Photosynthesis and antioxidant enzymes of phyllodes of
Acacia
mangium
Hua Yu, Bee-Lian Ong *
Department of Biological Sciences,National Uni6ersity of Singapore,Lower Kent Ridge Road,Singapore119260,Singapore
Received 11 January 2000; received in revised form 22 June 2000; accepted 3 July 2000
Abstract
Physiological processes are influenced by environmental factors and plant characteristics. The distribution of photosynthetic capacity of phyllodes ofAcacia mangiumWilld. seedlings was studied in relation to the in vivo photosystem II (PSII) function, photosynthetic gas exchange, chlorophyll fluorescence and activities of antioxidant enzymes (superoxide dismutase (SOD) and ascorbate peroxidase (APX)) of phyllodes at different positions on seedlings. There was a vertical gradient in photosynthetic capacity of phyllodes along the shoot. Phyllode 1 (at the apex) showed negative carbon uptake at PPFD lower than 400mmol m−2s−1. High photosynthetic capacities, chlorophyll concentrations,DF/F%m, andq
Pwere observed in phyllodes 4, 6 and 8. The high photosynthetic capacities of mature phyllodes could be attributed to the enhanced availability of CO2and the high efficiency of PSII in energy absorption and utilization. Total SOD and APX activities (on a dry weight basis) were highest at phyllode 1 and decreased as the phyllodes matured. The high photosynthetic capacity and low respiration loss in mature phyllodes could be important factors, responsible for the rapid establishment and fast growth of A.mangium in reforestation programs. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Acacia mangium; Ascorbate peroxidase; Chlorophyll fluorescence; Photosynthesis; Phyllode position; Superoxide dismutase
www.elsevier.com/locate/plantsci
1. Introduction
Leaves undergo many anatomical, physiological and metabolic changes during development. Indi-vidual leaves, located at different heights of a plant, contribute differently to the photosynthetic production of the whole plant. The rate of photo-synthesis in teak (Tectona grandis) was low in immature leaves, peaked in third or fourth leaves (from shoot apices) and declined in lower leaves [1]. In Acacia auriculiformis, the maximum rate of CO2 assimilation was observed in the eighth leaf
(from the shoot apex) and the changes in the rate
of photosynthetic CO2assimilation paralleled that
of stomatal conductance [2]. Light-saturating rates of photosynthetic O2 evolution of leaves of
Chenopodium album decreased more steeply with
decreasing leaf position than the chloroplast num-ber per cell; the loss of photosynthesis in lower leaves was ascribed both to the decrease in chloro-plast number and the reduced photosynthetic ca-pacity of the remaining chloroplasts [3].
Reactive oxygen species (ROS) are formed in leaves during the whole ontogenetic process and in response to stress [4], more so in immature leaves, as the lack of photosynthesis could result in a smaller sink for energy. Both enzymatic and non-enzymatic defense systems in plants have evolved to suppress the production and removal of such toxic active molecules [5 – 7]. Enzymatic defense systems in plants involve the action of superoxide dismutase (SOD), peroxidases including ascorbate peroxidases (APX) and reductases. Both
chloro-Abbre6iations: APX, ascorbate peroxidase; DF/F%m, effective PSII efficiency under light-adapted conditions; Fv/Fm, maximal potential photochemical efficiency of PSII under dark-adapted conditions; 1/Fo– 1/Fm, the excitation energy trapping capacity at the reaction center of PSII; PPFD, photosynthetic photon flux density;qP, photo-chemical quenching; NPQ, non-photophoto-chemical quenching.
* Corresponding author. Fax: +65-7792486.
E-mail address:[email protected] (B.-L. Ong).
plastic and cytoplasmic compartments of leaves contain these enzymes. The two important antioxi-dant enzymes in chloroplasts are SOD and APX, which allow the efficient detoxification of ROS generated from photooxidation [7]. Though many studies have focused on the antioxidant responses of plants to environmental stresses [4], not much work has been devoted to study such changes in relation to leaf development.
In the present study, the photosynthetic perfor-mance and antioxidant responses of phyllodes (ex-panded petioles that form simple, photosynthezing laminae [8]) of 220 day-old A. mangium Willd. seedlings were investigated. The aims of this study were to understand the distribution of phyllode photosynthetic capacity in seedlings and its rela-tionship with the in vivo PSII (photosystem II) function and antioxidant response. A. mangium is a fast growing tree, with an increasingly important role in reforestation programs. This study should enable an understanding of the physiological fac-tors that contribute to the rapid growth and estab-lishment of the seedlings.
2. Material and methods
2.1. Growth of seedlings and site description
Seeds of A. mangium were collected from Hainan Province, P.R. China. Seedlings were grown under natural conditions in the Department of Biological Sciences, National University of Sin-gapore. The temperature was 2996°C. Daily at-mospheric relative humidity ranged from 70 to 100%. The daily photosynthetic photon flux den-sity (PPFD) ranged from a minimum of 80 mmol m−2 s−1 at 08:00 and 18:00 h to 1650 mmol m−2
s−1 at 12:00 h.
Seeds were immersed in boiling water for 30 s and then soaked in cold water for 24 h to facilitate uniform germination. Pretreated seeds were sown in vermiculite in plastic pots (diameter 32.5 cm, height 26.0 cm). Three to four seeds were sown in each pot. Plants were kept well watered and were fertilized twice a week with Hoagland’s solution. Experiments were conducted when the plants were 220-days-old and 65 cm tall, on average. All experiments were repeated three times. A total of six seedlings were used for each experiment.
2.2. Position of phyllodes
Seedlings were selected for experiments based on their uniform appearance, in terms of the aver-age height of the plants, the total number of phyllodes present on each plant and the size of emerging phyllodes. The emerging phyllode at the shoot apex, with a length of 5 – 6 cm, was consid-ered as the phyllode at position 1 (phyllode 1). Phyllodes 2, 4, 6, 8, 10 and 12 were labeled in all seedlings, counting down from the top of the plant. Seedlings used for experiments had a total of 13 – 14 phyllodes.
2.3. Determination of photosynthetic pigments
Six discs (diameter 55 mm) were randomly re-moved from each phyllode and were ground with 100% (v/v) acetone. The concentrations of chloro-phylls and carotenoids were determined by the methods of Arnon [9] and Embry and Nothnagel [10], respectively.
2.4. Gas exchange measurements
Changes in photosynthetic CO2 exchange of
phyllodes at different positions (still attached to plants), in response to changes in PPFD levels, were determined with a CIRAS-1 portable differ-ential CO2/H2O infrared gas analyzer connected to
a Parkinson broad leaf cuvette equipped with an automatic light unit (PP Systems, Hitchin, UK). A phyllode area of 2.5 cm2 was enclosed in the leaf
cuvette. Gas exchange rates were determined at an airflow rate of 200 cm3 min−1 with ca. 365 mmol
mol−1 CO
2 in the cuvette and a constant leaf
temperature of 29°C. After the determination of dark respiration rate, the rates of CO2 exchange
over a range of PPFD from 25 to 1400 mmol m−2
s−1 were determined.
2.5. Chlorophyll fluorescence measurements
Chlorophyll fluorescence was determined with a pulse amplitude modulation fluorometer (PAM 101 – 103, Waltz, Effeltrich, Germany); experi-ments were conducted at 25°C. Following a 30-min dark adaptation, Fo was determined at a light
level lower than 0.01mmol m−2s−1. After Fowas
recorded, a 600-ms saturating light pulse (5000
Saturating light was supplied by a Schott flash lamp (KL 1500, Schott, Mainz, Germany). After the measurement of Fo and Fm, the actinic light (300 mmol m−2 s−1) was switched on. To
deter-mine maximum fluorescence (F%m) under
light-satu-rating conditions, a 600-ms saturation light pulse of 5000 mmol m−2 s−1 was switched on at 60 s
intervals. After 30 min, the actinic light was switched off and a far-red light was turned on for the accurate measurement ofF%o. Values of various
chlorophyll fluorescence parameters were calcu-lated according to Van Kooten and Snel [11] and Genty et al. [12].
2.6. Phyllode area and dry weight
After the measurement of chlorophyll fluores-cence, the phyllode was used for the determination of phyllode area and dry weight. For the determi-nation of phyllode area, the outline of each lamina was traced on graph paper and weighed. Phyllode area was calculated according to an area-weight standard curve. The phyllodes were dried in an oven at 80°C for 7 days until dry weight was constant.
2.7. Determinations of enzyme acti6ities and total
soluble protein content
For the determination of total SOD activity, fresh phyllode tissues were removed from the mid-dle portion of each phyllode and ground in a chilled mortar in 50 mM phosphate buffer (pH 7.8), containing 0.1 mM EDTA, for the extraction of total SOD enzyme. Similarly, for the extraction of total APX, phyllode tissues were ground in 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA, 5 mM ascorbate, 0.5% (w/v) PVP (molecu-lar weight 10 000), 0.1% (v/v) Triton X-100 and 0.05% (v/v)b-mercaptoethanol. After centrifuging at 11 700×g at 4°C for 15 min, the supernatant of each extract was used as the crude enzyme extract for determinations of soluble protein con-centration and activities of SOD and APX.
The SOD activities of phyllodes 1, 4, 8 and 12 were assayed by determining the ability of the extracted enzymes to inhibit the photochemical reduction of nitro-blue tetrazolium (NBT) using the method of Beauchamp and Fridovich [13]. The 3-ml reaction mixture contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 56 mM NBT, 13
mM methionine, 0.02 mM NaCN, 1.2 mM ri-boflavin and 15 ml enzyme extract. The reaction was started by illuminating the reaction mixture (PPFD=120mmol m−2 s−1) at 28°C for 15 min.
The absorbance of the reaction mixture at 560 nm was then recorded. One unit of SOD (U) was defined as the amount of SOD that caused 50% inhibition of the photo-reduction of NBT [13].
The APX activities of phyllodes 1, 4, 8 and 12 were determined according to Nakano and Asada [14] by monitoring the rate of ascorbate oxidation at 290 nm (extinction coefficient=2.8 mM−1
cm−1). The 3-ml reaction mixture contained 50
mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM ascorbate, 0.3 mM H2O2 and 40 ml enzyme extract. One unit of APX (U) was defined as the amount of enzyme that oxidized 1
mmol of ascorbate per min at room temperature [14].
The soluble protein concentration was measured by the protein – Coomassie dye binding method using bovine serum albumin as a standard [15].
2.8. Statistical analysis
Each result is presented as the mean9S.E. Multiple analysis of variance (Tukey test, P5
0.05,n=6) was conducted to analyze the effect of leaf position on the different physiological parameters determined.
3. Results and discussion
Besides a vertical gradient in developmental stage of the phyllodes, there was also a vertical gradient in light microclimate from the shoot apex to the base along the stem of each seedling. The level of PPFD incident on the surfaces of phyl-lodes 1 – 8 ranged between 1200 and 1500 mmol
m−2 s−1 on sunny days; it was 400 and 180 in
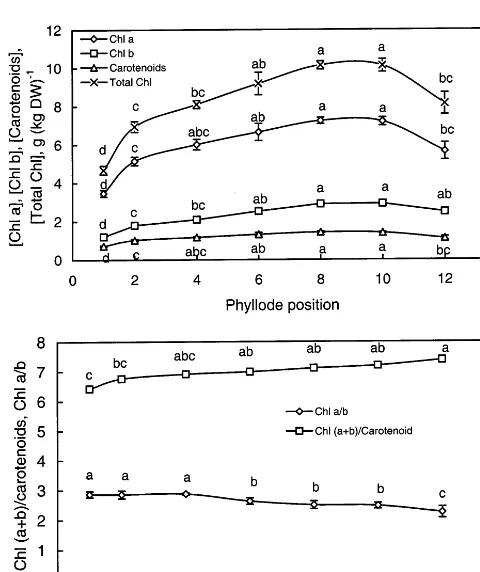
phyllodes 10 and 12, respectively. This probably contributed to the decrease in chlorophyll a/b ratio of the phyllodes (Fig. 1B). A low chlorophyll a/b ratio indicated that the pigment antenna bed for light absorption was enlarged [24]. At the same time, relatively lower chlorophyll/carotenoids ra-tios in phyllodes 1 – 4 indicated that younger phyl-lodes were protected from photodamage by a relatively higher amount of carotenoids [16].
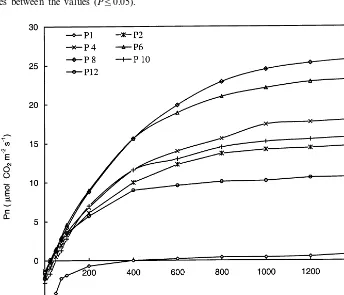
The characteristics of photosynthetic CO2
ex-change of A. mangiumphyllodes at different posi-tions on the stem are shown in Table 1. A set of typical photosynthetic light-response curves of phyllodes at different positions is depicted in Fig. 3. There was a vertical gradient in photosynthetic capacity (the rate of CO2uptake under light
satu-ration) of the phyllodes along the stems of A.
mangium seedlings (Fig. 3, Table 1). The youngest
phyllode (phyllode 1) did not show any positive CO2 uptake until the light supplied was increased
to 400 mmol m−2s−1 (Fig. 3). In phyllode 2, the
rate of photosynthetic CO2uptake was \50% of
that observed in phyllodes 4, 6 and 8 (Fig. 3, Table 1). High rates of light-saturated photosyn-thetic CO2fixation were observed in phyllodes 4, 6
and 8. This, probably, was related to the increased availability of the substrate, CO2, as stomatal
con-ductance of these phyllodes was high (Table 1). The qP value of those phyllodes were also high,
indicating low levels of oxidized PSII reaction centers (high qP) in mature phyllodes (Table 2).
This was accompanied by a low level of energy dissipated as heat (low NPQ) (Table 2). The high photosynthetic capacity of phyllodes 4 – 8 could also be attributed to their higher concentration of chlorophylls and the higher capacity of trapping excitation energy by PSII reaction centers (1/Fo−
1/Fm) (Table 2) [17,18].
The older phyllodes 10 and 12 exhibited positive carbon gain but their rates of photosynthetic CO2
fixation were lower than those of phyllodes 4, 6 and 8 (Table 1). Although the chlorophyll concen-tration of phyllode 10 was higher than that of phyllode 6, its photosynthetic capacity, however, decreased significantly to :36% of that of phyl-lode 6 (Fig. 1, Table 1). Light-saturating rate of photosynthetic CO2 uptake decreased further in
phyllode 12. The low photosynthetic capacity of phyllodes 10 and 12 was accompanied by a re-duced efficiency of PSII function (Fv/Fm) and an Fig. 1. Changes in concentrations of chlorophyll a,
phyll b, total chlorophylls and carotenoids (A), and chloro-phyll a/b ratio and ratio of total chlorophyll to carotenoids (B) in phyllodes at different positions along the stems ofA.
mangium seedlings. Identical letters indicate no significant differences between the values (P50.05). Error bars indicate S.E. (n=6).
Fig. 2. Changes in area ofA.mangiumphyllodes at different positions. Identical letters indicate no significant differences between the values (P50.05). Error bars indicate S.E. (n=
Table 1
Changes in dark respiration rate, apparent quantum yield, rate of photosynthetic CO2uptake, transpiration rate and stomatal conductance ofA.mangiumphyllodes at different positionsa
Rd Pn Gs E
a LCP
Phyllodeposition 1/2LSP
7.8690.59a 0.4490.35c 40.894.8d 1.0190.08c 400.09200a
1 – 450.0950.0a
3.1090.32b 13.2890.70b 213.6915.7bc
59.691.3ab 3.9290.17ab
2 48.695.4b 247.0910.4b
1.7890.40bc 19.9891.39a 261.6935.9ab
4 62.293.1a 4.2490.47a 27.494.0b 261.0911.9b
1.7690.17bc 23.3490.38a 366.6930.7a
64.991.7a 5.1590.22a
6 23.891.7b 296.0915.0b
8 60.792.0a 1.4290.24c 22.4691.23a 310.8925.7ab 5.1090.35a 23.093.0b 301.2911.6b 1.5090.43bc 14.5291.46b 228.6935.6bc
50.192.5b 3.9290.45ab
10 25.096.2b 266.2925.9b
0.8690.14c 12.1090.78b
12 25.592.5c 122.8910.1cd 2.5890.17b 30.894.7b 226.7931.8b
aThe latter three parameters were determined under light-saturating conditions with a PPFD of 1400mmol quanta m−2s−1 (data presented as means9S.E.,n=6).a, apparent quantum yield (mmol CO2(mol quanta)−1); Rd, dark respiration rate (mmol CO2 m−2 s−1); Gs, stomatal conductance (mmol H2O m−2 s−1); E, transpiration rate (mmol H2O m−2 s−1); Pn, rate of photosynthetic CO2uptake (mmol CO2m−2s−1); LCP, light compensation point (mmol m−2s−1); 1/2LSP, PPFD level at which photosynthesis was 50% of the maximum rate (mmol quanta m−2 s−1). Identical letters within the same column indicate no significant differences between the values (P50.05).
Fig. 3. Typical photosynthetic light-response curves ofA.mangiumphyllodes at different positions (P1, P2, P4, P6, P8, P10 and P12 represent phyllode 1, phyllode 2, phyllode 4, phyllode 6, phyllode 8, phyllode 10 and phyllode 12).
increase in NPQ (Table 2). Total chlorophyll con-centration of phyllode 12 was significantly lower than that of phyllodes 6 – 8 (Fig. 1).
Changes in stomatal conductance and the rate of transpiration of the phyllodes, under light-satu-rating conditions, paralleled those of photosyn-thetic CO2 uptake (Table 1). The rate of
photosynthetic CO2 uptake under light-saturating
conditions was linearly correlated with stomatal conductance (R2=0.92) (Fig. 4). The light
satura-tion point of phyllodes 2 – 10 was :1000 mmol m−2 s−1 (Fig. 3). Typical light saturation points
of phyllodes 1 and 12 were :400 mmol m−2s−1
phyl-Table 2
Changes in maximal potential photochemical efficiency of PSII under dark-adapted conditions (Fv/Fm), effective PSII efficiency under light-adapted conditions (DF/F%
m), photochemical quenching (qP), non-photochemical quenching (NPQ), excitation-energy trapping capacity at the reaction center of PSII (1/Fo-1/Fm) ofA.mangiumphyllodes at different positionsa
Phyllode position Fv/Fm DF/F%m qP NPQ 1/Fo-1/Fm Fv/Fo
aAll parameters are expressed in terms of relative units (data presented as means9S.E.,n=6). Identical letters within the same column indicate no significant differences between the values (P50.05). See text for details of abbreviations.
lodes to photosynthesize at half of the maximum value was lower in mature phyllodes compared with the youngest phyllode (phyllode 1) (Table 1). Light compensation point was highest in the youngest phyllode (400 mmol m−2 s−1) and it
ranged between 20 and 36 mmol m−2 s−1 in the
fully expanded phyllodes (Table 1). The light pensation points of mature phyllodes were com-parable to that of other light-demanding species; for instance, the light compensation point of a light-demanding pioneer species, Macaranga hy
-poleuca, was 38.796.2 mmol m−2 s−1 [19]. The
apparent quantum yield of phyllodes 2 – 8 ranged from 60 – 65 mmol CO2 (mol quanta)−1 (Table 1),
significantly higher than that of phyllode 10 (50
mmol CO2(mol quanta)−
1). A much lower
appar-ent quantum yield was observed in phyllode 12 (26
mmol CO2 (mol quanta)−1) (Table 1). Because of
the high rate of dark respiration, the light compen-sation point of the youngest phyllode varied (4009200 mmol m−2 s−1) (Table 1); it was an
extremely high value. Dark respiration rates de-creased with increasing age of the phyllodes (Table 1). It was highest at 7.9mmol m−2s−1in phyllode
1 and decreased significantly by \50% in lode 2. The high rate of dark respiration in phyl-lode 1 (Table 1) was likely due to the large cost for structural construction and metabolic activities of growth [20,21]. Dark respiration rate of the phyl-lodes decreased in older phylphyl-lodes (Table 1).
Chlorophyll fluorescence parameters of phyl-lodes at different positions along the stem are shown in Table 2. The maximal potential photo-chemical efficiency of PSII under dark-adapted conditions (Fv/Fm) did not vary much in phyllodes 2 – 12 (Table 2); the lowest value was obtained in
phyllode 1. Another indicator of quantum effi-ciency of PSII, Fv/Fo [12], was lowest in the youngest phyllode and increased in older phyl-lodes (Table 2). Effective PSII efficiency under light-adapted conditions (DF/F%m) and photochem-ical quenching (qP) increased with increasing age
of phyllodes and were lowest in phyllode 1 (youngest phyllode) (Table 2). Non-photochemical quenching (NPQ) was higher in phyllodes 1 and 12 (Table 2). The excitation-energy trapping ca-pacity at the reaction center of PSII (1/Fo−1/Fm)
[17] increased as the phyllodes matured (Table 2). The effective PSII efficiency under light-adapted conditions (DF/F%m) could be linearly correlated
with the rates of light-saturated photosynthetic CO2uptake (R2=0.94) (Fig. 5A). An inverse
rela-tionship, however, was observed between the rate of photosynthetic CO2 uptake and NPQ (R
2=
0.92) (Fig. 5C).
The simple chlorophyll fluorescence parameter,
Fv/Fm, has been frequently used as an easy and
Fig. 5. The relationship between the rates of light-saturated photosynthetic CO2uptake (Pn) andDF/F%m(A), the rates of
light-saturated photosynthetic CO2uptake (Pn) andFv/Fo(B) and the rates of light-saturated photosynthetic CO2 uptake (Pn) and NPQ (C) of A. mangium phyllodes in different positions. The numbers in the figures indicate the positions of the different phyllodes.
changes in photosynthetic capacity of A. mangium
phyllode were linearly related to Fv/Fo (R
2=0.68)
(Fig. 5B); no such relationship was found for
Fv/Fm.
In general, the data obtained indicated that photosynthetic capacity and efficiency of PSII function of the phyllodes increased as they ma-tured from phyllode 1 to phyllode 8 and decreased as they aged further (as in phyllodes 10 – 12). Such an age-related phenomenon has been observed in other plant species like Larix occidentalis[23] and
Oryza sati6a [24], for instance. Moreover, the
degradation of chloroplasts in senescent leaves was reported to provide an extra source of nitrogen for the syntheses of proteins in young leaves and the remobilization of nitrogen is important in main-taining a high photosynthetic productivity on a whole plant level [24,25].
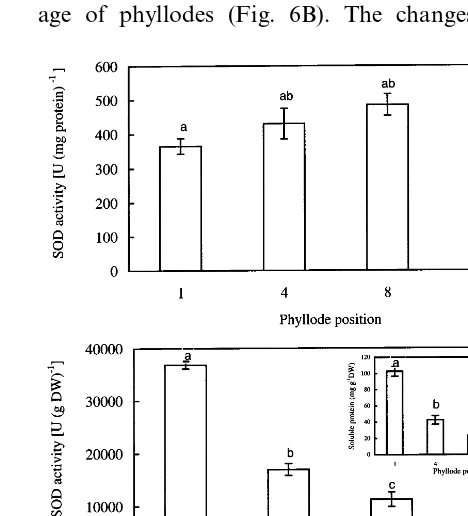
Total SOD activity, expressed on a soluble protein basis, steadily increased with phyllode age (Fig. 6A). When the SOD activity was expressed on a dry weight basis, a different trend was ob-served; it decreased significantly with increasing age of phyllodes (Fig. 6B). The changes in the
Fig. 6. Total SOD activity expressed on a protein basis (A) and dry weight basis (B) ofA.mangiumphyllodes at different positions. The insert shows the changes in soluble protein concentration (on a dry weight basis) of phyllodes. Identical letters indicate no significant differences between the values (P50.05). Error bars indicate S.E. (n=6).
non-destructive indicator of the maximal photo-chemical efficiency of PSII in plants [22]. Although
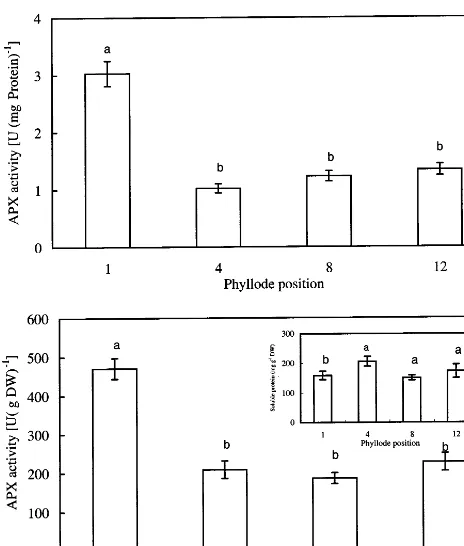
Fv/Fm readings were lower in phyllodes 1 and 2, they did not differ significantly for the rest of the phyllodes (Table 2). However, the values of Fv/Fo
Fig. 7. Total APX activity expressed on a protein basis (A) and dry weight basis (B) ofA.mangiumphyllodes at different positions. The insert shows the changes in soluble protein concentration (on a dry weight basis) of phyllodes. Identical letters indicate no significant differences between the values (P50.05). Error bars indicate S.E. (n=6).
mean time, the decreased SOD and APX activities were accompanied by lower dark respiration rates of the phyllodes (Figs. 6 and 7, Table 1).
Activities of SOD and APX were recalculated on a dry weight basis primarily because the con-centrations of total soluble proteins of phyllodes were different when they were extracted with dif-ferent buffers; such differences in protein levels were expected, as different extraction media and pH would facilitate the isolation of different proteins. The concentrations of soluble proteins, extracted by the SOD extraction buffer, decreased with phyllode age (insert, Fig. 6B). The concentra-tion of soluble proteins, extracted by the APX extraction buffer, was highest in phyllode 4 (insert, Fig. 7B).
In conclusion, mature phyllodes of A. mangium
seedlings, grown under natural light conditions, showed low light compensation points, low dark respiration rates, high photosynthetic capacities compared with that of young phyllodes. SOD and APX activities (on a dry weight basis) were, how-ever, highest in the youngest phyllodes. The high photosynthetic capacity of mature phyllodes could be attributed to the enhanced availability of CO2
and the high efficiency of PSII reaction centers in energy absorption and utilization. The changes in the physiological parameters determined seemed to be more related to the aging of the phyllodes rather than the changes in light microclimate. The low respiration loss and high photosynthetic ca-pacity in mature phyllodes would have resulted in increased resources for growth zones, thus allow-ing fast growth of the seedlallow-ings, an important attribute for rapid establishment of the plants in reforestation programs.
References
[1] G. Rajendrudu, C.V. Naidu, Leaf gas exchange capacity in relation to leaf position on the stem in field grown teak (Tectona grandis L.f.), Photosynthetica 34 (1997) 45 – 55.
[2] U.V. Pathre, K.K. Singh, P.V. Sane, Gas exchange and stomatal conductance in Acacia auriculiformis: effect of leaf position, Photosynthetica 24 (1990) 151 – 154. [3] T. Yamasaki, T. Kudon, Y. Kamimura, S. Katoh, A
vertical gradient of the chloroplast abundance among leaves of Chenopodium album, Plant Cell. Physiol. 37 (1996) 43 – 48.
[4] C. Bowler, M. Van Montagu, D. Inz´e, Superoxide dis-mutase and stress tolerance, Ann. Rev. Plant Physiol. Plant Mol. Biol. 43 (1992) 83 – 116.
activity of APX are presented in Fig. 7. Expressed on the basis of either soluble protein concentration or dry weight, the activity of APX of phyllode 1 was significantly higher than that of other phyl-lodes (Fig. 7). On a dry weight basis, the highest total SOD and total APX activities were observed in phyllode 1; thereafter, activities of these antiox-idant enzymes declined and they were not signifi-cantly different among the expanded phyllodes (Figs. 6 and 7). Phyllode 1 exhibited negative photosynthetic CO2 exchange rate and high dark
[5] K. Asada, Production and action of active oxygen spe-cies in photosynthetic tissues, in: C.H. Foyer, P.M. Mullineasx (Eds.), Causes of Photooxidative Stress and Amelioration of Systems in Plants, CRC Press, Boca Raton, FL, 1994, pp. 77 – 104.
[6] R.R. Wise, Chilling-enhanced photooxidation: the product, action and study of active oxygen species pro-duced during chilling in the light, Photosynth. Res. 45 (1995) 79 – 97.
[7] C.H. Foyer, M. Lelandais, K.J. Kunert, Photooxidative stress in plants, Physiol. Plant 92 (1994) 696 – 717. [8] K. Esau, Plant Anatomy, Wiley, New York, London,
Sydney, 1965, pp. 1 – 767.
[9] D.I. Arnon, Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta 6ulgaris, Plant Physiol. 24
(1949) 1 – 14.
[10] J.L. Embry, E.A. Nothnagel, Leaf development and senescence in Panicum miliaceum L., a cereal with a short seed-to-seed cycle, Plant Sci. 55 (1988) 129 – 136. [11] O. Van Kooten, J.F.H. Snel, The use of chlorophyll
fluorescence nomenclature in plant stress physiology, Photosynth. Res. 25 (1990) 147 – 150.
[12] B. Genty, J.M. Briantais, N.R. Baker, The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence, Biochim. Biophys. Acta 990 (1989) 87 – 92.
[13] C.O. Beauchamp, I. Fridovich, Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels, Anal. Biochem. 44 (1971) 276 – 287.
[14] Y. Nakano, K. Asada, Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts, Plant Cell. Physiol. 22 (1981) 867 – 880.
[15] M.M. Bradford, A rapid and sensitive method for the quantification of microgram quantities of protein utiliz-ing the principle of protein dye bindutiliz-ing, Anal. Biochem. 72 (1976) 248 – 254.
[16] B.A. Logan, D.H. Barker, W.W. Adams III, B. Dem-mig-Adams, The response of xanthophyll cycle-depen-dent energy dissipation in Alocasia brisbanensis to sunflecks in a subtropical rainforest, Aust. J. Plant Phys-iol. 24 (1997) 23 – 33.
[17] M. Havaux, R.J. Strasser, H. Greppin, A theoretical and experimental analysis of the qP and qN coefficients of chlorophyll fluorescence quenching and their relation to photochemical and nonphotochemical events, Photo-synth. Res. 27 (1991) 41 – 55.
[18] H.Y. Lee, W.S. Chow, Y.N. Hong, Photoinactivation of photosystem II in leaves of Capsicum annuum, Physiol. Plant 105 (1999) 377 – 384.
[19] M.G. Barker, M.C. Press, N.D. Brown, Photosynthetic characteristics of dipterocarp seedlings in three tropical rain forest light environments: a basis for niche parti-tioning?, Oecologia 112 (1997) 453 – 463.
[20] Z. S&esta´k, Changes in electron transport chain composi-tion, and activities of photosystems and photophospho-rylation during leaf ontogeny, in: Z. S&esta´k (Ed.), Photosynthesis During Leaf Development, W. Junk, Dordrecht, Boston, Lancaster, 1985, pp. 128 – 144. [21] J. C.atskyˆ, Z. S&esta´k, Photosynthesis during leaf
develop-ment, in: M. Pessarakli (Ed.), Handbook of Photosyn-thesis, Marcel Dekker, New York, Basel, Hong Kong, 1996, pp. 633 – 660.
[22] U. Schreiber, W. Bilger, C. Neubauer, Chlorophyll fluorescence as a nonintrusive indicator for rapid assess-ment of in vivo photosynthesis, in: E.D. Schulze, M.M. Caldwell (Eds.), Ecophysiology of Photosynthesis, Springer-Verlag, Berlin, Heidelberg, New York, 1995, pp. 49 – 67.
[23] S.I. Rosenthal, E.L. Camm, Photosynthesis decline and pigment loss during autumn foliar senescence in western larch (Larix occidentalis), Tree Physiol. 17 (1997) 767 – 775.
[24] C.Z. Jiang, K. Ishihara, K. Satoh, S. Katoh, Loss of the photosynthetic capacity and protein in senescing leaves at top positions of two cultivars of rice in relation to the source capacities of the leaves for carbon and nitrogen, Plant Cell. Physiol. 40 (1999) 496 – 503.
[25] T.L. Pons, W. Jordi, Induction of leaf senescence and shade acclimation in leaf canopies-variation with leaf longevity, in: H. Lambers, H. Poorter, M.M.I. Van Vuuren (Eds.), Inherent Variation in Plant Growth, Physiological Mechanisms and Ecological Conse-quences, Backhuys, Leiden, 1998, pp. 121 – 137.