]).
Congratulations!
![Fig. 1. Two common UV-induced photoproducts: a cyclobutanepyrimidine (in this example T-T) dimer and a pyrimidine [6-4]pyrimidinone (in this example T-C) dimer](https://thumb-ap.123doks.com/thumbv2/123dok/1038377.929738/2.858.44.293.80.179/common-induced-photoproducts-cyclobutanepyrimidine-example-pyrimidine-pyrimidinone-example.webp)