Summary A cost--benefit approach was developed to ana-lyze the carbon budget of the lowest Scots pine (Pinus sylvestris L.) branches subject to abscission. In addition to withbranch growth and respiratory costs, the budget in-cluded an estimation of a branch’s share of the maintenance respiration of the stem and root. A branch was considered productive if the budget was positive.
Foliar gas exchange and woody-tissue respiration were non-destructively measured at monthly intervals during the grow-ing season on the six lowest branches of 10-year-old Scots pine trees, to the moment when the branches died naturally. Photo-synthetic light response and temperature response of respira-tion, together with measurements of canopy light conditions and meteorological data, were used to calculate seasonal car-bon budgets for the branches. Maintenance respiration of stems and roots was estimated from published data.
All but one of the branches studied were found to be nonpro-ductive over the growing season. Following a decrease in photosynthetic capacity in July, the cumulative budget became negative and the branches died, indicating that a negative carbon budget corresponds with the onset of abscission of the lowest branches.
Keywords: branch abscission, branch autonomy, Pinus sylvestris, respiration.
Introduction
For a better understanding of the ecological significance of the architectural patterns of woody plants, it is important to link the photosynthetic benefits associated with different crown geometries to the structural costs of supporting and supplying the crown arrangements (Givnish 1986). Competition should favor plants that maximize the difference between carbon gain and the related costs. Thus, cost--benefit analyses are essential for the interpretation of individual plant success (Küppers 1989). I applied the cost--benefit approach to the analysis of carbon fixation by the lowest, shaded branches of young Scots pine (Pinus sylvestris L.) trees.
In conifers, the lowest, shaded branches apparently contrib-ute little carbohydrate to the rest of the tree and fix just enough carbon to meet their own needs, thus being autonomous with respect to carbon (Sprugel et al. 1991). This conclusion is
supported by the results of pruning studies showing that growth does not increase after lower branches are removed (e.g., Uotila and Mustonen 1994), and by defoliation experi-ments that provide evidence for a mechanism that prevents carbohydrate compensation to damaged branches (Honkanen and Haukioja 1994). Additional evidence is provided by stud-ies of the movement of 14C-labeled carbon among branches (for references see Sprugel et al. 1991). Theoretical considera-tions concerning the adaptive benefits of plant modularity (Watson and Casper 1984, Hardwick 1986, Sachs et al. 1993) may be applied to coniferous branches. However, there are few published data on the contribution of particular branches (or whorls) to the total carbon budget of Scots pine. Existing studies (Ågren et al. 1980, Linder and Axelsson 1982) are based on extrapolation of the photosynthetic measurement from one whorl to the whole crown, which does not provide the accuracy needed to assess whether the carbon budget of the lowest branches is positive.
I have attempted to assess, from the whole-tree perspective, the costs of branch maintenance versus branch photosynthetic gain. A seasonal branch carbon budget was calculated for the six lowest branches subject to abscission. The budget estimate was based on seasonal gas exchange measurements, published data on stem and root respiration, and meteorological data recorded for the growing period.
Theory: determination of costs of carbon acquisition by a branch
On an annual basis, it can be assumed that the carbon assimi-lated by a tree is entirely consumed in respiration and total growth, with the latter including turnover rates of various tree parts. Denoting annual total growth by G (definitions of sym-bols and their units are given in Table 1) and annual respiration by R, the annual gross photosynthesis, A, is therefore:
A=R+G. (1)
Respiration can be divided into growth (RG) and maintenance (RM) components:
R=RG+RM. (2)
Gas exchange of the lowest branches of young Scots pine: a
cost--benefit analysis of seasonal branch carbon budget
JAN WITOWSKI
Forest Research Institute, Department of Ecology and Environmental Protection, 00-973 Warsaw, Bitwy Warszawskiej 3, Poland
Received December 18, 1996
Let R and G with subscripts N, B, S and R denote the respira-tion and growth of the corresponding tree funcrespira-tional parts: needles, branches, stem and roots, respectively. For the whole tree, we have:
A=RN+GN+RB+ GB+RSM
+RRM+RSG+RRG+GS +GR, (3)
where growth and maintenance components of respiration were distinguished only for stem (RSG, RSM) and roots (RRG, RRM). Let A, R and G with subscripts B* and N* denote the gross photosynthesis, respiration and growth of a particular branch and its needles. Following Sprugel et al. (1991) the branch is defined as autonomous with respect to carbon if:
AN∗≥RN∗+GN∗+RB∗+GB∗, (4)
i.e., the branch does not drain carbohydrates from the stem and roots. If the photosynthetic production of the branch exceeds its carbohydrate utilization, the branch contributes carbohy-drate to the rest of the tree. This does not mean, however, that the branch is productive from the whole-plant perspective, because a fraction of stem and root respiration can be attributed to the supply of water and nutrients to the branch. It is the total carbon budget of the tree, rather than of the branch, that must balance, and if all of the branches of a tree were just autono-mous the tree could not survive. In this study a branch is said to be productive if:
AN∗ > RN∗+GN∗+RB∗+GB∗+(RS∗M +RRM)MN∗/MN, (5)
where MN∗ denotes the needle biomass of the branch, MN the total needle biomass of the tree and RS∗M maintenance respira-tion of the stem part located below the branch. Following the pipe model theory (Shinozaki et al. 1964), it is assumed that the unit amount of leaves (branch) is associated with the downward continuation of non-photosynthetic tissue (a pipe) serving both as a vascular passage and as mechanical support to the branch. The productive branch is not only autonomous, but pays the cost of maintenance respiration of the pipe. More-over, the productive branch participates in tree growth; i.e., supplies photosynthate for some of the last four components in Equation 3. The branch’s share in stem and root maintenance respiration can be estimated as the relative biomass of a branch’s needles to the total needle biomass of a tree.
Equation 5, characterizing a productive branch, involves the maintenance respiration of the stem section located between the branch and the ground, and it holds only for the lowermost tree branches. If it is to be generalized to hold for any branch, each internodal stem section would have to be treated sepa-rately, because each supports a different population of needles.
Materials and methods Study site
The study site was a 10-year-old Pinus sylvestris (Scots pine) stand located about 25 km south of Warsaw (central Poland). The soil is rusty and podsolized, with the texture of weak loamy sand on loose sand. No silvicultural treatments had been carried out in the stand, which was planted after a clearcut. The stand, which had a stocking of 8400 trees ha−1, had formed a closed dense canopy comprising 4 to 5-m high Scots pine trees with some birches and larches, and fairly low ground vegeta-tion. Most of the Scots pine trees in the stand had two whorls of dead branches when the study started in spring 1995.
Climatic data
Climatic data for the study site were collected at the Forest Research Institute Station located within a mature Scots pine stand 9 km from the study area. Incident photosynthetically active radiation (PAR) was measured every minute by a point quantum sensor placed above the stand canopy, and 5-min averages were recorded with a data logger (Delta-T Devices, Table 1. Definitions of symbols and their units.
Symbol Definition Unit
A, AN∗ Photosynthesis of tree or of a g C year−1 particular branch
G Total growth of tree including g C year−1 turnover rates of tree parts
GB, GB∗ Growth of all branches or of g C year−1 a particular branch
GN, GN∗ Growth of all needles or of g C year−1 needles of a particular branch
GS, GR Growth of stem or root g C year−1 MN, MN∗ Needle biomass of tree or of g C
a particular branch
R, RB, Respiration of tree, all branches g C year−1 RB∗ or a particular branch
RG, RM Growth respiration or mainte- g C year−1 nance respiration of tree
RN, RN∗ Respiration of all needles or of g C year−1 needles of a particular branch
RSG, RRG Growth respiration of stem g C year−1 or root
RSM, RRM, Maintenance respiration of g C year−1 RS∗M stem, root or stem part located
below a particular branch
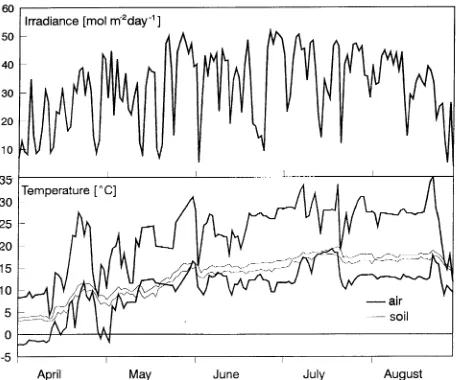
Cambridge, U.K.). Air temperatures were recorded every hour at heights of 0.05 and 6 m above the ground, and soil tempera-tures were measured at depths of 10 and 20 cm by an RC-10 logger (Trax, Cracow, Poland). For this study, soil temperature at 10 and 20 cm depths, and air temperatures at 0.05 and 6 m heights were averaged, and represent average soil temperature in the root zone and air temperature, respectively. The climatic data collected for the period April--August 1995 are presented in Figure 1.
Gas exchange measurements
Six codominant trees were selected in the stand that had a live branch satisfying the following criteria: (1) the branch is lo-cated on the lowermost live whorl; (2) the tree has at least one dead whorl below the branch; (3) the branch does not have reproductive organs; (4) the branch has at least one live bud; and (5) the branch has no visible signs of injury by herbivores or insects. The six branches chosen for gas exchange measure-ments (one on each tree) are denoted as MBs (measured branches) in the text. Criteria (1) and (2) allowed for an expectation that at least some of the MBs would be self-pruned by the trees during the oncoming growing season; however, the fates of the MBs were not definitely determined, because they had an opportunity for further growth (4)--(5). Criterion (3) was set to avoid involving non-photosynthetic benefits from the branch.
In situ gas exchange of each MB was determined with a closed, battery-operated portable LI-6200 photosynthesis sys-tem (Li-Cor, Inc., Lincoln, NE). The measuring equipment included a quantum sensor attached outside an assimilation chamber to monitor PAR during gas exchange measurements, and a thermocouple to measure air temperature in the chamber. The system was used with two Li-Cor 4-dm3 Plexiglas cham-bers, one of which was modified for thicker branches by cutting notches in the chamber wall. The use of large chambers
allowed the 13-cm long shoot sections to be located within the chamber without disturbance to the needles. Care was taken to keep the shoots in their natural position during measurements. Two locations were selected on each MB: one on the 1-year-old shoots and the second on the non-needle-bearing part of the branch. Needle gas exchange rates reported in this study in-clude the needle-bearing branch parts, and the branch respira-tion values refer to the non-needle-bearing branch parts only.
During the period April--August, the diurnal course of need-le CO2 exchange was monitored once a month for each MB. The diurnal course consisted of three measurements repeated approximately every 2 h from dawn to sunset. The temperature dependences of MB needle dark respiration and branch respi-ration were determined for each MB by making measurements at predawn and midday, because ambient temperatures dif-fered most between these two periods. Needle dark respiration rates were measured 10 min after the chamber was positioned in a cardboard box blacked inside (cf. Sprugel et al. 1995), whereas the assimilation chamber was left uncovered during the branch respiration measurements.
Harvest measurements
When all needles on an MB turned yellow or brown, the MB was harvested and returned to the laboratory where the number of needles, needle dry weight (48 h, 85 °C), and branch dry weight were determined. Following the MB harvest, PAR measurements were performed in the stand (see below) and then, in late September and October, the six sample trees were felled and returned to the laboratory where the dry weights of needles and stems were determined.
Measurements of PAR
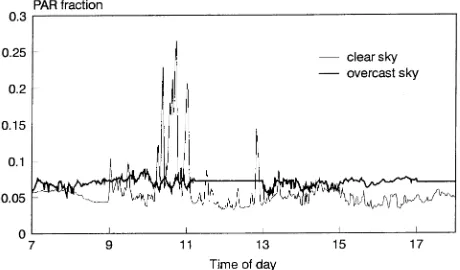
As in the case of forest floor vegetation, the utilization of sunflecks by a shaded Scots pine shoot may substantially contribute to its carbon gain. The amount of PAR reaching the MB shoot at a given time of day was mainly influenced by three factors: sun elevation, sky conditions and wind move-ment of the canopy. Each of these factors was included in the model. However, no attempt was made to account for seasonal changes in sun elevation and canopy dynamics. Measurements of PAR in the stand were made once during the growing season, within a week following the death of an MB. After an MB was harvested, a point quantum sensor was placed hori-zontally above the forest floor exactly at the previous location of the MB shoot section used for photosynthesis measure-ments. A reference sensor was located above the canopy, and both sensors were connected to a Li-Cor LI-1000 data logger. The sensor readings were recorded every 5 s, and 1-min aver-ages were stored every minute from 0700 to 1800 h. The measurements were carried out for at least 3 days for each MB location. The measurements were then divided into two groups depending on whether the sky was overcast or clear during the measurements (see Messier and Puttonen 1995). As a result, two daily curves of 1-min PAR percentage (%PAR) reaching the MB were obtained for each MB location: one for clear skies and one for overcast weather. The use of a 5-s sampling interval allowed short-term light variations to be incorporated Figure 1. Seasonal course of daily sum of irradiance, and
in the model, assuming that these variations are averaged for the shoot and the photosynthetic rate achieved is at the mean (1-min) irradiance (Gross 1982).
Simulation model
For a given month and MB, the recorded values of the daily course of photosynthesis were plotted against simultaneously recorded PAR and the regression parameters of the response curve (Landsberg 1977) were fitted using the Marquardt itera-tive least-square method available in the Statgraphic software package (STSC, Rockville, MD):
Ai =Amax(1 −exp[−α(I−Io)]), (6) where Ai = instantaneous photosynthesis, I = PAR reaching the MBs, and Amax, α, Io are parameters. The measured values of needle and branch respiration were used to find the regression parameters of the standard exponential curve of temperature dependence of respiration:
Ri(T)=R10(Q10)(T − 10)/10, (7) where Ri(T) = instantaneous respiration, T = temperature, R10 = respiration rate at T = 10 °C, and Q10 is the proportional change in respiration resulting from a 10 °C increase in tem-perature.
To estimate the parameters in Equation 7 for the temperature dependence of maintenance respiration of the stem and root of the experimental trees, I used the data on the annual course of Scots pine stem and root respiration published by Linder and Troeng (1981). It was assumed that the late September meas-urements of Linder and Troeng (1981) at T = 10 °C represented stem maintenance respiration rates, and that higher values recorded earlier in the season included growth plus mainte-nance (the ‘subtraction’ method, Sprugel 1990). Similarly, late-October measurements of root respiration at T = 10 °C were used to estimate the R10 parameter for maintenance res-piration of the root. The parameter Q10 was set at 2 during the growing season (Linder and Troeng 1981). The data on root biomass of young Scots pines provided by Vyskot (1983) were used to estimate the root biomass of the investigated trees. It was assumed that the ratio of aboveground biomass to root biomass was the same for the trees as for those harvested by Vyskot (1983).
The photosynthetic production of 2- and 3-year-old MB shoots was calculated from the measured photosynthesis of a 1-year-old shoot of the same MB, by assuming the efficiencies of the 2- and 3-year-old shoots to be 75 and 50% of the efficiency of the 1-year-old shoot, respectively (Linder and Troeng 1980).
To calculate the branch carbon budget from the data, a simulation model was written in Turbo Pascal 6.0. The input variables of the simulation model were incident PAR above the canopy, air temperature and soil temperature, and the input parameters were the coefficients of Equations 6 and 7. The data on %PAR reaching an MB (Figure 2) were used to calculate the amount of PAR reaching an MB from the incident PAR
data. The amount of PAR reaching an MB was then used to calculate MB photosynthesis from Equation 6. Needle dark respiration, branch respiration and maintenance respiration of the stem were calculated from the air temperature records by means of Equation 7 and the corresponding coefficients. Simi-larly, maintenance respiration of the root was calculated from the record of root temperature by means of Equation 7. The budget was calculated independently for each MB with time steps of 1 min and 1 h for the calculation of photosynthesis and respiration, respectively. From the six sets of MB data an ‘average branch’ was then constructed to illustrate the dynam-ics of the budget during the growing season.
Results
Dimensions and biomass of the sample trees and MBs are presented in Table 2. All of the MBs died and were harvested between July 27 and August 25. Three of the MBs did not develop any current-year shoots during the growing season, and the remainder had very small current-year shoot incre-ments. No measurable annual growth ring production was detected during the period of study for any of the MBs. This is a commonly observed phenomenon for branches located in the lower portions of coniferous crowns (Roberts 1994).
The %PAR reaching the MB locations varied more under clear skies than under overcast skies (Figure 2). A similar result was obtained by Messier and Puttonen (1995) for young Scots pine stands. Representative examples of the relationship be-tween irradiance and CO2 assimilation and the dependence of branch and needle respiration rates on temperature are given in Figures 3 and 4, respectively. For the period April--June, light-saturated photosynthesis was relatively stable, but in July a rapid decline in light-saturated photosynthesis occurred for all MBs, and it decreased even further in August (Figure 3, Ta-ble 3).
For the calculation of a seasonal MB carbon budget (Ta-ble 4), it was assumed that, for each MB, current growth respiration balanced its photosynthesis, the error introduced by this assumption is relatively small because the total biomass of current-year growth was very small. All MBs but one were
found to be autonomous with respect to carbon. Branch No. 5, which failed to be autonomous, had a high respiration rate per branch biomass unit compared with the other MBs. For all MBs, the branch respiration cost was the highest component of the budget, and was as a result of the high branch/needle biomass ratio (Table 2), rather than a high respiration rate per MB biomass unit. Of the six MBs, MB No. 1 was the only productive branch, but it contributed little carbon for tree growth, because it was only just productive.
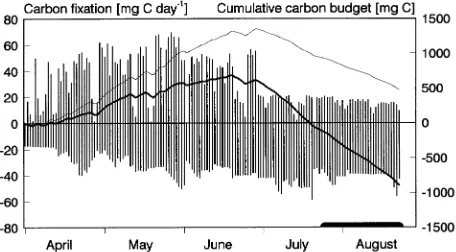
The cumulative budget of an ‘average branch’ (Figure 5) peaked in late June and then decreased as daily respiratory costs outbalanced photosynthetic gain. The budget became negative in late July, shortly before the MBs began to die.
Discussion
Measurement procedure
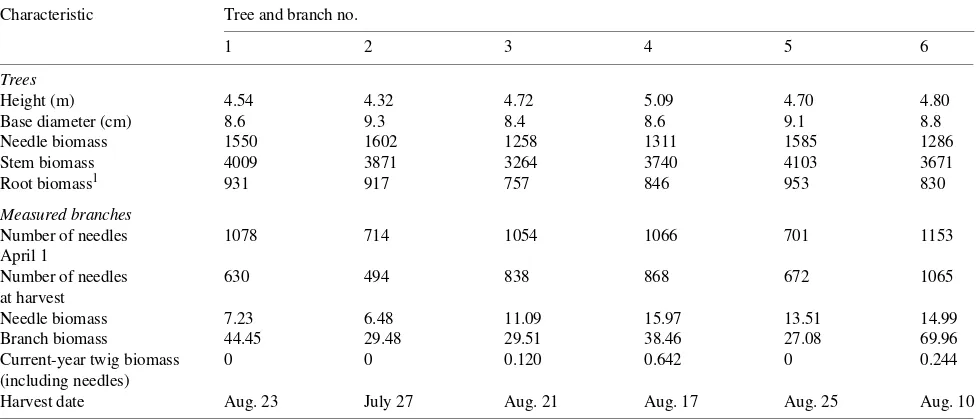
Because the MB gas exchange measurements were made solely for the purpose of MB carbon budget estimation, the measurement procedure was set accordingly. As a conse-quence, the curves used to estimate MB seasonal photosynthe-sis (Figure 3) are not true ‘light response curves,’ because factors other than light varied significantly during the day-long measurements (e.g., temperature, ambient CO2 concentration, and air humidity). Moreover, the PAR measured by the quan-tum sensor during the photosynthesis measurements was lower than the light actually intercepted by the shoot, because the sensor was set horizontally and did not track the sun. For low-radiation regimes, when the share of dispersed light in Table 2. Characteristics of sample trees and measured branches. All biomass values are given in grams of dry weight.
Characteristic Tree and branch no.
1 2 3 4 5 6
Trees
Height (m) 4.54 4.32 4.72 5.09 4.70 4.80
Base diameter (cm) 8.6 9.3 8.4 8.6 9.1 8.8
Needle biomass 1550 1602 1258 1311 1585 1286
Stem biomass 4009 3871 3264 3740 4103 3671
Root biomass1 931 917 757 846 953 830
Measured branches
Number of needles 1078 714 1054 1066 701 1153
April 1
Number of needles 630 494 838 868 672 1065
at harvest
Needle biomass 7.23 6.48 11.09 15.97 13.51 14.99
Branch biomass 44.45 29.48 29.51 38.46 27.08 69.96
Current-year twig biomass 0 0 0.120 0.642 0 0.244
(including needles)
Harvest date Aug. 23 July 27 Aug. 21 Aug. 17 Aug. 25 Aug. 10
1 Estimated from Vyskot (1983).
Figure 3. Relationship between irradiance and CO2 assimilation for the shoot of branch No.1 measured on May 5, 1995. Inset shows relationship averaged for all branches in monthly steps.
total light is high, the measured light could underestimate the radiation received by a shoot as well. Thus, the curve would not be the most suitable for comparative purposes; e.g., for calculation of quantum yield. However, it served well for the prediction of photosynthesis because it involved changes in ambient microclimate that most likely accompany changes in irradiance. For instance, when PAR at the MB location was low (morning or evening hours), the air temperatures correspond-ing to the photosynthesis measurements were low and CO2 concentration of the air was high.
Sources of error in the simulation model
The assumption that the temperature record from the weather station is valid for the experimental stand could have intro-duced error to the respiration estimates in the simulation model. This assumption was verified only for the days of gas exchange measurements; however, the differences between air temperatures recorded by the weather station and those in the study stand were low (less than 2 °C for most of the measure-Table 3. Parameters of photosynthetic response to irradiance (monthly steps), and respiration response to temperature (whole season). Values are given as means ± SD for the six branches, notation as in Equations 6 and 7, units are given in Table 1.
Parameter April May June July August
Photosynthetic response
Amax 5.63 ± 0.61 5.01 ± 0.68 5.45 ± 1.17 1.61 ± 0.44 0.98 ± 0.10
α 0.0088 ± 0.004 0.0106 ± 0.005 0.0072 ± 0.004 0.0163 ± 0.015 0.0310 ± 0.026 Io 12.82 ± 2.57 10.58 ± 4.52 11.13 ± 5.26 16.56 ± 5.46 12.18 ± 6.79
Tree part R10 Q10
Needles 0.123 ± 0.049 3.32 ± 0.45 Branches 0.073 ± 0.042 1.58 ± 0.16
Stem 0.0201 21
Roots 0.0851 21
1 Estimated from Linder and Troeng (1981), see text for details.
Table 4. Estimated seasonal carbon budget for the six measured branches (MB). All values are given in mg of carbon, notation as in Equation 5.
Branch no. 1 2 3 4 5 6
Photosynthesis (AN∗) 4135 2223 3767 5888 5274 4163
Needle respiration (RN∗) 461 577 473 568 1257 648
Current growth1 (GN∗) 0 0 60 321 0 122
Branch respiration (RB∗) 2573 1070 2932 3773 5395 2262
Branch growth (GB∗) 0 0 0 0 0 0
Available for export2 1100 575 303 1226 0 1132
(Ex = AN∗ -- (RN∗ + GN∗ + RB∗ + GB∗)
Branch’s share of 217 178 497 258 524 347
maintenance respiration of stem (RS∗MM
N∗/MN)
Branch’s share of 775 486 995 991 1152 1226
maintenance respiration of root (RRMMN∗/MN)
Available for tree growth2 109 0 0 0 0 0
(T = Ex -- (RSM∗+ RRM)MN∗/MN)
1 Assuming a 50% carbon content of biomass. 2 Zero stands for negative values.
ments, data not presented) on these days. In the case of root respiration estimates, there was an additional error associated with the use of literature-based estimations of root biomass.
The partition of stem and root respiration into growth and maintenance components was made on the assumption that maintenance respiration at a constant temperature remains constant throughout the year. This assumption is necessary for most methods of partitioning respiration (Sprugel et al. 1995), but its validity has been questioned (Sprugel and Benecke 1991).
Measurements of %PAR reaching an MB were made once during the growing season, after the MB had died, and thus did not include seasonal canopy and sun angle dynamics. The %PAR reaching an MB had been higher earlier in the season, i.e., before new canopy shoots had fully expanded. Also higher sun elevation earlier in the season allowed for better light penetration in the canopy. As a result, the model calculations underestimated MB photosynthesis, which means that more than one branch could have been on the threshold of produc-tivity (Table 4).
Although the MB photosynthesis model (Equation 7) lacks a temperature component, within-day and seasonal tempera-ture variations were included in the measurement design. Be-tween-day variation in air temperature during a month could have introduced an error to the MB photosynthesis estimate; however, this error will be minor because the temperature effect on photosynthesis was most pronounced at light satura-tion, which was seldom attained by the shaded shoots.
All of these sources of error in the simulation model resulted from limitations of the collected data, and must be considered when analyzing the budget. Nevertheless, the budget analysis provides some insight into the carbon economy of self-pruning in Scots pine trees, because it is unlikely that the physiological mechanism underlying self-pruning responds to the minute changes in amounts of exported carbohydrates that may turn a productive branch into a nonproductive one. Therefore, even though the numbers obtained are approximations, some con-clusions can be drawn from the budget analysis.
The budget
Because the carbon exported by a Scots pine branch to the stem and root is not redistributed to the branch during the next growing season (Hansen and Beck 1990), a single growing season is an appropriate time unit of reference for branch carbon budget analysis. The modeled budget (Table 4) re-vealed that all but one MB failed to be productive. This means that the branches did not provide an adequate carbon return for the water and nutrient supply they received. That is, this sup-ply, or part of it, could have been available for other branches. In accordance with this observation, green pruning of the lower part of the crown has been found to improve water (Margolis et al. 1988) and mineral (Nuorteva and Kurkela 1993) status in the remaining upper crown.
In Scots pine, current-year shoot growth is supplied by photosynthates from the 1-year-old needles of the same branch (Ericsson 1978, Hansen and Beck 1994). In the case of an MB, the carbon investment to develop a current-year shoot of
regu-lar size (a few grams of C) would be made at the expense of imported carbohydrates (Table 4). The MBs had poor current-year growth, and in this way avoided becoming parasitic on the tree, a result that confirms the theoretical predictions of Can-nell and Morgan (1990).
This cost--benefit analysis of a branch carbon budget did not include changes in carbon storage within the branch. For this reason, the corresponding values in the budget are referred to as carbon available for export and growth, rather than amounts actually exported or utilized for tree growth.
The cumulative amount of carbon available for export de-creased after the late June peak (Figure 5, fine line), which means that, after late June, the MBs were using more carbon than they fixed. This short-term negative carbon balance could possibly lead by itself to later MB death, without invoking the branch’s share in the maintenance respiration of stem and root. However, the time span of MB death begins when the cumula-tive total branch carbon budget becomes negacumula-tive, i.e., when seasonal carbon export becomes lower than seasonal respira-tory demands of stem and root tissue supporting the MB. Thus, the branch’s share in the maintenance respiration of stem and root (Figure 5, thick line) is of relevance when considering the links between branch carbon economy (cost--benefit analysis) and the observed self-pruning strategy of a tree. No attempts were made to determine the actual causes of MB deaths.
Implications and improvements
The concept of branch productivity proposed here may lead to a better understanding of the mechanisms determining leaf area index (LAI, area of foliage per unit land surface) and leaf area efficiency (E, stemwood volume increment per unit leaf area). Leaf area index and E are important indices of canopy structure and tree growth efficiency. The LAI of different conifer species varies by a factor of more than five (Leverenz and Hinckley 1990). Species differences have also been ob-served in the relationship between E and the leaf area of individual trees within stands (Roberts et al. 1993). Because E of a nonproductive branch equals zero, an increase in number of nonproductive whorls of branches would decrease E and increase LAI.
of the respiratory costs (Tables 2 and 4). Shade-shoot geometry and needle longevity combined with self-pruning of nonpro-ductive branches may determine the maximum LAI of Scots pine.
To obtain more detailed information about the productivity of a branch, the simulation model could be improved by incorporating a more accurate simulation of photosynthesis. The empirical model proposed by Küppers and Schulze (1985) provides a diurnal course of net photosynthesis for Scots pine using climatic factors as input, and taking into account tem-perature and stomatal limitations on photosynthesis. The model parameterization would require measurements of vari-ous photosynthetic, stomatal and respiration response curves in the field. As in the research reported here, seasonal changes in the response parameters would have to be measured. The input meteorological data (PAR, air temperature, air humidity, air CO2 concentration) would also have to be recorded in the study stand together with the soil temperature needed for the calculation of root respiration.
The precise assessment of branch productivity also requires a knowledge of belowground respiration costs, which are dif-ficult to estimate, either directly or indirectly (Sprugel et al. 1995). However, for the purpose of developing satisfactory process-based tree growth models, measurements of photosyn-thesis must be linked to the respiration and growth of tree parts (Ford and Bassow 1989). Therefore, further studies are needed to couple branch photosynthesis with carbohydrate utilization by roots.
Conclusions
Because the simulation model contains several simplifying assumptions, considerable error may exist in some compo-nents of the modeled MB carbon budgets. I have shown, however, that MB photosynthetic gain approximates the corre-sponding respiratory costs (Table 4). The results suggest that these lowest branches abscise when they are no longer photo-synthetically useful to the tree. Further growth of these branches would require a substantial import of carbohydrates from the rest of the tree. Apparently, there is a physiological mechanism in a Scots pine tree that prevents this import, indicating that young Scots pine trees are able to optimize their self-pruning strategy with respect to carbon acquisition.
Acknowledgments
Study supported by General Directorate of the State Forests (DGLP) Grant No. BLP-654.
References
Ågren, G., B. Axelsson, J.G.K. Flower-Ellis, S. Linder, H. Persson, H. Staaf and E. Troeng. 1980. Annual carbon budget for a young Scots pine. In Structure and Function of Northern Coniferous For-ests--An Ecosystem Study. Ed. T. Persson. Ecol. Bull. 32:307--313. Cannell, M.G.R. and J. Morgan. 1990. Theoretical study of variables affecting the export of assimilates from branches of Picea. Tree Physiol. 6:257--266.
Ericsson, A. 1978. Seasonal changes in translocation of 14C from different age-classes of needles on 20-year-old Scots pine trees (Pinus sylvestris). Physiol. Plant. 43:351--358.
Ford, E.D. and S.L. Bassow. 1989. Modeling the dependence of forest growth on environmental influences. In Biomass Production of Fast-Growing Trees. Eds. J.S. Pereira and J.J. Landsberg. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 209--229. Givnish, T.J. 1986. Economics of support. In On the Economy of Plant
Form and Function. Ed. T.J. Givnish. Cambridge University Press, Cambridge, U.K., pp 413--420.
Gross, L.J. 1982. Photosynthetic dynamics in varying light environ-ments: a model and its application to whole leaf carbon gain. Ecology 63:84--93.
Hansen, J. and E. Beck. 1990. The fate and path of assimilation products in the stem of 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 4:16--21.
Hansen, J. and E. Beck. 1994. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 8:172--182.
Hardwick, R.C. 1986. Physiological consequences of modular growth in plants. Philos. Trans. R. Soc. Lond. B. 313:161--173.
Honkanen, T. and E. Haukioja. 1994. Why does a branch suffer more after branch-wide than after tree-wide defoliation? Oikos 71:441--450.
Küppers, M. 1989. Ecological significance of above-ground architec-tural patterns in woody plants: a question of cost--benefit relation-ships. Trends Ecol. Evol. 4:375--379.
Küppers, M. and E.-D. Schulze. 1985. An empirical model of net photosynthesis and leaf conductance for the simulation of diurnal courses of CO2 and H2O exchange. Aust. J. Plant Physiol. 12:513--526.
Landsberg, J.J. 1977. Some useful equations for biological studies. Exp. Agric. 13:273--286.
Leverenz, J.W. 1992. Shade shoot structure and productivity of ever-green conifer stands. Tests of three alternative hypotheses. Scand. J. For. Res. 7:345--353.
Leverenz, J.W. and T.M. Hinckley. 1990. Shoot structure, leaf area index and productivity of evergreen conifer stands. Tree Physiol. 6:135--149.
Linder, S. and B. Axelsson. 1982. Changes in carbon uptake and allocation patterns as a result of irrigation and fertilization in a young Pinus sylvestris stand. In Carbon Uptake and Allocation in Subalpine Ecosystems as a Key to Management. Ed. R.H. Waring. Proc. IUFRO Workshop, Forest Research Laboratory, Oregon State Univ., Corvallis, OR, pp 38--44.
Linder, S. and E. Troeng. 1980. Photosynthesis and transpiration of 20-year-old Scots pine. In Structure and Function of Northern Coniferous Forests----An Ecosystem Study. Ed. T. Persson. Ecol. Bull. 32:165--181.
Linder, S. and E. Troeng. 1981. The seasonal variation in stem and coarse root respiration of a 20-year-old Scots pine (Pinus sylvestris L.). Mitt. Forstl. Bundesversuchsanst Wien. 142:125--139.
Margolis, H.A., R.R. Gagnon, D. Pothier and M. Pineau. 1988. The adjustment of growth, sapwood area, heartwood area, and sapwood saturated permeability of balsam fir after different intensities of pruning. Can. J. For. Res. 18:723--727.
Nuorteva, H. and T. Kurkela. 1993. Effects of crown reduction on needle nutrient status of scleroderris-canker-diseased and green-pruned Scots pine. Can. J. For. Res. 23:1169--1178.
Reich, P.B., T. Koike, S.T. Gower and A.W. Schoettle. 1995. Causes and consequences of variation in conifer leaf life-span. In Ecophysi-ology of Coniferous Forests. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, CA, pp 225--254.
Roberts, S.D. 1994. The occurrence of non-ring producing branches in Abies lasiocarpa. Trees 8:263--267.
Roberts, S.D., J.N. Long and F.W. Smith. 1993. Canopy stratification and leaf area efficiency: a conceptualization. For. Ecol. Manag. 60:143--156.
Sachs, T., A. Novoplansky and D. Cohen. 1993. Plants as competing populations of redundant organs. Plant Cell Environ. 16:765--770. Shinozaki, K., K. Yoda, K. Hozumi and T. Kira. 1964. A quantitative analysis of plant form----the pipe model theory. I. Basic analyses. Jpn. J. Ecol. 14:97--104.
Sprugel, D.G. 1990. Components of woody-tissue respiration in young Abies amabilis (Dougl.) Forbes trees. Trees 4:88--98. Sprugel, D.G. and U. Benecke. 1991. Measuring woody-tissue
respi-ration and photosynthesis. In Techniques and Approaches in Forest Tree Ecophysiology. Eds. J.P. Lassoie and T.M. Hinckley. CRC Press, Boca Raton, FL, pp 329--355.
Sprugel, D.G., T.M. Hinckley and W. Schaap. 1991. The theory and practice of branch autonomy. Annu. Rev. Ecol. Syst. 22:309--334. Sprugel, D.G., M.G. Ryan, J.R. Brooks, K.A. Vogt and T.A. Martin.
1995. Respiration from the organ level to the stand. In Resource Physiology of Conifers: Acquisition, Allocation and Utilization. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, CA, pp 255--299.
Stenberg, P. 1996. Simulations of the effects of shoot structure and orientation on vertical gradients in intercepted light by conifer canopies. Tree Physiol. 16:99--108.
Stenberg, P., T. Kuuluvainen, S. Kellomäki, J.C. Grace, E.J. Jokela and H.L. Gholz. 1994. Crown structure, light interception and produc-tivity of pine trees and stands. In Environmental Constraints on the Structure and Productivity of Pine Forest Ecosystems: A Compara-tive Analysis. Eds. H.L. Gholz, S. Linder and R.E. McMurtrie. Ecol. Bull. 43:20--34.
Uotila, A. and S. Mustonen. 1994. The effect of different levels of green pruning on the diameter growth of Pinus sylvestris L. Scand. J. For. Res. 9:226--232.
Vyskot, M. 1983. Young Scotch pine in biomass. Academia Nak-ladatelstvi Ceskoslovenske Akademie Ved. Praha, 156 p.